Abstract
Background
Delirium affects 20–30% of patients after cardiac surgery and is associated with increased mortality and persistent cognitive decline. Hyperoxic reperfusion of ischemic tissues increases oxidative injury, but oxygen administration remains high during cardiac surgery. We tested the hypothesis that intraoperative hyperoxic cerebral reperfusion is associated with increased postoperative delirium and that oxidative injury mediates this association.
Methods
We prospectively measured cerebral oxygenation with bilateral oximetry monitors in 310 cardiac surgery patients, quantified intraoperative hyperoxic cerebral reperfusion by measuring the magnitude of cerebral oxygenation above baseline after any ischemic event, and assessed patients for delirium twice daily in the ICU following surgery using the confusion assessment method for ICU (CAM-ICU). We examined the association between hyperoxic cerebral reperfusion and postoperative delirium, adjusted for the extent of cerebral hypoxia, the extent of cerebral hyperoxia prior to any ischemia, and additional potential confounders and risk factors for delirium. To assess oxidative injury mediation, we examined the association between hyperoxic cerebral reperfusion and delirium after further adjusting for plasma levels of F2-isoprostanes and isofurans at baseline and ICU admission, the association between hyperoxic cerebral reperfusion and these markers of oxidative injury, and the association between these markers and delirium.
Results
Ninety of the 310 patients developed delirium following surgery. Every 10%·hour of intraoperative hyperoxic cerebral reperfusion was independently associated with a 65% increase in the odds of delirium (OR, 1.65 [1.12 to 2.44]; P=0.01). Hyperoxia prior to ischemia was also independently associated with delirium (1.10 [95% CI, 1.01 to 1.19]; P=0.02), but hypoxia was not (1.12 [95% CI, 0.97 to 1.29]; P=0.11). Increased hyperoxic cerebral reperfusion was associated with increased concentrations of F2-isoprostanes and isofurans at ICU admission, increased concentrations of these markers were associated with increased delirium, and the association between hyperoxic cerebral reperfusion and delirium was weaker after adjusting for these markers of oxidative injury.
Conclusions
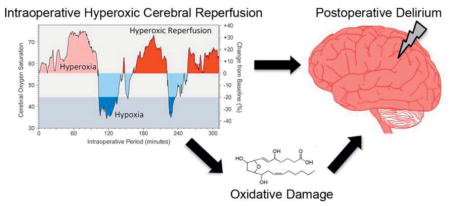
Intraoperative hyperoxic cerebral reperfusion was associated with increased postoperative delirium, and increased oxidative injury following hyperoxic cerebral reperfusion may partially mediate this association. Further research is needed to assess the potential deleterious role of cerebral hyper-oxygenation during surgery.
Keywords: brain oxygenation, oxidative injury, oxidative stress, delirium, oxygen, hyperoxia, ischemia reperfusion, surgery, f2-isoprostanes, isofurans
Graphical abstract
Introduction
Delirium is a manifestation of acute brain dysfunction and affects 20–30% of patients following cardiac surgery.1, 2 Delirium is associated with increased mortality, pulmonary dysfunction, and duration of hospitalization following cardiac surgery, and is an independent predictor of long-term cognitive decline in other medical and surgical patient populations.3–6
During cardiac surgery impaired heart function, exposure to cardiopulmonary bypass, and rapid changes in temperature, intravascular pH, and arterial pressure lead to abrupt changes in cerebral perfusion, oxygen extraction, and oxygen consumption. These changes in brain oxygenation, along with exposure to anesthetics, systemic and cerebral inflammation, and microemboli may precipitate delirium following cardiac surgery, although precise mechanisms are poorly understood.
In preclinical studies tissue hypoxia, hyperoxia, ischemia, and hyperoxic reperfusion – all common in patients undergoing cardiac surgery – increase the production of reactive oxygen species and induce oxidative injury.7–10 Intraoperative oxidative injury may contribute to postoperative brain injury, as it has been demonstrated that intraoperative oxidative injury contributes to postoperative kidney injury, another organ susceptible to ischemia reperfusion injury.11 Hyperoxia may contribute to this phenomenon. Indeed, in other clinical scenarios of cerebral ischemia and reperfusion injury, including cardiac arrest and stroke, hyperoxia during reperfusion is a strong predictor of neurologic damage.12, 13 Hyperoxia during surgery remains standard clinical practice despite these potential deleterious effects.
We conducted this study to test the hypothesis that hyperoxic cerebral reperfusion is associated with the development of delirium following cardiac surgery and that increased oxidative injury may mediate this association.
Methods
Patients
We performed a cohort study using participants from the Statin AKI Cardiac Surgery RCT, a randomized clinical trial conducted to test the hypothesis that perioperative atorvastatin treatment compared to placebo reduces acute kidney injury, intensive care unit (ICU) delirium, and additional organ dysfunctions following cardiac surgery.14 We used the Statin trial cohort because study participants were assessed for delirium by research personnel twice daily while in the ICU, had detailed preoperative, intraoperative, and postoperative prospective data collected, and were phlebotomized at baseline and following surgery to quantify perioperative oxidative injury. Adults undergoing elective coronary artery bypass grafting, heart valve surgery, or ascending aortic surgery at Vanderbilt University Medical Center (Nashville, TN) were eligible to participate. Patients with acute coronary syndrome, liver dysfunction, prior statin intolerance, use of CYP3A4 inhibitors, current use of cyclosporine, current renal replacement therapy, history of kidney transplant, or pregnancy were excluded. Patients who had detailed measurements of intraoperative cerebral oximetry saved in the perfusionist database comprised the study cohort. The Vanderbilt Institutional Review Board approved the study, and all patients provided written informed consent.
Baseline Assessments
Past medical history, vital signs, and baseline laboratory data were obtained. To characterize baseline mental function, each subject was administered the Mini Mental State Exam and scored from 0 to 30.15 The Charlson comorbidity index, a 17 component mortality prediction score, was calculated for each subject.16
Standardized Patient Management
Anesthetic and surgical management were conducted according to institutional protocols as outlined below and previously described.17 Pulse oximetry, electrocardiography, and cerebral oximetry (INVOS, Medtronic, Minneapolis, MN) probes were placed on the finger, chest, and both sides of the forehead, respectively, prior to induction of general anesthesia and remained until completion of surgery. Baseline pulse oximetry and cerebral oximetry measurements were recorded prior to supplemental oxygen administration. Following induction of anesthesia and tracheal intubation, patients were mechanically ventilated with a fraction of inspired oxygen (FIO2) between 60–100%, tidal volumes of 8±2 ml/kg ideal body weight, respiratory rate titrated to an end-tidal carbon dioxide partial pressure between 30 and 35 mmHg, and positive end expiratory pressure of 5 cm H2O. Anesthesiologists did not use cerebral oximetry measurements to adjust patient ventilation.
Institutional protocol directed the perfusionist to set the oxygen/carbon dioxide blender on the cardiopulmonary bypass oxygenator to an FIO2 of 0.8 and titrate the sweep rate to maintain a PaCO2 between 45 and 50 mmHg during bypass. If cerebral oximetry values decreased below 80% of baseline during cardiopulmonary bypass the perfusionist performed the following sequence: requested the surgeon to check and adjust arterial and venous cannulas to confirm central cannulation and increase venous drainage, decreased sweep speed on oxygenator to increase patient partial pressure of carbon dioxide to 50–55 mmHg, increased arterial flow to as high as 3 L/min/m2, increased FIO2 to 1.0, and requested the anesthesiologist to transfuse one unit of packed red blood cells if the hematocrit was less than 24%. If cerebral oximetry values rose above baseline prior to or following any decrease in values, perfusionists and anesthesiologist did not change patient management to reduce cerebral oxygenation.
Cerebral Oxygenation Measurements
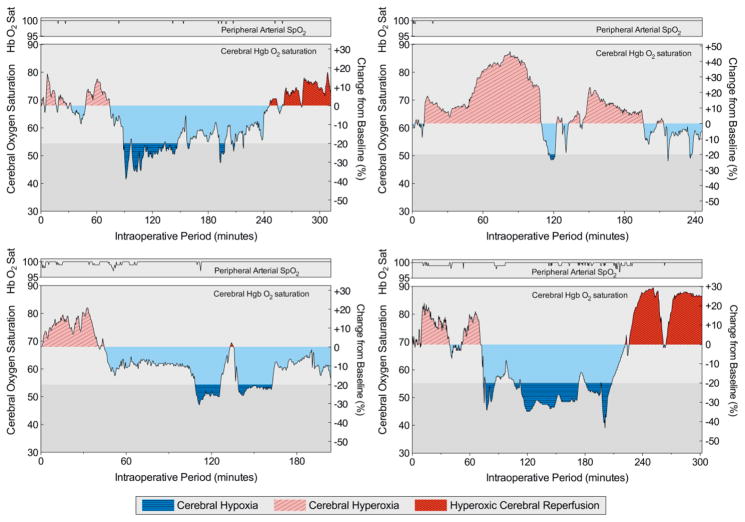
Cerebral oximetry continuously measures regional cerebral tissue oxygenation using near-infrared spectroscopy. Sensors are designed to measure approximately one and a half cubic centimeters in the outer cortical layers of the brain.18, 19 Cerebral oximetry measurements were automatically recorded every 5 seconds and stored in the INVOS monitor, and the perfusionist saved the data in the clinical database after surgery. The cerebral oxygenation values used for analyses were taken as the average of the right and left probes. These values were used to quantify baseline cerebral oxygenation, ischemia, and the cumulative (over the course of surgery) levels of hypoxia, hyperoxia, and hyperoxic reperfusion. These levels were quantified using areas under the oxygenation-time curves (AUCs). Examples of these AUC oxygenation metrics are presented (Figure 1).
Figure 1. Illustration of cerebral oxygenation metrics.
Four different patients’ peripheral arterial hemoglobin oxygen saturations (SpO2) and cerebral hemoglobin oxygen saturations plotted during surgery were chosen to illustrate oxygenation metrics and observed patterns. Hyperoxic cerebral reperfusion was defined as any cerebral oxygenation greater than baseline that followed a period of cerebral ischemia. Cerebral ischemia was defined as cerebral oxygenation 80% of baseline for five or more minutes or the equivalent AUC for desaturations to less than 80% of baseline (e.g., 60% for 2.5 minutes). Cerebral hypoxia was quantified by calculating the AUC below 80% of baseline throughout surgery, and cerebral hyperoxia by calculating the AUC above baseline throughout surgery prior to any ischemia or the AUC above baseline throughout surgery in patients that never experienced ischemia.
Hyperoxic cerebral reperfusion was defined as any cerebral oxygenation greater than baseline that followed a period of cerebral ischemia, and we quantified hyperoxic cerebral reperfusion by calculating the oxygenation AUC above baseline after any period of ischemia. We chose a decrease to 80% of baseline as a threshold for cerebral hypoxia and ischemia because this value has been reported as the threshold for interventions to increase cerebral oxygenation during surgery,20, 21 and we chose 5 minutes of cerebral hypoxia or an equivalent AUC for data less than 80% of baseline (e.g., 60% of baseline for 2.5 minutes) to define ischemia because this duration has been associated with neuron injury and poor neurologic recovery in other settings.22, 23 We quantified cerebral hyperoxia independent of hyperoxic cerebral reperfusion by measuring the oxygenation AUC above baseline throughout surgery prior to any ischemia or in patients that never experienced ischemia.
Because associations between cerebral hyperoxia and outcomes have not previously been investigated, criteria for cerebral oximetry hyperoxia are not established. Some degree of cerebral oxygenation above baseline may still be in the normal range. As a sensitivity analysis, we used a higher threshold (10% above baseline) to define and calculate AUC magnitudes of hyperoxic reperfusion and hyperoxia.
AUCs are reported as 10%·hours for ease of interpretation. For example, a 20% change for one half hour equals one 10%·hour.
Delirium Assessment
Research personnel assessed delirium twice daily while patients were in the ICU using the Confusion Assessment Method for ICU delirium (CAM-ICU) and Richmond Agitation-Sedation Scale (RASS). The CAM-ICU is a validated diagnostic algorithm that assesses cardinal features of delirium by determining fluctuations in mental status, inattention, disorganized thinking, and altered level of consciousness.24 The RASS uses a 10-point scale to assess level of consciousness with negative scores indicating decreased responsiveness, a score of zero indicating a calm state, and positive scores indicating agitation.25
Oxidative injury measurements
Blood was sampled at induction of anesthesia and at ICU admission to assess oxidative injury biomarkers at baseline and immediately following surgery per protocol. Arterial blood was collected in 0.105 M sodium citrate–coated tubes, immediately placed on ice, centrifuged for 20 minutes at 1000 g, and plasma frozen at −80°C. We quantified oxidative injury by measuring non-esterified, free plasma concentrations of F2-isoprostanes and isofurans, established markers of oxidative injury in vivo,26 using gas chromatography-mass spectrometry as previously described.27, 28 We used the sum of F2-isoprostanes and isofurans to comprehensively measure oxidative injury because F2-isoprostanes and isofurans originate from the same lipid radical intermediate, both classes of molecules reflect the free radical-induced arachidonic acid peroxidation we sought to measure, and F2-isoprostanes and isofurans are differentially expressed in tissues.29, 30 These compounds are produced from a relatively limitless pool of arachidonic acid in direct proportion to the concentration of inciting radicals, which include ROS and lipid radicals. This process is unidirectional, and there is no equilibrium between the substrate (arachidonic acid) and the product (F2-isoprostanes and isofurans) in this non-enzymatic reaction.
Statistical Analysis
Prior to accessing data and performing any analysis, we created a statistical analysis plan by defining the independent and dependent variables, selecting confounders and risk factors for multiple regression analysis, and by developing the regression modeling technique and associated diagnostics. Descriptive statistics of categorical data are summarized using their count (%), and quantitative data are summarized using their median (10th percentile, 90th percentile). Pre-specified cerebral oxygenation variables included magnitudes of cerebral hyperoxic reperfusion, cerebral hypoxia, and cerebral hyperoxia. The primary endpoint was presence or absence of delirium (any CAM-ICU positive exam) in the ICU. We used multiple logistic regression to test the association of these markers of cerebral oxygenation and delirium adjusting for possible confounders and known risk factors selected a priori including age, preoperative mini-mental state exam score (MMSE), Charlson comorbidity index, valvular heart surgery, use of cardiopulmonary bypass during surgery, duration of cardiopulmonary bypass, intraoperative circulatory arrest, and nasal temperature following cardiopulmonary bypass rewarming. We did not include any risk factors for delirium that occurred after intraoperative measurement of cerebral oxygenation, since these factors may mediate the effect of intraoperative cerebral reperfusion, and adjusting for a downstream mediator has the potential to mask the target association (i.e., the association between intraoperative cerebral reperfusion and delirium). For hyperoxic cerebral reperfusion and other quantitative variables, the possibility of nonlinear association with the log-odds of delirium was investigated using a five-knot restricted cubic spline basis, where the knots were assigned at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of the independent variable. The significance of nonlinear effects was assessed using a likelihood ratio test; nonlinear terms were omitted where there was insufficient statistical evidence of nonlinear associations.
To investigate whether oxidative injury statistically mediates the associations between intraoperative cerebral oxygenation and the subsequent development of delirium, we examined the associations between cerebral oxygenation parameters and delirium in models with and without the oxidative injury biomarkers. Mediation is supported if there is a strong association between oxidative injury markers and delirium, adjusting for cerebral oxygenation parameters, and simultaneously a strong association between oxidative injury markers and cerebral oxygenation.31 The associations between intraoperative cerebral oxygenation metrics and ICU admission F2-isoprostane and isofuran concentrations (oxidative injury markers) were estimated using linear regression, adjusting for the same baseline and intraoperative covariates. The product-of-coefficients method was also used to assess mediation.32, 33
Results
The cohort included 310 patients. Demographic and intraoperative data are shown in Table 1. The median (10th percentile to 90th percentile) age of the cohort was 67 (47 to 81) years, 30.6% of patients were diabetic, and 79.7% had surgery with the use of cardiopulmonary bypass. Ninety patients (29.0%) developed delirium after surgery for a median of 1.0 (1.0 to 4.0) days. Treatment assignment of the parent trial (atorvastatin vs. placebo) did not affect oxygenation or delirium outcomes in this study.
Table 1.
Baseline and intraoperative patient characteristics.
| Patient Characteristic | Total (n = 310) |
|---|---|
| Age, years | 67 (47, 81) |
| Female gender | 111 (35.8%) |
| African American ancestry | 13 (4.2%) |
| Medical history | |
| Atrial fibrillation | 84 (27.1%) |
| Congestive heart failure | 119 (38.4%) |
| Left ventricular ejection fraction, % | 60 (35, 60) |
| Chronic obstructive pulmonary disease | 34 (11.0%) |
| Current smoking | 44 (14.2%) |
| Former smoking | 115 (37.1%) |
| Obstructive sleep apnea | 45 (14.5%) |
| Charlson comorbidity index | 2 (0, 5) |
| Diabetes | 95 (30.6%) |
| Body mass index, kg/m2 | 27.6 (22.5, 36.6) |
| Peripheral vascular disease | 82 (26.5%) |
| Stroke | 17 (5.5%) |
| Transient ischemic attack | 11 (3.5%) |
| Mini Mental State Exam score | 29 (26, 30) |
| Trails B score, seconds | 110 (70, 211) |
| Medication use | |
| Baseline angiotensin-converting enzyme inhibitor | 96 (31%) |
| Baseline statin use | 185 (59.7%) |
| Perioperative statin treatment | 159 (51.3%) |
| Baseline laboratory and hemodynamic data | |
| Blood glucose, mg/dl | 108 (88, 157) |
| Hematocrit, % | 35 (29, 43) |
| Arterial pH | 7.4 (7.34, 7.47) |
| PaCO2, mmHg | 41 (35, 49) |
| Arterial lactate, mg/dl | 0.7 (0.4, 1.4) |
| Mean arterial Pressure, mmHg | 70 (57, 86) |
| Heart rate, beats/minute | 64 (49, 84) |
| Cardiac index, liters/min/m2 | 2.2 (1.5, 3.1) |
| Central venous pressure, mmHg | 13 (7, 20) |
| Peripheral arterial hemoglobin oxygen saturation | 98 (95, 100) |
| Cerebral hemoglobin oxygen saturation | 68 (54, 81) |
| Oxidative injury biomarkers | |
| F2-isoprostanes, plasma, baseline, pg/ml | 28.8 (14.7, 58.4) |
| Isofurans, plasma, baseline, pg/ml | 45.17 (21.4, 102.8) |
| F2-isoprostanes, plasma, ICU admission, pg/ml | 31.3 (17.8, 61.8) |
| Isofurans, plasma, ICU admission, pg/ml | 63.1 (31.7, 123.6) |
| Procedure characteristics | |
| Duration of surgery, minutes | 312 (222, 479) |
| Coronary artery bypass surgery | 124 (40%) |
| Valve surgery | 225 (72.6%) |
| Mean intraop arterial hemoglobin oxygen saturation, % | 100 (99,100) |
| Cardiopulmonary bypass use | 247 (79.7%) |
| Cardiopulmonary bypass time, min | 136 (89.8, 237) |
| Aorta cross-clamp use | 164 (66.4%) |
| Circulatory arrest use | 20 (8.1%) |
Binary characteristics are reported as n (%) and continuous characteristics as median (10th percentile, 90 percentile). PaCO2, arterial partial pressure of carbon dioxide; intraop, intraoperative; min, minutes.
The median baseline cerebral hemoglobin O2 saturation at induction of anesthesia was 68 (50 to 80.5) percent. During surgery 208 of the 310 patients (67.1%) developed cerebral hypoxia (cerebral oxygenation <80% of baseline) for a median duration of 15.5 (0.6 to 108) minutes, and 159 of these patients (51.3% of the total cohort) developed cerebral hypoxia that met the definition of ischemia (hypoxia >5 minutes). Ninety-four of the 159 patients that experienced cerebral ischemia (30.3% of the total cohort) subsequently became hyperoxic (cerebral oxygenation above baseline) for a median duration of 27.3 (3.0 to 118) minutes. Despite these changes in cerebral oxygenation, the median value of each patient’s mean arterial hemoglobin oxygen saturation during surgery was 100 (99, 100) percent, and no patient experienced more than five minutes of arterial hemoglobin desaturation below 95%.
Thirty-eight of the 94 patients (40.4%) that experienced intraoperative cerebral hyperoxia following ischemia (hyperoxic cerebral reperfusion) developed delirium following surgery compared to 16 of the 65 ischemic patients (24.6%) that did not experience hyperoxic cerebral reperfusion and 36 of the 151 patients (23.8%) that did not experience ischemia.
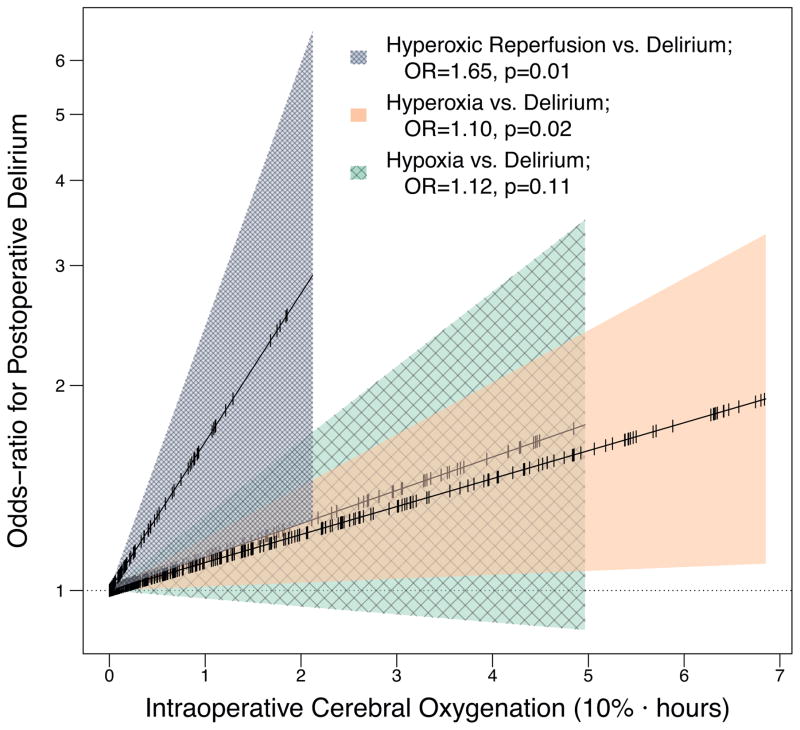
Every 10%·hour of hyperoxic cerebral reperfusion was independently associated with a 65% increase in the odds of postoperative delirium (OR, 1.65 [95% CI, 1.12 to 2.44]; P=0.01; Table 2, Figure 2). Hyperoxia outside of hyperoxic reperfusion, that is the AUC of cerebral oxygenation above baseline that occurred prior to ischemia or in patients that never experienced ischemia, was also associated with postoperative delirium (OR for delirium for every 10%·hour of hyperoxia, 1.10 [95% CI, 1.01 to 1.19]; P=0.02). In contrast, the extent of intraoperative hypoxia was not independently associated with postoperative delirium (OR for delirium for every 10%·hour of hypoxia, 1.12 [95% CI, 0.97 to 1.29]; P=0.11).
Table 2.
Intraoperative cerebral oxygenation and postoperative delirium.
| Variable | Odds Ratio | 95% CI | P value | |
|---|---|---|---|---|
| Hyperoxic cerebral reperfusion, 10%·hour | 1.65 | 1.12 | 2.44 | 0.01 |
| Cerebral hyperoxia, 10%·hour | 1.10 | 1.01 | 1.19 | 0.02 |
| Cerebral hypoxia, 10%·hour | 1.12 | 0.97 | 1.29 | 0.11 |
| Age, years | 1.04 | 1.01 | 1.06 | 0.01 |
| Preoperative MMSE score | 0.75 | 0.63 | 0.88 | <0.001 |
| Charlson comorbidity index | 1.18 | 0.99 | 1.40 | 0.07 |
| Valve surgery | 0.36 | 0.11 | 1.25 | 0.11 |
| Use of cardiopulmonary bypass | 5.66 | 1.14 | 28.11 | 0.03 |
| Duration of cardiopulmonary bypass, min | 1.00 | 0.99 | 1.00 | 0.99 |
| Use of circulatory arrest | 0.24 | 0.03 | 1.88 | 0.18 |
| Intraoperative nasal temp after rewarming, °C | 0.77 | 0.50 | 1.20 | 0.25 |
Hyperoxic cerebral reperfusion was quantified as the AUC of cerebral oxygenation above baseline after any ischemia. Cerebral hypoxia was quantified as the AUC of cerebral oxygenation below 80% of baseline and ischemia as hypoxia for five minutes or the equivalent AUC for desaturations to less than 80% of baseline (e.g., 60% for 2.5 minutes). Cerebral hyperoxia was quantified as the AUC of cerebral oxygenation above baseline exclusive of reperfusion. Delirium odds ratios are reported for each 10%·hour of oxygenation metric (e.g., 20% above baseline for 0.5 hours equals one 10%·hour). MMSE, mini mental state exam.
Figure 2. Associations between intraoperative cerebral hyperoxic reperfusion, cerebral hyperoxia, and cerebral hypoxia and postoperative delirium.
Odds ratios (OR) represent the odds of postoperative delirium for every 10%·hour (e.g., 20% above baseline for 30 minutes equals one 10%·hour hyperoxia) intraoperative oxygenation metric, adjusted for potential confounders, risk factors, and the other oxygenation metrics. For example, a patient with two 10%·hours of hyperoxic reperfusion had a 65% increase in the odds of delirium compared to the patient with one 10%·hours of hyperoxic reperfusion independent of the effects of hyperoxia prior to reperfusion, hypoxia, confounders, and risk factors on delirium. Cerebral oxygenation parameters (x-axis) were truncated at the 90th percentile to simplify exposition.
Using a higher cerebral oxygenation threshold for calculating the magnitudes of hyperoxic reperfusion and hyperoxia (cerebral oxygenation >10% above baseline following or prior to any cerebral ischemia, respectively) as a sensitivity analysis, both intraoperative hyperoxic reperfusion and hyperoxia remained associated with postoperative delirium. Every 10%·hour of hyperoxic reperfusion defined as the AUC of cerebral oxygenation >10% above baseline following ischemia was independently associated with a 59% increase in the odds of delirium (OR, 1.59 [95% CI, 1.06 to 2.39]; P=0.02), and every 10%·hour of hyperoxia defined as the AUC of cerebral oxygenation >10% above baseline exclusive of ischemia was independently associated with a 10% increase in the odds of delirium (OR, 1.10 [95% CI, 1.02 to 1.20]; P=0.02).
The median plasma concentrations of F2-isoprostanes and isofurans increased 15.5 (10th to 90th percentile, −35.4 to 73.1) pg/ml from baseline to ICU admission. F2-isoprostanes increased 1.9 (−14.6 to 21.7) pg/ml, and isofurans increased 14.6 (−33.7, 61.2) pg/ml. To assess whether the relationship between cerebral hyperoxic reperfusion and delirium could be mediated by oxidative injury, we examined 1) the association between hyperoxic reperfusion and delirium after additionally adjusting for baseline and ICU admission F2-isoprostane and isofuran concentrations, 2) the independent associations between ICU admission F2-isoprostanes and isofurans and delirium, and 3) the association between intraoperative hyperoxic reperfusion and ICU admission F2-isoprostanes and isofurans.
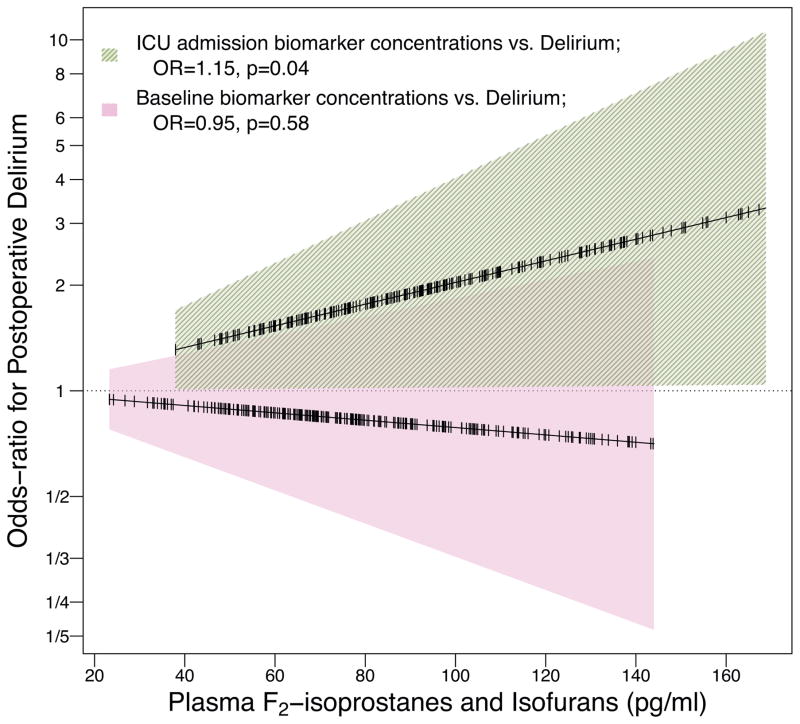
The association between hyperoxic reperfusion and delirium was diminished after adjusting for baseline and ICU admission plasma concentrations of F2-isoprostanes and isofurans, consistent with oxidative injury mediating the association between hyperoxic reperfusion and delirium. In addition, ICU admission F2-isoprostanes and isofurans were independently associated with subsequent delirium, and intraoperative hyperoxic reperfusion was associated with increased F2-isoprostanes and isofurans. Specifically, 1) the odds ratio for delirium associated with each 10%·hour of hyperoxic reperfusion was smaller by 22% and no longer statistically significant (OR, 1.43 [95% CI 0.96 to 2.12]; P=0.08) when oxidative injury markers were added to the model; 2) each 20 pg/ml increase in ICU admission F2-isoprostanes and isofurans was associated with a 15% increase in the odds of delirium (OR, 1.15 [95% CI 1.00 to 1.32]; P=0.04; Figure 3); and 3) every 10%·hour of hyperoxic reperfusion was independently associated with a 8.5 pg/ml (95% CI 1.1 to 16.0, P=0.03) increase in ICU admission F2-isoprostanes and isofurans. Despite this evidence of statistical mediation, the product-of-coefficients method did not meet statistical significance.
Figure 3. Associations between baseline and ICU admission concentrations of F2-isoprostanes and isofurans and postoperative delirium.
Odds ratios (OR) represent the odds of postoperative delirium for every 20 pg/ml increase in plasma concentrations of F2-isoprostanes and isofurans measured at baseline or at ICU admission, adjusted for potential confounders, risk factors, and ICU admission or baseline oxidative injury biomarkers, respectively. For example, a patient with an ICU admission plasma concentration of 120 pg/ml F2-isoprostanes and isofurans had a 30% increase in the odds of delirium compared to a patient with a concentration of 80 pg/ml, independent of the effects of baseline oxidative injury concentrations, potential confounders, and risk factors on delirium.
Discussion
Reperfusion injury is frequently blamed for postoperative organ injury,22, 34 but clinicians do not limit hyper-oxygenation following intraoperative ischemia. In a well-phenotyped cohort of cardiac surgery patients intraoperative cerebral hyper-oxygenation following ischemia correlated strongly with an increased incidence of postoperative delirium, and we found some evidence that increased oxidative injury may partially mediate this association. Intraoperative cerebral hyper-oxygenation independent of ischemia (prior to ischemia or in patients that did not develop ischemia) was also associated with delirium, while intraoperative cerebral hypoxia was not associated with delirium.
A deleterious association between high levels of oxygen during surgery and postoperative organ injury has not previously been reported. Harms of hyper-oxygenation following ischemia, however, have been established in other clinical scenarios. After return of spontaneous circulation following cardiac arrest, for example, hyper-oxygenation increased mortality in a dose-dependent fashion,12 and the administration of supplemental oxygen to normoxic patients following ST-elevation-myocardial infarction increased infarct size.35 Hyper-oxygenation is a common practice in the perioperative period, especially in cardiac surgery, and excess oxygen administration requires further investigation.
Most prior studies of surgical patients have focused on the hazards of cerebral hypoxia rather than hyperoxia or on the use of cerebral hemoglobin O2 saturation monitoring to affect outcomes. Low baseline cerebral hemoglobin O2 saturations have been associated with increased mortality, delirium, and cognitive decline after cardiac surgery.36–38 In a clinical trial, patients randomized to cerebral oxygenation monitoring and an intervention for desaturations less than 75% of baseline had decreased ICU length of stay, compared to patients randomized to hidden monitoring and no intervention.20, 39 It is unknown if hyper-oxygenation after desaturation contributed to the observations that desaturations were associated with worse outcomes in these previous investigations since hyper-oxygenation was not measured. In the current study we found no evidence that cerebral hypoxia was associated with postoperative delirium.
Cerebral ischemia and hyperoxia may occur during surgery following changes in systemic perfusion or associated with the use of cardiopulmonary bypass. Interestingly, arterial hemoglobin saturation did not decline in any study patients or correlate with cerebral oxygenation, and the initiation or termination of cardiopulmonary bypass did not have a consistent positive or negative effect on cerebral oxygenation. Nonetheless, to account for procedure related phenomena we adjusted for use of cardiopulmonary bypass, duration of cardiopulmonary bypass, valvular heart surgery, and use of circulatory arrest in all regression analyses. Despite this, there remains the potential for unmeasured confounders to affect the observed associations between cerebral oxygenation and delirium.
Potential mechanisms for the association between hyperoxic cerebral reperfusion and delirium include hyperoxic reperfusion-induced vasoconstriction or oxidative injury.9, 40 We found marginal evidence that increased oxidative injury mediates the association between hyperoxic reperfusion and ICU delirium. Intraoperative hyperoxic reperfusion was associated with increased plasma concentrations of F2-isoprostanes and isofurans at ICU admission; including F2-isoprostanes and isofurans in the model decreased the association between hyperoxic reperfusion and delirium; and increased F2-isoprostane and isofuran concentrations at ICU admission were independently associated with increased delirium. These findings are necessary evidence for mediation, but the weaknesses of these findings suggest additional factors contribute to the association between hyperoxic cerebral reperfusion and delirium. The perioperative changes in circulating concentrations of F2-isoprostanes and isofurans are consistent with in vitro and preclinical studies that demonstrate that hyperoxic reperfusion increases oxidative injury and that increased oxygen tension increases the production of isofurans to a greater extent than F2-isoprostanes.30, 41 F2-isoprostanes and isofurans have cerebral biologic effects. In pigs, for example, F2-isoprostane administration induced brain arteriole vasoconstriction,42 and in mice, isofurans have been implicated as mediators between hyperoxia and cognitive dysfunction.43 In humans we do not know the rate of F2-isoprostane and isofuran hydrolysis and release into plasma following cerebral production, nor do we know if systemic plasma levels of these markers reflect cerebral oxidative injury.
The study design and analyses completed in an observational study limit the conclusions that can be drawn. The associations observed among cerebral oxygenation, oxidative injury biomarkers, and delirium are not evidence of causality, and despite adjustment for potential baseline and intraoperative confounders, there remains the potential for residual confounding. In addition, the use of a cerebral oximeter to measure cerebral oxygenation is a limitation of this study. Recent investigations indicate that cerebral near-infrared spectroscopy can be impacted by extra-cranial cutaneous blood flow,44 although validation studies and more recent investigations that simultaneously directly measured internal jugular vein hemoglobin saturations have validated near-infrared spectroscopy as a technique to measure brain oxygenation.45–48 Also, cerebral oxygenation and near-infrared spectroscopy can be affected by carboxyhemoglobin, which was not measured in all patients. To limit any subject-specific interference and consistent with common practice we measured oxygenation change in each subject. Despite any limitations of this monitor, this study is one of the first to suggest that the common practice of hyperoxygenation may not be protective – and in fact may be harmful – to the brain. The study is also limited by the lack of precedent for defining hyperoxia during surgery since the focus in clinical practice is placed on hypoxia. Baseline reflects a patient’s normoxic conditions at the beginning of surgery, so oxygenation saturations above baseline may be a reasonable criterion for hyperoxia. Alternatively the normal range of cerebral oxygenation may exist within a range around baseline. To address this limitation, we performed a sensitivity analysis in which we defined hyperoxia as the AUC above 110% of baseline that lead to similar results. The present study suggests specific thresholds for monitoring cerebral hyper-oxygenation may need to be established.
In conclusion, the magnitude of intraoperative hyperoxic cerebral reperfusion was independently associated with delirium following cardiac surgery and increased oxidative injury may contribute to this association. The magnitude of cerebral hyperoxia independent of ischemia reperfusion was also associated with delirium. The magnitude of cerebral hypoxia, however, was not associated with delirium. These conclusions diverge from common perioperative patient management strategies that lead to hyper-oxygenation but are consistent with a large body of preclinical evidence. Future studies are needed to assess the effects of excess oxygen delivery during major surgery and to determine if interventions that limit hyperoxic cerebral reperfusion and hyperoxia decrease postoperative brain dysfunction.
Highlights.
Cerebral oxygenation fluctuates considerably during cardiac surgery.
Cerebral hyperoxia after hypoxia was strongly associated with delirium.
Hyperoxia independent of hypoxia was also associated with postoperative delirium.
Hypoxia was not associated with delirium.
Intraoperative oxidative injury may partially mediate these effects.
Acknowledgments
We acknowledge cardiopulmonary bypass perfusionists Matthew Warhoover, MS, MMHC, and Dane A. Fornero, BS, CCP, from the Vanderbilt Heart and Vascular Institute for assistance in obtaining cerebral oximetry data; Patty Hendricks, R.N., for nursing support; Will Hardeman, B.A., Cleo Carter, B.A., Kiersten Card, and Damon Michaels, B.S., of the Vanderbilt Department of Anesthesiology Perioperative Clinical Research Institute, for assisting us in data collection; Anthony DeMatteo, B.S., Stephanie Sanchez, and the Vanderbilt Eicosanoid Core for technical support; Russ Beebe, from the Center for Research and Innovation in Systems Safety for assisting in graphics; and Nancy J. Brown, M.D., Professor, Vanderbilt Department of Internal Medicine for manuscript review; Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sources of Funding
This work was supported by K23GM102676, R01GM112871, and UL1TR000445 from the National Institutes of Health, the Foundation for Anesthesia Education and Research, and the Vanderbilt University Medical Center Department of Anesthesiology.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McPherson JA, Wagner CE, Boehm LM, Hall JD, Johnson DC, Miller LR, Burns KM, Thompson JL, Shintani AK, Ely EW, Pandharipande PP. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Critical care medicine. 2013;41:405–13. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph JL, Jones RN, Levkoff SE, Rockett C, Inouye SK, Sellke FW, Khuri SF, Lipsitz LA, Ramlawi B, Levitsky S, Marcantonio ER. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW Investigators B-IS. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369:1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangusan RF, Hooper V, Denslow SA, Travis L. Outcomes associated with postoperative delirium after cardiac surgery. Am J Crit Care. 2015;24:156–63. doi: 10.4037/ajcc2015137. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, Selnes OA, McKhann GM. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–44. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stransky M, Schmidt C, Ganslmeier P, Grossmann E, Haneya A, Moritz S, Raffer M, Schmid C, Graf BM, Trabold B. Hypoactive delirium after cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25:968–74. doi: 10.1053/j.jvca.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–63. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- 8.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–31. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazelton JL, Balan I, Elmer GI, Kristian T, Rosenthal RE, Krause G, Sanderson TH, Fiskum G. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. 2010;27:753–62. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317:798–801. doi: 10.1136/bmj.317.7161.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billings FT, IV, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23:1221–8. doi: 10.1681/ASN.2011090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S Emergency Medicine Shock Research Network I. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–22. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 13.Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, Jallo J, Pineda CC, Tzeng D, McBride W, Bell R. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Critical care medicine. 2014;42:387–96. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 14.Billings FT, IV, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ. High-Dose Perioperative Atorvastatin and Acute Kidney Injury Following Cardiac Surgery: A Randomized Clinical Trial. JAMA. 2016;315:877–88. doi: 10.1001/jama.2016.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Billings FT, IV, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24:913–20. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia. 2002;57:999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–87. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 20.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, Cleland A, Schaefer B, Irwin B, Fox S. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 21.Yao FS, Tseng CC, Ho CY, Levin SK, Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:552–8. doi: 10.1053/j.jvca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Abramson NS, Safar P, Detre KM, Kelsey SF, Monroe J, Reinmuth O, Snyder JV. Neurologic recovery after cardiac arrest: effect of duration of ischemia. Brain Resuscitation Clinical Trial I Study Group. Critical care medicine. 1985;13:930–1. [PubMed] [Google Scholar]
- 23.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. The Journal of clinical investigation. 2000;106:723–31. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 25.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 26.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2013;59:36–44. doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fessel JP, Jackson Roberts L. Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid Redox Signal. 2005;7:202–9. doi: 10.1089/ars.2005.7.202. [DOI] [PubMed] [Google Scholar]
- 29.Fessel JP, Hulette C, Powell S, Roberts LJ, 2nd, Zhang J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J Neurochem. 2003;85:645–50. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 30.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–8. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Sobel ME. Social Methodology. Washington, D.C: American Sociological Association; 1982. Asymptomatic confidence intervals for indirect effects in structural equation models; pp. 290–312. [Google Scholar]
- 33.Tofighi D, MacKinnon DP. Monte Carlo Confidence Intervals for Complex Functions of Indirect Effects. Structural Equation Modeling: A Multidisciplinary Journal. 2016;23:194–205. [Google Scholar]
- 34.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, 3rd, Rodriguez AL, Magovern CJ, Zaubler T, Freundlich K, Parr GV. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. The Annals of thoracic surgery. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. discussion 44–5. [DOI] [PubMed] [Google Scholar]
- 35.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM Investigators A. Air Versus Oxygen in ST-Segment Elevation Myocardial Infarction. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 36.Heringlake M, Garbers C, Kabler JH, Anderson I, Heinze H, Schon J, Berger KU, Dibbelt L, Sievers HH, Hanke T. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011;114:58–69. doi: 10.1097/ALN.0b013e3181fef34e. [DOI] [PubMed] [Google Scholar]
- 37.Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger KU. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care. 2011;15:R218. doi: 10.1186/cc10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Ellis J, Corso PJ, Hill PC, Lowery R, Chen F, Lindsay J. Mortality predicted by preinduction cerebral oxygen saturation after cardiac operation. The Annals of thoracic surgery. 2014;98:91–6. doi: 10.1016/j.athoracsur.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, Fierro G, De Cosmo G, Servillo G. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101:740–7. doi: 10.1213/01.ane.0000166974.96219.cd. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Watson NA, Beards SC, Altaf N, Kassner A, Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur J Anaesthesiol. 2000;17:152–9. doi: 10.1046/j.1365-2346.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–86. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- 42.Hou X, Roberts LJ, 2nd, Gobeil F, Jr, Taber D, Kanai K, Abran D, Brault S, Checchin D, Sennlaub F, Lachapelle P, Varma D, Chemtob S. Isomer-specific contractile effects of a series of synthetic f2-isoprostanes on retinal and cerebral microvasculature. Free Radic Biol Med. 2004;36:163–72. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Arendash GW, Cox AA, Mori T, Cracchiolo JR, Hensley KL, Roberts LJ., 2nd Oxygen treatment triggers cognitive impairment in Alzheimer’s transgenic mice. Neuroreport. 2009;20:1087–92. doi: 10.1097/WNR.0b013e32832e6459. [DOI] [PubMed] [Google Scholar]
- 44.Hirasawa A, Yanagisawa S, Tanaka N, Funane T, Kiguchi M, Sorensen H, Secher NH, Ogoh S. Influence of skin blood flow and source-detector distance on near-infrared spectroscopy-determined cerebral oxygenation in humans. Clin Physiol Funct Imaging. 2015;35:237–44. doi: 10.1111/cpf.12156. [DOI] [PubMed] [Google Scholar]
- 45.Abdul-Khaliq H, Troitzsch D, Berger F, Lange PE. Regional transcranial oximetry with near infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygenation. Biomed Tech (Berl) 2000;45:328–32. doi: 10.1515/bmte.2000.45.11.328. [DOI] [PubMed] [Google Scholar]
- 46.Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F. Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurol Res. 1995;17:89–93. doi: 10.1080/01616412.1995.11740293. [DOI] [PubMed] [Google Scholar]
- 47.Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput. 2000;16:191–9. doi: 10.1023/a:1009940031063. [DOI] [PubMed] [Google Scholar]
- 48.Nagdyman N, Ewert P, Peters B, Miera O, Fleck T, Berger F. Comparison of different near-infrared spectroscopic cerebral oxygenation indices with central venous and jugular venous oxygenation saturation in children. Paediatr Anaesth. 2008;18:160–6. doi: 10.1111/j.1460-9592.2007.02365.x. [DOI] [PubMed] [Google Scholar]