Abstract
Purpose
To measure TACE utilization and survival benefit among patients with HCC in the SEER patient population.
Method and Methods
A retrospective study identified 37, 832 patients with HCC diagnosed between 1991 and 2011. Survival was estimated by Kaplan-Meier method and compared using the log rank test. Propensity score matching was used to address imbalance of covariates.
Results
Over 75% of HCC patients did not receive any HCC directed treatment. TACE was the most common initial therapy (15.9%). Factors associated with TACE utilization included younger age, more HCC risk factors, more comorbidities, higher socio-economic status, intrahepatic tumor, unifocal tumor, vascular invasion, and smaller tumor size (all p<0.001). Median survival was improved in patients treated with TACE compared to those not treated with TACE (20.1 vs. 4.3 months, p<0.0001). Similar findings were demonstrated in propensity scoring analysis (14.5 vs. 4.2 months, p<0.0001) and immortal time bias sensitivity analysis (9.5 vs. 3.6 months, p<0.0001). There was a significantly improved survival hazard ratio in patients treated with TACE [HR 0.42 (95%CI 0.39 – 0.45)].
Conclusions
HCC patients treated with TACE experienced significant survival advantage compared to those not treated with TACE. Over 75% of SEER-Medicare patients diagnosed with HCC received no identifiable oncologic treatment. There is a significant public health need to increase awareness of efficacious HCC treatments such as TACE.
INTRODUCTION
The overall prognosis for patients with HCC is poor, with a 5 year survival less than 5%; survival is worse for patients who do not receive any liver specific therapy.1 The majority of the HCC patients present at intermediate or advanced stage, precluding them from potentially curative treatments.2 Trans-arterial chemoembolization (TACE) is recommended treatment for intermediate-stage HCC.2,3 TACE is the most common oncologic treatment for HCC patients in the United States.4 TACE is also the most common bridging therapy offered to >70% of patients on the waiting list for liver transplantation in the United States.5 According to the American Association for the Study of Liver Diseases Guidelines, TACE is the recommended first line non-curative therapy for non-surgical patients who do not have vascular invasion or extrahepatic spread.6,7
Despite clinical trials and single center studies supporting the use of TACE in selected patients with HCC, little is known about population-based survival efficacy of TACE in the treatment of HCC in unselected patients.8,9 Prior analysis has identified variation in the management of HCC, suggested to be as important as clinical and tumor related characteristics in determining the extent and type of HCC therapy.4 The objective of this analysis was to measure the survival benefit of TACE in the Medicare cohort. The hypothesis of this study is that TACE confers a survival advantage when compared to matched controls.
MATERIALS AND METHODS
Data Source
The Institutional Review Board approved this study. The Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database was the source of data for this study (http://healthcaredelivery.cancer.gov/seermedicare/). The 2012 SEER linkage version was utilized, which includes outcome data through 12/31/2011. The SEER cancer registries collected clinical, demographic and cause of death information for Medicare patients with cancer. Medicare claims for health care services were captured from the time of a person's Medicare eligibility until death. The SEER-Medicare linked database used in this study contains information on HCC cases diagnosed between 1991 and 2009 from 16 cancer registries in eight states (Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah) and 9 metropolitan areas (Atlanta, Greater California, Detroit, Los Angeles, San Francisco/Oakland, San Jose-Monterey, Seattle-Puget Sound, and Rural Georgia), accounting for about 25% of the Medicare-insured population in the United States. Geographically-based socioeconomic information from the US Census Bureau is also included in this database. The procedure codes, the dates of procedures from 1991–2011 for those HCC patients, and their living status were included in the Medicare claims. These data include inpatient and outpatient procedures, physician-generated and laboratory-generated claims, home health, and hospice claims.
Study cohort
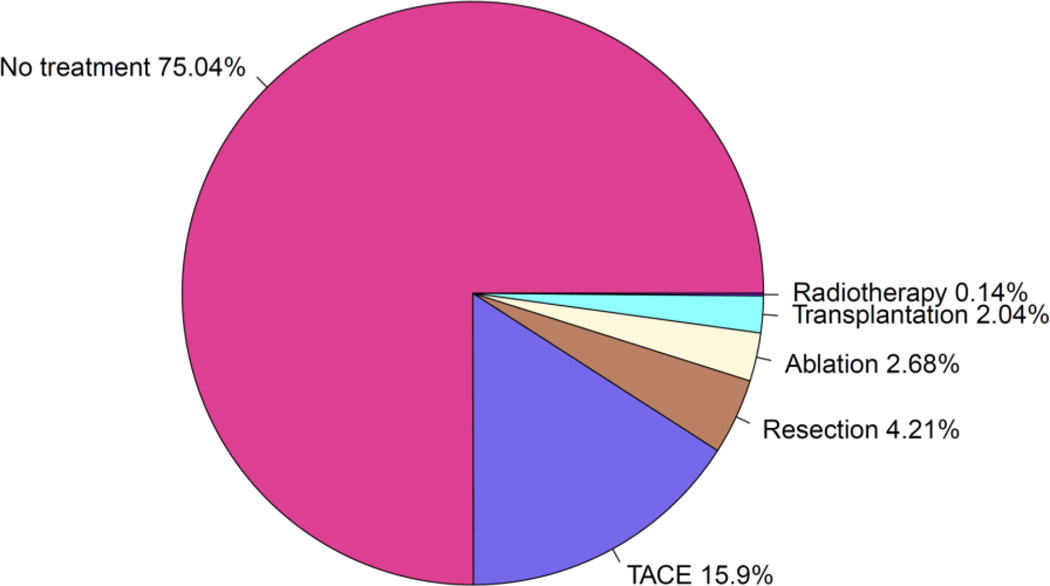
A total of 43,594 patients with HCC were identified from the SEER cancer registries. Patients were excluded from this study if they were diagnosed with an intrahepatic bile duct carcinoma only, if the diagnosis date was before 1991, or if the diagnosis date was missing. After applying these exclusions, the study cohort comprised 37,832 HCC patients diagnosed between 1991 and 2009. In the data analyses, either the total study cohort or subsamples from the total cohort were used depending on the study objectives. The majority of HCC patients (75.0%) with Medicare coverage received no oncologic treatment (Figure 1). Among patients who received HCC treatment, TACE was the most common initial therapy utilized (15.9%), followed by surgical resection (4.2%), ablation (2.7%), liver transplantation (2.0%), and radiotherapy (0.1%).
Figure 1.
Oncologic interventions in Hepatocellular Carcinoma patients in SEER-Medicare linked database 1991–2010. For patients who received more than one intervention, only the first intervention is depicted.
The characteristics of the study cohort have been summarized in Table 1. TACE was administered in isolation or in concert with other modalities in 6432 (17%) patients compared to 31,400 (83%) patients who did not receive TACE (Table 1). The mean age of the TACE group was 70.3 years old, 69.7% were male, and 61.9% were White. HCC patients undergoing TACE were younger than those were not treated with TACE (70.3yrs ± 9.4 vs. 72.4yrs ± 11.2, p<0.0001). There were ethnic differences in HCC patients who were treated with TACE including fewer Whites (61.9% vs. 66.9%, p<0.0001) and more Asians (15.6% vs. 9.5%, p<0.0001). The HCC risk factors hepatitis C virus, hepatitis B virus, alcohol history, obesity/diabetes, and rare diseases were documented in a statistically greater proportion of HCC patients who underwent TACE. Cirrhosis was present in 74.3% of TACE patients compared to 41.0% of those not treated with TACE. More comorbidities were reported in HCC patients who were treated with TACE compared to HCC patients who did not receive TACE (p<0.0001). Over half of HCC patients receiving TACE had a Charlson Score ≥3. HCC patients who were treated with TACE had a higher socioeconomic status (SES) compared to HCC patients not treated with TACE. (Table 1)
Table 1.
Basic Demographics of HCC patients in SEER-Medicare linked database 1991–2010
| TACE + | TACE − | Significance | |
|---|---|---|---|
| Population | 6432 (17.00%) | 31400 (83.00%) | |
| Age | 70.3 (9.4) | 72.4 (11.2) | <.0001 |
| Gender | 0.0044 | ||
| Male | 4482 (69.68%) | 21310 (67.87%) | |
| Female | 1950 (30.32%) | 10090 (32.13%) | |
| Ethnicity | <.0001 | ||
| White | 3982 (61.91%) | 21006 (66.90%) | |
| Black | 588 (9.14%) | 3133 (9.98%) | |
| Hispanic | 330 (5.13%) | 1489 (4.74%) | |
| Asian | 1001 (15.56%) | 2985 (9.51%) | |
| Other | 531 (8.26%) | 2787 (8.88%) | |
| HCC Risk Factors35 | |||
| HCV | 3443 (53.53%) | 7876 (25.08%) | <.0001 |
| HBV | 1281 (19.92%) | 2237 (7.12%) | <.0001 |
| Alcohol | 2200 (34.20%) | 6083 (19.37%) | <.0001 |
| Diabetes/ Obesity | 4246 (66.01%) | 14724 (46.89%) | <.0001 |
| Rare Diseases | 401 (6.23%) | 907 (2.89%) | <.0001 |
| Cirrhosis Present | 4776 (74.25%) | 12868 (40.98%) | <.0001 |
| Charlson Score | <.0001 | ||
| 0 | 1835 (28.53%) | 14808 (47.16%) | |
| 1 | 446 (6.93%) | 1691 (5.39%) | |
| 2 | 752 (11.69%) | 2599 (8.28%) | |
| ≥3 | 3399 (52.85%) | 12302 (39.18%) | |
| SES Quartile | <.0001 | ||
| 1 | 1390 (22.82%) | 7583 (25.49%) | |
| 2 | 1495 (24.54%) | 7442 (25.02%) | |
| 3 | 1509 (24.77%) | 7435 (25.00%) | |
| 4 | 1698 (27.87%) | 7285 (24.49%) |
HCC Hepatocellular Carcinoma; SEER Surveillance, Epidemiology and End Results; TACE Transarterial Chemoembolization; HCV Hepatitis C Virus; HBV Hepatitis B Virus; SES Socioeconomic Status
Outcomes of Interest
Sociodemographic and tumor characteristics
The sociodemographic characteristics include age at diagnosis, gender, ethnicity and median household incomes. The age at diagnosis was calculated by subtracting the birth year from the diagnosis year. The median household income was generated from the Census 2000 data (included in the 2012 SEER linkage version). The following known HCC risk factors were identified via ICD- diagnosis codes: hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholism, diabetes and obesity, rare genetic diseases associated with HCC, and the presence of cirrhosis (Table 1 in Supplemental Materials).10 The Klabunde modification of the Charlson comorbidity index was used to quantify non-liver comorbidities.11 The comorbidity index was calculated from hospital and physician claims in the 12 months before the HCC diagnosis. Specific tumor characteristics include tumor size (maximal diameter of the largest tumor), tumor number, intrahepatic location vs. extrahepatic extension, presence of metastasis, presence of vascular invasion, and American Joint Committee on Cancer (AJCC) HCC stage.
Treatment Modalities
HCC-related treatments were identified using the ICD-9 procedure codes and Healthcare Common Procedure Coding System (HCPCS) codes (Table 2 in in Supplemental Materials). These treatments include TACE, surgical resection, ablation, liver transplantation, and radiotherapy. For patients who received multiple treatments, the initial treatment and the order of successive treatments were identified by the Medicare claim dates.
Statistical analysis
Descriptive statistics were conducted including median and range or mean and standard deviation for continuous variables, as well as frequencies for categorical variables. Sociodemographic and tumor characteristics were compared between patients receiving TACE and patients not receiving TACE using a two-sample t-test, Wilcoxon-Mann-Whitney test, or chi-square test where appropriate. Survival was calculated as the time from diagnosis to death from any cause. Patients who were still alive were censored on 12/31/2011, the date of last follow-up in Medicare claims. The overall survival was estimated by the Kaplan-Meier method and group comparisons performed using the log-rank test. All the data analyses were performed using SAS 9.4 (Cary, NC).
The primary outcome of interest was survival from the time of diagnosis. Comparisons were made among the following subsamples: 1) all patients treated with a TACE vs. all patients who did not receive TACE (includes patients who received other therapies or no treatment), and 2) patients who were treated with TACE-only vs. patients without any HCC-related treatments (we removed patients who received any oncologic therapy). The SEER dataset includes Medicare Part D data, which allows for identification of chemotherapy and exclusion of treated patients when appropriate for analysis. To address the imbalance of covariates in the survival outcome analysis, propensity score matching was performed. In the propensity score analysis, patients receiving TACE-only were selected as cases and patients without any HCC-related treatments were selected as controls. The propensity score for each patient’s likelihood of receiving TACE was calculated from a logistic regression with sociodemographic and tumor characteristics as covariates. Based upon the propensity score, a 1:1 case-control match without replacement was performed using a greedy algorithm.12 To account for the properties of the matched pairs, the balance of covariates between cases and controls were evaluated using paired t-test for continuous variables and Wald F-test from generalized estimating equations models for categorical variables. The survival outcomes of cases and controls were estimated by the Kaplan-Meier curves and group comparisons performed by a F-test in a Cox proportional hazards model stratified for the matched pairs.
To minimize the immortal time bias,13 a sensitivity analysis was performed using the patients receiving TACE-only within 90 days of HCC diagnosis. In observational studies there may be a delay or waiting period before a subject receives treatment. If treated subjects entered a cohort at the time of their first treatment but untreated subjects entered the cohort at an earlier time, such as their first assessment at clinic, then the time period between the first clinic visit (baseline) and the first treatment corresponds to “immortal time”. This delay period is labeled “immortal” because, by design, subjects in the treated group cannot develop an outcome before receiving the treatment. Therefore, if some subjects develop the outcome before getting the opportunity to initiate treatment, they will be assigned to the untreated group.
RESULTS
Tumor Characteristics
The tumor characteristics are summarized in Table 2. Among patients receiving TACE, the HCC tumor burden was more likely to be intrahepatic (81.1% vs. 60.0%, p<0.0001) and unifocal (48.9% vs. 45.9%, p<0.0001) compared to HCC patients who were not treated with TACE. The mean HCC tumor size was smaller among TACE patients (5.8cm ± 3.8 vs. 6.4cm ± 4.4, p<0.0001). Vascular invasion was more common among TACE patients (15.8% vs. 11.4%, p<0.0001).
Table 2.
Hepatocellular Carcinoma Tumor Characteristics
| TACE + | TACE − | Significance | |
|---|---|---|---|
| Population | 6432 (17.00%) | 31400 (83.00%) | |
| Intrahepatic Disease | 5219 (81.14%) | 18849 (60.03%) | <0.0001 |
| Unifocal | 2554 (48.94%) | 8646 (45.87%) | |
| Multifocal | 2146 (41.12%) | 7966 (42.26%) | |
| Unspecified | 519 (9.94%) | 2237 (11.87%) | |
| Extrahepatic Disease | 367 (5.71%) | 3272 (10.42%) | |
| Unspecified | 846 (13.15%) | 9279 (29.55%) | |
| Vascular Invasion | <0.0001 | ||
| YES | 1019 (15.84%) | 3572 (11.38%) | |
| NO | 3814 (59.30%) | 13301 (42.36%) | |
| UNKNOWN | 1599 (24.86%) | 14527 (46.26%) | |
| Tumor Size | < 0.0001 | ||
| Mean Size (cm) | 5.8 (3.8) | 6.4 (4.4) | <0.0001 |
| Median Size (cm) | 5.0 | 5.3 | <0.0001 |
| <2cm | 360 (5.60%) | 1401 (4.46%) | |
| 2–5cm | 2412 (37.52%) | 7320 (23.32%) | |
| >5cm | 2307 (35.88%) | 9082 (28.93%) | |
| UNKNOWN | 1350 (21.00%) | 13593 (43.30%) | |
| AJCC | < 0.0001 | ||
| I | 1488 (23.15%) | 4325 (13.78%) | |
| II | 914 (14.22%) | 2038 (6.49%) | |
| III | 789 (12.27%) | 2574 (8.20%) | |
| IV | 207 (3.22%) | 2510 (7.99%) | |
| UNKNOWN | 3031 (47.15%) | 19949 (63.54%) |
TACE Transarterial Chemoembolization; cm Centimeter; AJCC American Joint Committee on Cancer
Survival After TACE
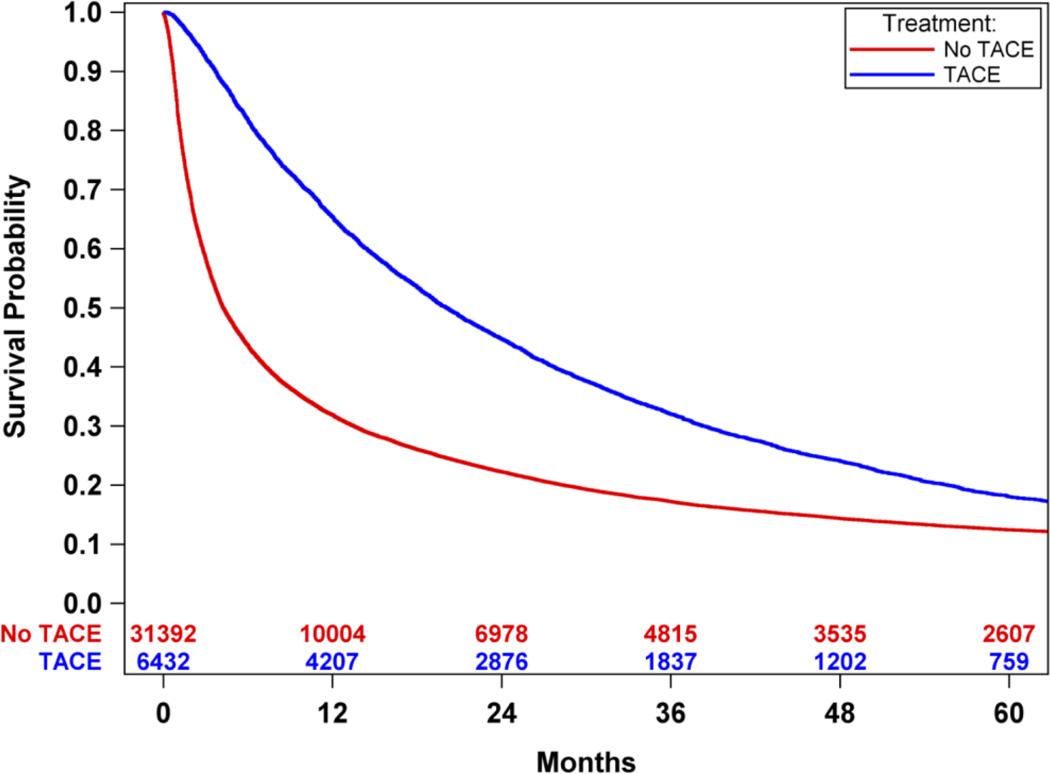
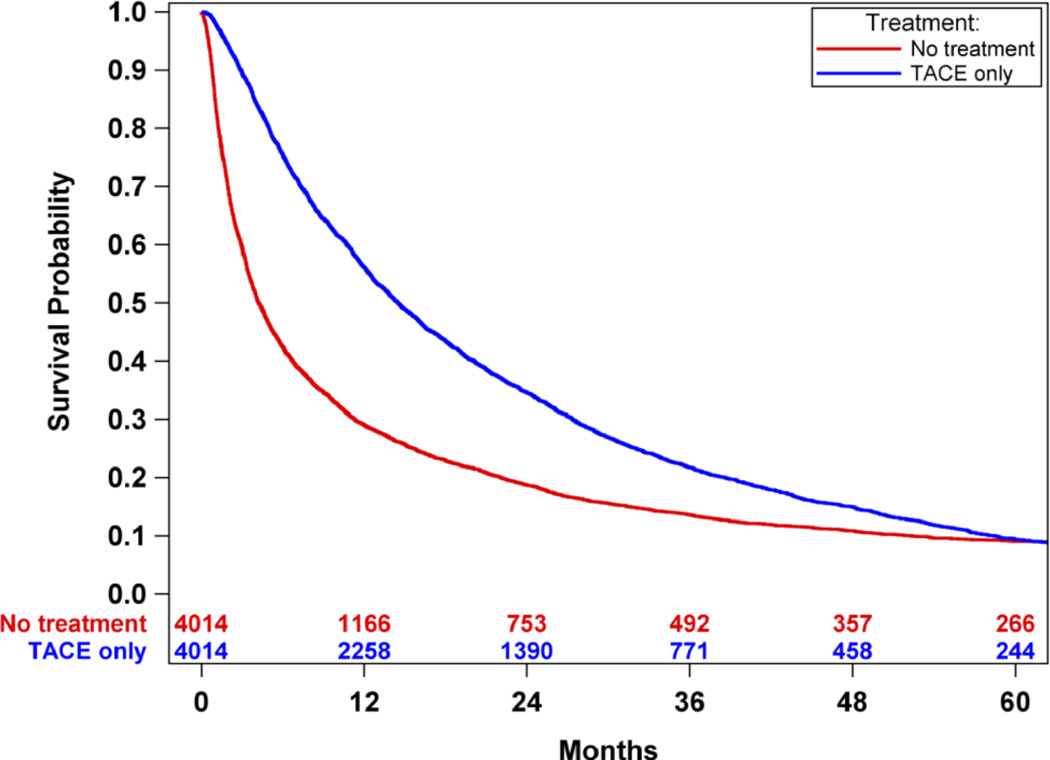
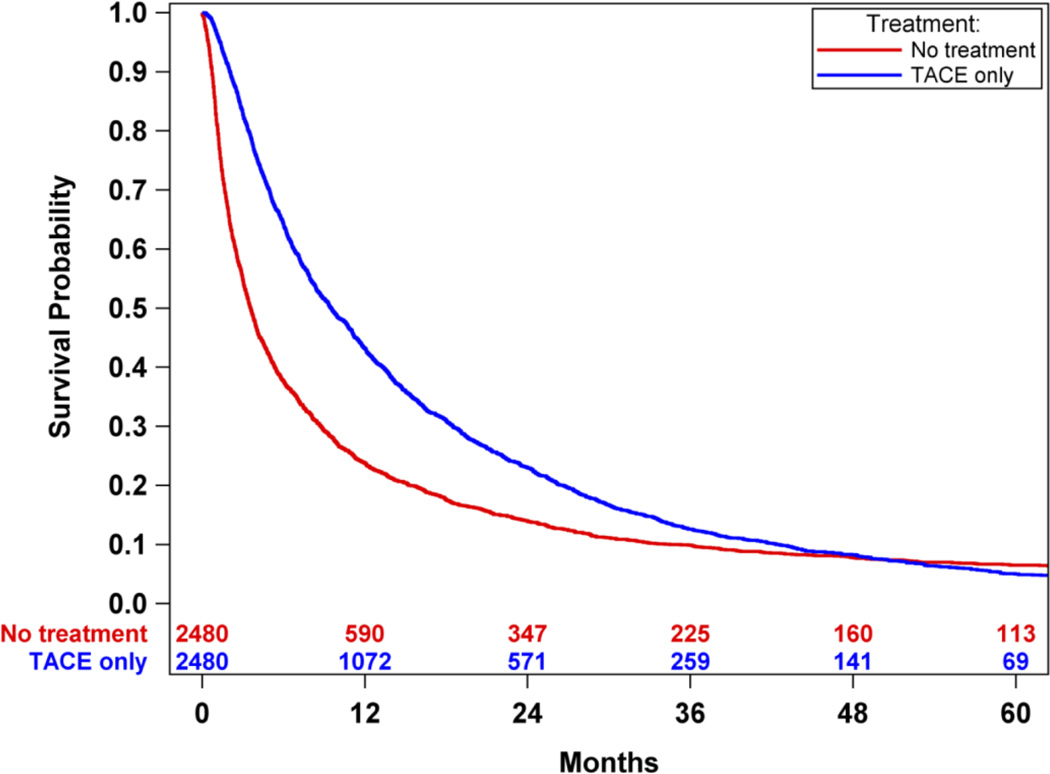
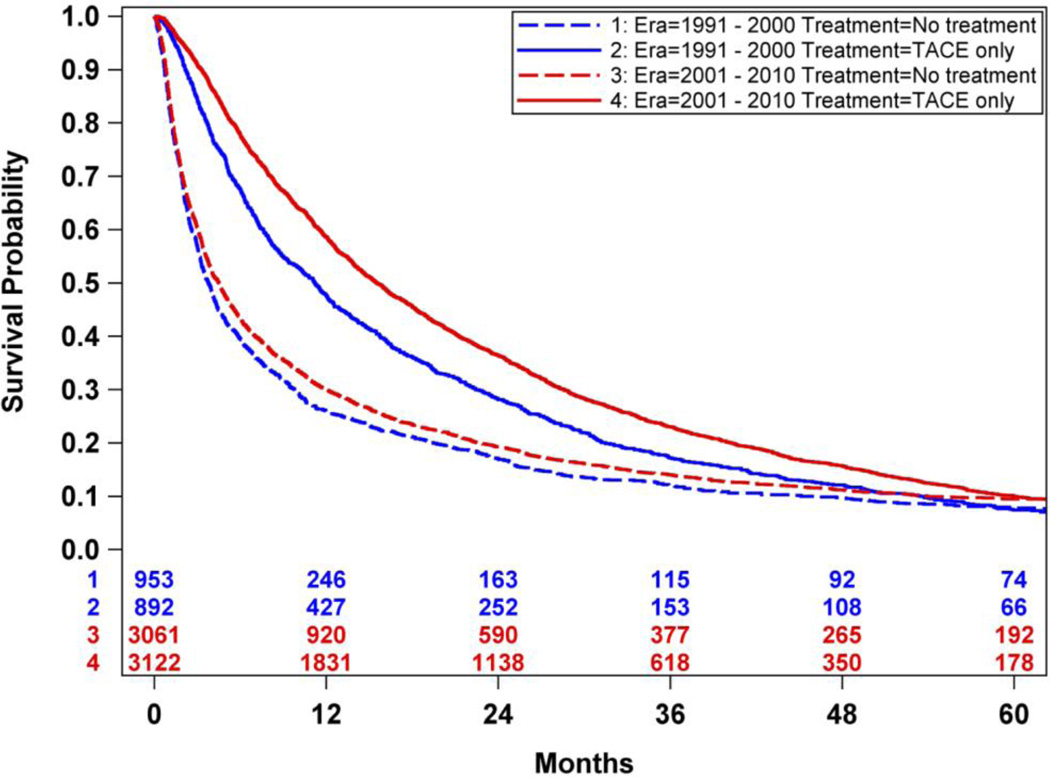
An overall survival analysis of Medicare HCC patients was performed where patients were dichotomized based upon whether or not they were treated with TACE treatment, irrespective of other non-TACE treatments. Survival was significantly improved in patients treated with TACE compared to those not treated with TACE (median survival 20.1 months-TACE vs. 4.3 months-no TACE, p<0.0001). (Figure 2) There was an improved survival hazard ratio of HCC patients treated with TACE compared to those not treated with TACE [HR 0.59 (95% CI 0.57 – 0.61)]. To address the imbalance of covariates in the survival outcome analysis, propensity score matching was performed resulting in 4014 cases of patients receiving TACE-only and 4014 matched controls of patients who did not receive any oncologic treatment. There were no significant difference in the balance of covariates between cases and controls (Tables 3 and 4 in Supplemental Materials). Propensity score analysis also demonstrated significantly improved survival in HCC patients treated with TACE-only compared to those HCC patients who did not receive any oncologic treatments (median survival 14.5 months-TACE vs. 4.2 months-no TACE, p<0.0001). (Figure 3) There was an improved survival hazard ratio of HCC patients treated with TACE compared to those not treated with TACE as determined in the propensity score analysis [HR 0.42 (95%CI 0.39 – 0.45)]. A sensitivity analysis was then performed utilizing the propensity match pairs to minimize the immortal time bias by limiting the TACE patients to those who were treated within 90 days after the HCC diagnosis date. Overall survival remained significantly improved in HCC patients treated with TACE within 90 days of diagnosis compared to patients not treated with TACE (median survival 9.5 months-TACE within 90 days vs. 3.6 months-no TACE, p<0.0001). (Figure 4) There was an improved survival hazard ratio of HCC patients treated with TACE compared to those not treated with TACE as determined in the sensitivity analysis [HR 0.48 (95%CI 0.44 – 0.52)]. Finally, propensity score matched TACE survival analyses were performed to measure the era effect on TACE outcomes. The survival hazard ratio decreased from the initial decade era 1991–2000 [HR 0.47 (95%CI 0.38 – 0.60)] to the most recent decade era 2001–2010 [HR 0.40 (95%CI 0.37 – 0.44)], but this decrease was not statistically significant (p=0.13). (Figure 5)
Figure 2.
Overall survival analysis of Medicare HCC patients, dichotomized based upon whether or not they were treated with TACE, irrespective of other non-TACE treatments. Survival was significantly improved in patients treated with TACE compared to those not treated with TACE (median survival: 20.1 months-TACE vs. 4.3 months-no TACE, p<0.0001).
Figure 3.
Propensity score-matched survival analysis of Medicare HCC patients only receiving TACE compared with patients not receiving any treatment. Survival was significantly improved in patients treated with TACE compared to those not treated with TACE (median survival: 14.5 months-TACE vs. 4.2 months-no treatment, p<0.001).
Figure 4.
A sensitivity analysis was performed limiting the TACE patients to those who were treated within 90 days after the HCC diagnosis date in effort to minimize the immortal time bias. Overall survival remained significantly improved in HCC patients treated with TACE within 90 days of diagnosis compared to patients not treated with TACE (median survival: 9.5 months-TACE within 90 days vs. 3.6 months-no treatment, p<0.0001).
Figure 5.
TACE significantly improved survival in both eras. The hazard ratio of TACE vs. No-treatment decreased from the initial decade era 1991–2000 [HR 0.47 (95%CI 0.38 – 0.60)] to the most recent decade era 2001–2010 [HR 0.40 (95%CI 0.38 – 0.44)].
DISCUSSION
The principal finding of this propensity-matched study is a 6–14 month improved survival in HCC patients treated with TACE compared to patients not treated with TACE. Although TACE was the most common oncologic intervention, >75% of HCC Medicare patients did not receive any oncologic therapies (Figure 1). The characteristics of many patients who did not receive any oncologic therapies were similar to those who were treated with TACE suggesting a significant underutilization of TACE in the SEER-Medicare HCC population.
This study may underestimate the survival benefit of TACE because all TACE were weighted as equivalent, despite prior studies demonstrating that the tumor response to a TACE affects further treatment options and survival.14–17 For example, it was not possible to radiographically assess the adequacy of the TACE response. Other studies demonstrate an increased patient survival with a complete or partial response compared to HCC patients with stable or progressive disease following TACE.15 Furthermore, this analysis was unable to determine if the TACE was accomplished via a non-selective “lobar” or selective fashion, and whether conventional lipiodol or newer drug eluting beads were used as the embolic agent. The SEER-Medicare HCC population in this study undoubtedly represents a mixture of these TACE techniques. Despite this heterogeneity, a significant survival advantage was demonstrated with TACE utilization.
TACE resulted in palliative life prolongation. Prior analysis of HCC SEER-Medicare data reported a significant survival improvement in HCC from 1973–2010.18 The improved survival was associated with earlier detection of HCC (at a curative stage) and greater utilization of curative modalities.18 Earlier presentation in HCC is evidenced by increased diagnosis of tumors ≤5.0 cm in diameter during 2000–2010, surpassing diagnosis of larger tumors.19 Utilization of curative (or potentially curative) therapies liver transplantation, surgical resection and ablation all have increased over time.18 However, TACE is employed more frequently than all these curative therapies combined, highlighting the central role of TACE in HCC treatment.4,18
Despite these favorable outcomes in early stage HCC, another SEER analysis found that nearly 50% of HCC patients meeting Milan criteria (AJCC Stage I and II) received only supportive care.20 Similarly, other publications show a underutilization of surgical therapy in patients with HCC.21 There is a clear public health need to increase curative and life prolonging HCC interventions in the SEER-Medicare population. It is likely that many HCC oncologic therapies are administered at specialty or tertiary care facilities. Studies demonstrate that the referral to specialized care varies significantly and may account for the disparity in receipt of liver directed intervention.22 Further investigation is needed to identify opportunities to improve referral and treatment of patients with HCC.23
As with all large database studies, these analyses are limited by a general lack of granularity in the data, including why clinicians chose to not treat the majority of Medicare patients diagnosed with HCC in this cohort. Prior studies have demonstrated that utilization of multidisciplinary tumor directed care is associated with improved HCC patient outcomes, unfortunately our analysis does not account for tumor board involvement.24 The HCC patient cohort was large, thus some reported statistical differences might not be clinically meaningful. Furthermore, missingness of patient and tumor characteristics occurs at a higher frequency in HCC patients who did not receive any oncologic intervention, which may bias the propensity score matching process. Misclassification may bias the results toward the null, specifically when analysis attempted to exclude patients who received any oncologic treatment except TACE from analysis. This study is limited by the quality of the data collection and billing codes utilized in the registry dataset. The SEER-Medicare database does not include clinical variables required to calculate staging scores such as the Child-Turcotte-Pugh score or Barcelona-Clinic Liver Cancer staging system score. No laboratory data was available in the SEER-Medicare data, which significantly limits generalizability given that most HCC arise in the setting of cirrhosis and HCC incidence is higher in decompensated cirrhosis. It is likely that the etiology for non-treatment in some patients was liver decompensation.
In summary, this report describes population-based TACE utilization in the community. This epidemiology study of the SEER-Medicare registry demonstrates a significant palliative, life-prolonging, survival advantage in HCC patients treated with TACE. Although TACE was the most commonly utilized oncologic modality for HCC, over 75% of SEER-Medicare patients diagnosed with HCC receive no identifiable oncologic therapy. There is a public health need to increase awareness of efficacious HCC treatments in the Medicare population, including TACE utilization for intermediate-staged HCC.
Supplementary Material
Acknowledgments
Grant Support: This research was sponsored by the Deep South Resource Center for Minority Aging Research and the National Institute on Aging Award Number 3 P30 AG031054-08S1 (SG). This research was funded by National Institutes of Health grant numbers 1 K23 DK091514 (DD), 1 R03 DK106432 (DD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
SG, JW,PL, and DD designed the study, PL DR and MK collected the data and performed statistical analyzes and interpreted the data, SH, JW, PL analyzed and interpreted the data, SG, JW, and DD drafted the manuscript AA, HS, BM, and DE revised and edited the manuscript and approved its final version.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117(5):1019–1026. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 5.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver Transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10(4p2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M for the of Diseases A. Management of hepatocellular carcinoma: An update. Hepatology. 2011 doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman M, Bruix J, Porayko M, Tran T, Committee A. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology (Baltimore Md.) 2012;56(3):793–796. doi: 10.1002/hep.25869. [DOI] [PubMed] [Google Scholar]
- 8.Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 10.Welzel TM, Graubard BI, Quraishi S. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. The American journal of …. 2013 doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM. Development of a comorbidity index using physician claims data. Journal of clinical …. 2000 doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 12.Parsons L. Performing a 1: N Case-Control Match on Propensity Score: Proceedings of the Twenty-Ninth Annual SAS Users Group International Conference. SAS Institute Inc; Montreal, QB. 2004. [Google Scholar]
- 13.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. Bmj. 2010;340 doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Lee J, Oh D-H, Yim Y, Lee H. Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. European journal of gastroenterology & hepatology. 2012;24(6):640–645. doi: 10.1097/MEG.0b013e3283524d32. [DOI] [PubMed] [Google Scholar]
- 15.Haywood N, Gennaro K, Obert J, et al. Does the Degree of Hepatocellular Carcinoma Tumor Necrosis following Transarterial Chemoembolization Impact Patient Survival? Journal of oncology. 2016;2016:4692139. doi: 10.1155/2016/4692139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogl TJ, Trapp M, Schroeder H, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214(2):349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM. Evidence-based medicine in the treatment of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2002;17(s3) doi: 10.1046/j.1440-1746.17.s3.40.x. [DOI] [PubMed] [Google Scholar]
- 18.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology (Baltimore Md.) 2015;61(1):191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulahannan SV, Duffy AG, McNeel TS, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology (Baltimore Md.) 2014;60(5):1637–1644. doi: 10.1002/hep.27288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devaki P, Wong RJ, Marupakula V, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer. 2014;120(11):1725–1732. doi: 10.1002/cncr.28639. [DOI] [PubMed] [Google Scholar]
- 21.Nathan H, Hyder O, Mayo SC, et al. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Annals of surgery. 2013;258(6):1022–1027. doi: 10.1097/SLA.0b013e31827da749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyder O, Dodson RM, Weiss M, et al. Trends and patterns of utilization in post-treatment surveillance imaging among patients treated for hepatocellular carcinoma. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013;17(10):1774–1783. doi: 10.1007/s11605-013-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davila JA, Duan Z, McGlynn KA, El-Serag HB. Utilization and Outcomes of Palliative Therapy for Hepatocellular Carcinoma: A Population-based Study in the United States. Journal of Clinical Gastroenterology. 2012;46(1):71. doi: 10.1097/MCG.0b013e318224d669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chirikov VV, Mullins CD, Hanna N, Breunig IM, Seal B, Shaya FT. Multispecialist Care and Mortality in Hepatocellular Carcinoma. Am J Clin Oncol. 2015;38(6):557–563. doi: 10.1097/COC.0000000000000000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.