Abstract
Background/Objectives
To date, studies examining the association between warfarin therapy and incidence of ischemic stroke among patients with atrial fibrillation (AF) have not accounted for the competing risk of death. Competing risk analysis may provide greater understanding of the “real world” impact of anticoagulation on stroke risk over a multiyear time span.
Design
Cohort study
Setting
ATRIA Study community-based cohort
Participants
13,559 adults with nonvalvular AF between 1996 and 2003.
Measurements
All events were clinician-adjudicated. We used extended Cox regression with longitudinal warfarin exposure to estimate cause-specific hazard ratios (HR) for thromboembolism (TE) and the competing risk event (all cause death). The Fine and Gray subdistribution regression approach was used to estimate this association while accounting for competing death events. As a secondary analysis, follow-up was limited to 1, 3, and 5-years.
Results
The rate of death was much higher in the non-warfarin group (8.1 deaths/100 person-years) compared to the warfarin group (5.5 deaths/100 person-years). The cause-specific HR indicated a large reduction in TE with warfarin use (adjusted HR: 0.57, 95% CI: 0.50–0.65). However, after accounting for competing death events, this association was substantially attenuated (adjusted HR: 0.87, 95% CI: 0.77–0.99). In analyses limited to 1-year of follow-up with fewer competing death events, the results for models that did and did not account for competing risks were similar.
Conclusion
Analyses accounting for competing death events may provide a more realistic estimate of the longer-term stroke prevention benefits of anticoagulants for patients with AF, particularly those who are not currently treated with anticoagulants.
Keywords: anticoagulation, stroke, atrial fibrillation
INTRODUCTION
Atrial fibrillation (AF) is independently associated with an approximate five-fold increase in the risk of ischemic stroke, and is estimated to account for 15% of all strokes nationally, including more than 36% of strokes in those 80 years of age or older.1, 2 Warfarin anticoagulation therapy markedly reduces risk of ischemic stroke in patients with AF.3–5
To date, studies examining the association between warfarin therapy and incidence of ischemic stroke have not formally taken competing risks into account. A competing risk is an event whose occurrence precludes the ability to observe the outcome of interest for reasons such as dying from another cause.6 Given that the prevalence of AF is strongly associated with older age, rising to nearly 10% among those 80 years of age or older, deaths from comorbid conditions are common.7, 8 Accounting for competing risks may provide greater understanding of the longer-term, “real world” impact of AF on stroke risk and the expected preventative effect of anticoagulation in adults with AF.
Time to event statistical approaches such as Kaplan-Meier (KM) survival analysis and Cox proportional hazards regression are standard approaches that account for unequal follow-up time among participants based on different censoring events. Most often, competing risk events are ignored in these analyses; however, ignoring competing risks may result in overestimates of the actual incidence of the outcome of interest.6, 9, 10 AF patients not taking warfarin are more likely to be elderly, frail, and have higher rates of death than those taking warfarin.11 As a result, estimates of the longer-term impact of starting anticoagulants in AF patients not taking warfarin in usual clinical care may be particularly biased if competing risks are not taken into account.11
This study aimed to assess the effect of accounting for competing risk events when estimating the impact of warfarin anticoagulation on prevention of thromboembolism in a population of AF patients in clinical care.
METHODS
Source population
Assembly of the Anticoagulation and Risk Factors in Atrial fibrillation (ATRIA) cohort has been described in detail previously.12 In brief, the cohort included 13,559 adults aged 18 and older with diagnosed non-valvular AF who received care with Kaiser Permanente (KP) Northern California.5 Cohort members were identified by searching electronic inpatient, outpatient, and electrocardiographic databases for physician-assigned International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnostic codes of AF (427.31, 427.32) between July 1996 and December 1997. We included all patients ≥ 18 years old with either 2 or more outpatient AF diagnoses or 1 outpatient AF diagnosis with ECG validation. Included patients were followed through September 2003. Since we were interested in non-transient, nonvalvular AF, we excluded AF patients with diagnosed mitral stenosis, valvular repair or replacement, transient postoperative AF, or concurrent hyperthyroidism. All patients were followed prospectively from their index date (date of the first diagnosis of AF during the period of cohort assembly) until the event of interest, a competing risk event, withdrawal from the health plan, or end of follow-up.
Measures
Longitudinal warfarin exposure was assessed using an algorithm validated by chart review based on the number of days supplied per dispensed warfarin prescription from health plan pharmacies and intervening outpatient INR measurements.12 The outcome of interest was thromboembolism (TE). Cases of TE included both ischemic stroke and systemic arterial embolism and were identified by searching hospitalization and billing claims databases for relevant ICD-9 codes found in the primary discharge diagnosis position.5 A valid ischemic stroke was defined as a documented acute neurological deficit fitting a vascular distribution, lasting > 24 hours that was not explained by other causes (e.g., primary hemorrhage, trauma, infection, or vasculitis). A valid peripheral embolism was defined as an acute occlusion identified by radiographic imaging, intraoperative examination, or pathological findings in the absence of underlying atherosclerotic disease in the affected artery. Potential events were adjudicated by a Clinical Outcomes Committee composed of physicians using a structured medical records review. Only a patient’s first valid TE event was included in the analysis.
Deaths from any non-TE cause were considered competing risk events. TE-related deaths were not considered competing events since these subjects experienced the event of interest prior to death. Deaths were determined through reviewing medical charts, health plan databases, Social Security Administration vital status file, and the comprehensive California State death certificate registry.
Patient characteristics, including data on patient age (<65 years, 65–74 years, 75–84 years, ≥85 years), gender, and self-reported race/ethnicity (white, black/African American, Asian/Pacific Islander, Hispanic ethnicity, other/unknown) were obtained from administrative databases. History of comorbid conditions, including prior ischemic stroke, heart failure, coronary heart disease, peripheral artery disease, hypertension, and diabetes mellitus were collected from clinical inpatient and ambulatory databases as well as pharmacy dispensing data using validated algorithms and were assessed using data during the five years prior to the patient’s index date and were updated during the follow-up period.8, 12 Kidney dysfunction (defined as estimated glomerular filtration rate [eGFR] < 45 ml/min/1.73 m2) was calculated from outpatient serum creatinine values using the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration formula.13 Patients without an eGFR value in the prior year were considered to have normal renal function and patients on dialysis were considered to have kidney dysfunction. Proteinuria was defined as a urine dipstick protein result of ≥1+ (30 mg/dL or higher) in the absence of potential urinary tract infection found in laboratory databases.14 Patients without a urine dipstick protein laboratory result in the prior year were considered to not have proteinuria. All health-related variables were dichotomized. For all patients, the CHADS2,15 CHA2DS2-VASc,16 and ATRIA17 stroke risk scores were calculated.
Statistical analysis
Two of the mostcommonly used approaches to competing risk regression differ in construction of the risk set. The cause-specific hazard is estimated by constructing a proportional hazards model separately for each event type, where individuals who experience the competing event type are treated as censored observations. In this situation, the risk set is modified over time by removing individuals from remaining risk sets as they have either event, so the competing event influences the measure of association for the event of interest by removing at risk person-time from the risk set over time.18
Alternatively, Fine and Gray developed a regression modeling approach for competing risks analysis which is a modification of the Cox proportional hazards model and measures the subdistribution cumulative incidence function.10 This method considers the effect of predictors on the subdistribution hazard function accounting for the presence of competing risks.10 In contrast to the cause-specific hazard where individuals who have a competing event are censored, in the subdistribution hazard model these individuals remain in the risk set but can never experience the event of interest.18, 19 The subdistribution hazard is then defined as the probability of the event of interest given that an individual has survived up to that time without having the event of interest or has had the competing event prior to that time.18 Both the cause-specific and subdistribution approaches are informative. The cause-specific hazard approach addresses the potential etiologic association between an exposure and an event, while the subdistribution hazard approach seeks to demonstrate the actual benefit of an exposure or treatment in a given population.20
We assessed distributions of each covariate by exposure status (warfarin use) and by the occurrence of outcome events (TE events). Additionally, we calculated the rate of TE events and death by warfarin status. We used extended Cox regression to estimate unadjusted and adjusted cause-specific incidence rate ratios for both the outcome of interest (TE events) and the competing risk event (death) while accounting for different lengths of follow-up among patients and time-varying exposure status while adjusting for time-varying potential confounders. We then used the subdistribution hazard modeling approach, developed by Fine and Gray, and compared this result to our results for the outcome of interest from the cause-specific hazard models.10 We plotted the unadjusted cumulative incidence for the cause-specific model using the Simon and Makuch approach (which uses Kaplan-Meier product-limit calculations) for time-varying covariates, and by using the Fine and Gray’s extension of Cox regression for the subdistribution hazards model.21, 22 All deaths occurring in subjects who did not experience a TE event during follow-up were considered competing risk events. As a secondary analysis, we limited follow-up to 1, 3, and 5-years to have fewer competing risk events. Additionally, to examine the impact of competing risks in different age groups we added an interaction term between age and warfarin use to generate cause-specific and subdistribution hazard ratios.
This research was approved by the Institutional Review Boards at Massachusetts General Hospital and at Kaiser Foundation Research Institute. Waiver of informed consent was obtained because of the nature of the study.
RESULTS
Follow-up and characteristics of the study population
Patients were followed for an average of 4.77 years (Median: 5.89 years, IQR: 2.73–6.67 years). Patients not using warfarin were more likely to be younger than 65 years and also 85 years and older compared to patients using warfarin at baseline. In contrast, patients on warfarin were more likely to have traditional AF stroke risk factors, including prior ischemic stroke, diabetes, coronary artery disease, hypertension, and heart failure (Table 1). As a result, patients using warfarin had higher mean CHADS2 (2.02 vs. 1.64), CHA2DS2-VASc (3.68 vs. 3.22), and ATRIA (6.65 vs. 5.89) stroke risk scores at baseline than patients not using warfarin, respectively.
Table 1.
Characteristics of Patients with Atrial Fibrillation by Warfarin Status at Baseline, and by Time-varying Warfarin Status (Proportion of Person-years)
| No Warfarin | Warfarin | |||
|---|---|---|---|---|
| N (%) (n=6353) |
% Person-Years (32,610.6 PY) |
N (%) (n=7206) |
% Person-Years (32,132.4 PY) |
|
| Age category | ||||
| <65 years | 1491 (23.5%) | 23.8% | 1542 (21.4%) | 15.6% |
| 65–74 years | 1770 (26.1%) | 25.8% | 2614 (36.3%) | 31.5% |
| 75–84 years | 2165 (34.1%) | 34.1% | 2602 (36.1%) | 42.5% |
| ≥85 years | 927 (14.6%) | 16.3% | 448 (6.2%) | 10.4% |
| Women | 2857 (45.0%) | 42.8% | 2938 (40.8%) | 41.5% |
| Race/ethnicity | ||||
| White | 5430 (85.5%) | 84.6% | 6251 (86.8%) | 86.9% |
| Asian/Pacific Islander | 348 (5.5%) | 4.1% | 381 (5.3%) | 3.4% |
| Black/African American | 255 (4.0%) | 5.9% | 275 (3.8%) | 5.6% |
| Hispanic | 161 (2.5%) | 2.9% | 179 (2.5%) | 2.9% |
| Other/Unknown | 159 (2.5%) | 2.5% | 120 (1.7%) | 1.2% |
| Comorbid conditions | ||||
| Prior ischemic stroke | 366 (5.8%) | 5.0% | 886 (12.3%) | 9.8% |
| Diabetes mellitus | 930 (14.6%) | 17.0% | 1305 (18.1%) | 22.4% |
| Coronary artery disease | 1716 (27.0%) | 28.3% | 2210 (30.7%) | 32.9% |
| Peripheral artery disease | 131 (2.1%) | 2.9% | 190 (2.6%) | 4.0% |
| Hypertension | 3147 (49.5%) | 56.1% | 3760 (52.2%) | 60.8% |
| Chronic heart failure | 1658 (26.1%) | 26.1% | 2494 (34.6%) | 37.5% |
| Significant kidney dysfunction | 926 (14.6%) | 14.6% | 949 (13.2%) | 14.7% |
| Proteinuria | 782 (12.3%) | 15.0% | 901 (12.5%) | 17.1% |
PY = person-years
Warfarin use, mortality, and thromboembolism over the full follow-up period
During the full follow-up period for the ATRIA cohort of 13,559 individuals with AF, 4414 (32.5%) experienced competing death events. Patients taking warfarin had a markedly lower mortality rate with 1777 deaths on warfarin (5.5/100 person-years) versus 2637 deaths off warfarin (8.1/100 person-years) for an unadjusted mortality rate ratio of 0.68 (95% CI: 0.64–0.73).
Over the same period, there were 1092 TE events (1017 ischemic strokes and 75 systemic arterial emboli), with 407 occurring on warfarin (1.3/100 person-years) and 685 occurring off warfarin (2.1/100 person-years) for an unadjusted rate ratio of 0.60 (95% CI: 0.53–0.68). Figure 1a displays the estimated cumulative incidence of thromboembolism on and off warfarin from the cause-specific model. The adjusted cause-specific hazard ratio for thromboembolism was 0.57 (95% CI: 0.50–0.65), reflecting a large reduction in stroke rate with warfarin (Table 2). In the subdistribution hazard models which account for competing death events, the reduced rate of thromboembolism associated with warfarin was markedly attenuated in unadjusted (HR: 0.84, 95% CI: 0.74–0.95) and adjusted (HR: 0.87, 95% CI: 0.77–0.99) models (Table 2). This attenuated effect of warfarin due to competing death events is further demonstrated in the cumulative incidence curves estimated using the Fine-Gray subdistribution hazards model (Figure 1b).
Figure 1.
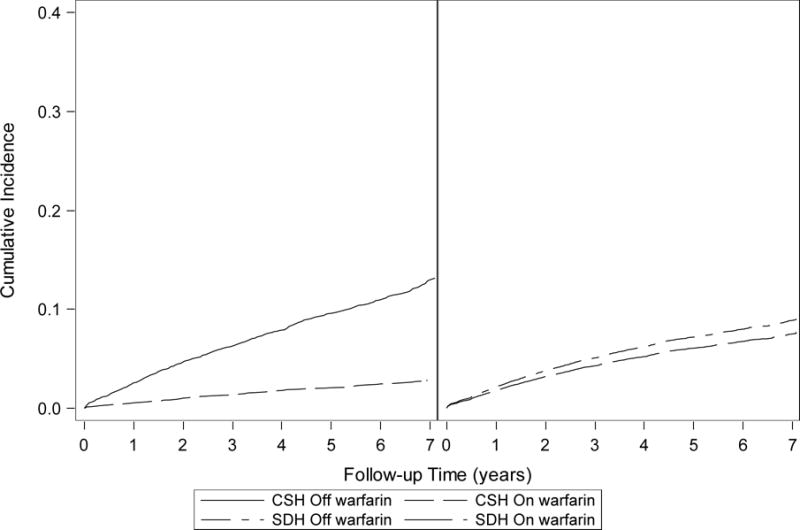
Unadjusted cumulative incidence curves estimated using the Simon and Makuch approach (cause-specific model)22 (Figure 1a) and the subdistribution hazards modeling approach (Figure 1b) for patients on and off warfarin: The unadjusted cumulative incidence at the end of follow-up is higher for patients off warfarin in the cause-specific model than in the subdistribution hazards model (cumulative incidence < 0.1 on and off warfarin), and the cumulative incidence curves for patients on and off warfarin are closer together in the subdistribution hazards model. In the cause-specific model, individuals who have a competing death event are censored and assumed to be at risk for the outcome, despite the outcome no longer being possible. In the subdistribution hazards model, patients with a competing death event remain in the risk set but can never experience the event of interest. The unadjusted curves for the subdistribution modeling approach were generated with the assumption of proportional hazards.
Table 2.
Association Between Time-varying Warfarin, Mortality, and Thromboembolism in Adults with Atrial Fibrillation During Follow-up (Cause-specific and Subdistribution Hazards)
| No Warfarin | Warfarin | |
|---|---|---|
| Mortality | ||
| Death events | 2637 | 1777 |
| Person-years | 32,610.6 | 32,132.4 |
| Rate | 8.1/100 person-years | 5.5/100 person-years |
| Cause-Specific Rate Ratio (Death) – unadjusted | – | 0.68 (0.64–0.73) |
| Thromboembolism | ||
| Thromboembolism events | 685 | 407 |
| Person-years | 32,610.6 | 32,132.4 |
| Rate | 2.1/100 person-years | 1.3/100 person-years |
| Cause-Specific Rate Ratio (TE) – unadjusted | – | 0.60 (0.53–0.68) |
| Cause-Specific Hazard Ratio (TE) – adjusteda | – | 0.57 (0.50–0.65) |
| Subdistribution Hazard Ratio – unadjusted | – | 0.84 (0.74–0.95) |
| Subdistribution Hazard Ratio – adjusteda | – | 0.87 (0.77–0.99) |
Adjusted for age, gender, race, prior stroke, diabetes, coronary disease, peripheral artery disease, hypertension, significant kidney dysfunction, proteinuria
Warfarin use, mortality, and thromboembolism over 1, 3, and 5-years of follow-up
In analyses limited to 1-year follow-up, there were 648 competing death events. The death rates in those on and those off warfarin were lower than in the longer-term follow-up. The rate of death in the warfarin group (4.3/100 person-years) was lower than in the off-warfarin group (5.6/100 person-years), for a rate ratio of 0.77 (95% CI: 0.66–0.90) (Table 3). In the first year of follow-up, there were 294 TE events. The rate of TE in this 1-year period was lower on warfarin (1.7/100 person-years) than off warfarin (2.8/100 person-years). Similar to the full follow-up period, the cause-specific rate ratio and hazard ratio during 1-year of follow-up indicated a markedly reduced risk of thromboembolism for those taking warfarin compared to those not using warfarin in unadjusted (RR: 0.61, 95% CI: 0.48–0.77) and adjusted (HR: 0.55, 95% CI: 0.43–0.71) analyses. With many fewer competing death events and more similar death rates on versus off warfarin during the first year of follow-up, the subdistribution hazard ratio during this time period was similar to the cause-specific hazard ratio in unadjusted (HR: 0.64, 95% CI: 0.51–0.82) and adjusted analyses (HR: 0.59, 95% CI: 0.46–0.75) (Table 3). As the number of competing death events increased over time (3-years: 2266 deaths; 5-years: 3503 deaths) and the difference in death rates off versus on warfarin grew, the adjusted subdistribution hazard ratio moved towards the markedly attenuated association we observed over the full 7-years of follow-up (Table 3).
Table 3.
Association Between Time-varying Warfarin Use, Mortality, and Thromboembolism in Adults with Atrial Fibrillation Over 1,3, 5, and 7-years of Follow-up (Cause-specific and Subdistribution Hazards)
| Length of follow-up | No Warfarin | Warfarin |
|---|---|---|
| 7-years (full follow-up) | ||
| Deaths (rate) | 2637 (8.1/100 person-years) | 1777 (5.5/100 person-years) |
| Thromboembolism events (rate) | 687 (2.1/100 person-years) | 405 (1.3/100 person-years) |
| Cause-specific HR (adjusteda) | – | 0.57 (0.50–0.65) |
| Subdistribution HR (adjusteda) | – | 0.87 (0.77–0.99) |
| 5 –years | ||
| Deaths (rate) | 2120 (7.8/100 person-years) | 1383 (5.4/100 person-years) |
| Thromboembolism events (rate) | 590 (2.2/100 person-years) | 344 (1.3/100 person-years) |
| Cause-specific HR (adjusteda) | – | 0.56 (0.49–0.64) |
| Subdistribution HR (adjusteda) | – | 0.81 (0.71–0.93) |
| 3-years | ||
| Deaths (rate) | 1388 (7.5/100 person-years) | 878 (5.3/100 person-years) |
| Thromboembolism events (rate) | 429 (2.3/100 person-years) | 243 (1.5/100 person-years) |
| Cause-specific HR (adjusteda) | – | 0.58 (0.49–0.73) |
| Subdistribution HR (adjusteda) | – | 0.73 (0.62–0.85) |
| 1-year | ||
| Deaths (rate) | 385 (5.6/100 person-years) | 263 (4.3/100 person-years) |
| Thromboembolism events (rate) | 191 (2.8/100 person-years) | 103 (1.7/100 person-years) |
| Cause-specific HR (adjusteda) | – | 0.55 (0.43–0.71) |
| Subdistribution HR (adjusteda) | – | 0.59 (0.46–0.75) |
Adjusted hazard ratio for thromboembolism adjusting for age, gender, race, prior stroke, diabetes, coronary disease, peripheral artery disease, hypertension, significant kidney dysfunction, proteinuria
Warfarin use and thromboembolism by age group
The difference between the cause-specific hazard ratio and the subdistribution hazard ratio is greater among older age groups, indicating a bigger impact of competing death events among older patients (Table 4).
Table 4.
Association Between Time-varying Warfarin Use and Thromboembolism in Adults with Atrial Fibrillation by Age Group (Cause-specific and Subdistribution Hazards)
| Cause-Specific Hazard Ratio a | Subdistribution Hazard Ratio a | |
|---|---|---|
| < 65 years | 1.08 (0.90–1.30) | 1.07 (0.73–1.57) |
| 65–74 years | 0.66 (0.59–0.73) | 0.96 (0.77–1.20) |
| 75–84 years | 0.57 (0.52–0.62) | 0.76 (0.63–0.92) |
| ≥ 85 years | 0.62 (0.53–0.72) | 0.91 (0.63–1.31) |
Adjusted hazard ratio for thromboembolism adjusting for age, gender, race, prior stroke, diabetes, coronary disease, peripheral artery disease, hypertension, significant kidney dysfunction, proteinuria
DISCUSSION
Within a large community-based cohort of patients with nonvalvular atrial fibrillation in clinical practice followed for up to seven years, warfarin was associated with a reduced rate of TE in cause-specific hazard models that did not account for competing risk events. These long-term, “real world” results extend our earlier findings and are consistent with those of randomized trials conducted for a shorter period of time with more selected patients.3, 5 The protective association between warfarin and thromboembolism was markedly attenuated over the full follow-up period when using a modeling approach that accounted for the large number of competing death events that occurred and the substantially higher death rate among patients not taking warfarin. When analyses were limited to 1-year of follow-up with many fewer competing death events, models that did and did not account for competing death events produced similar results and demonstrated a strong protective effect of warfarin for TE events. Further, our results demonstrate that the impact of competing death events is greatest among older patients, where death rates are higher.
Large fractions of patients with AF do not receive anticoagulant therapy.23 We were interested in estimating the potential stroke-preventive impact of anticoagulating ATRIA cohort patients not receiving warfarin. In contrast to randomized trials, long-term follow-up of AF patients in clinical care is marked by a high rate of competing death events, consistent with the patients’ older age (45% ≥ 75 years) and comorbidity burden. Indeed, competing death events (4414 deaths) were much more numerous than thromboembolic events (1092 TE events), and occurred more frequently in those off warfarin (8.1/100 person-years) than those on warfarin (5.5/100 person-years). While the cause-specific hazard approach demonstrated a strong effect of warfarin, the subdistribution hazard approach accounting for competing death events provides a more realistic population-level estimate of the stroke preventive benefit of warfarin therapy over a long follow-up period in predominantly older patients with AF in clinical care. In particular, our results highlight the reduced long-term expected benefit of anticoagulant therapy in patients not currently on anticoagulants. Older age, comorbidity, and frailty are among the reasons patients with AF are not treated with anticoagulants and these features are reflected in higher rates of death.23–25 When limiting our follow-up to 1-year, there were far fewer competing death events, the death rates in the warfarin and non-warfarin groups were more similar, and the results that did and did not account for competing risks were comparable. Between 1-year and full follow-up, the cause-specific results remained essentially constant demonstrating a large protective effect of warfarin but the competing risk analysis produced progressively smaller effects of warfarin.
Guidelines recommend use of anticoagulants in patients with AF based on untreated risk of stroke.26, 27 As this risk increases, the expected reduction in absolute stroke risk increases in parallel.28 But this benefit of anticoagulants is, of course, contingent upon patient survival. In predominantly older patients with AF, the competing risk of death reduces the expected longer-term benefit of anticoagulants. This is especially true in “real world” clinical care where the oldest patients with comorbid illness and frailty are less likely to be treated. Estimates of the individual patient or population level benefit of adding an anticoagulant to these currently untreated patients is more realistically estimated by competing risk analyses. Recent research has utilized competing risk analyses to assess stroke risk in AF patients with end-stage renal disease, a population with high rates of competing death events.29
Our analyses view non-thromboembolic deaths as competing risk events. One concern is that warfarin reduced the risk of these competing events, as well. The original warfarin AF trials did observe a reduction in all-cause death in the warfarin arms.30 However, in the later BAFTA trial among elderly patients with AF which also demonstrated a marked reduction in stroke risk with warfarin versus aspirin, there was no difference in overall mortality. Indeed, BAFTA observed a higher number of non-stroke vascular deaths in the warfarin arm.31 More recently, the AVERROES trial demonstrated a 55% reduction in stroke risk with the oral anticoagulant apixaban versus aspirin but with only a small reduction in non-stroke mortality risk.32 Taken together, this evidence from randomized trials argues that most of the large relative and absolute difference in non-stroke death rates that we observed in those using versus not using warfarin is independent of warfarin’s effect.
Our study was strengthened by examination of a large cohort of AF patients with long-term comprehensive follow-up and a large number of outcome events. All TE events were adjudicated by physicians using a standardized medical record review protocol. Competing death events were ascertained from multiple comprehensive databases and ascertainment is expected to be complete. In addition, we were able to assess longitudinal warfarin exposure using a validated algorithm which utilizes both comprehensive pharmacy and laboratory databases.
This study also has several potential limitations. There may still be residual confounding in our comparative analyses of stroke rates despite controlling for all the risk factors included in CHADS2, CHA2DS2-VASc, and ATRIA stroke risk scores. We also did not have information on aspirin use for patients not prescribed warfarin, so our non-warfarin group includes a mix of patients using aspirin and patients not taking aspirin. Additionally, our search strategy may have missed a small number of TE events that did not lead to hospitalization; however, we do not expect these missed events to differ by warfarin status. Finally, we know cause of death only for hospitalized TE and bleeding events and not for the vast majority of competing death events.
In conclusion, this study demonstrates the large impact of accounting for competing death events when evaluating the longer-term stroke prevention effect of anticoagulants in patients with AF in clinical care. The competing risk of death is common in patients with AF and higher than the risk of TE events. Further, the mortality rate of patients not prescribed anticoagulants is markedly higher than that for patients taking warfarin, reflecting the higher proportion of elderly patients and those with complex comorbidities among patients not prescribed warfarin. As a consequence, analyses accounting for competing death events result in a smaller estimated effect of warfarin than standard cause-specific approaches that treat deaths as censoring events, and the difference increases with length of follow-up. The cause-specific hazard ratio can provide a valid estimate of the etiologic effect of anticoagulants in patients with AF. However, the competing risk analyses presented in this study likely provide a more realistic estimate of the longer-term stroke-reducing benefits of warfarin for patients with AF not currently anticoagulated in clinical care. The stroke-preventive benefits of anticoagulation for patients with AF accumulate over time. For health care system quality improvement programs, our analyses emphasize the importance of particularly encouraging use of anticoagulants in AF patients in relatively good health. For the clinician and individual patient, our analyses highlight the importance of accounting for the individual’s life expectancy in weighing the benefits and harms of long-term anticoagulant therapy.
Acknowledgments
The authors acknowledge Long H. Ngo, PhD of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA for statistical guidance.
Funding Sources: This study was supported by the National Institute on Aging (R01 AG15478 and K23 AG028978), the National Heart, Lung and Blood Institute (U19 HL91179 and RC2HL101589), and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA). The funding sources had no role in study design, data collection, data analysis, data interpretation, or preparation of this manuscript. Dr. Ashburner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Meeting: This work was presented at the 2015 American Heart Association Scientific Sessions in Orlando, Florida; November 2015.
Conflicts of Interest: Dr. Singer serves as a consultant/advisory board member for Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson and Johnson, Pfizer, and St. Jude Medical on matters related to preventing stroke in atrial fibrillation and with CSL Behring related to reversal of warfarin anticoagulation. Additionally, Dr. Singer has research contracts with Medtronic, Inc related to atrial fibrillation and risk of stroke, with Johnson and Johnson related to stroke prevention in atrial fibrillation, and with Bristol-Myers Squibb related to atrial fibrillation and risk of stroke. Dr. Go has received research grants from CSL Behring and iRhythm Technologies.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561–4. [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457. [PubMed] [Google Scholar]
- 4.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999;281(19):1830–5. doi: 10.1001/jama.281.19.1830. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Pintilie M. An introduction to competing risks analysis. Rev Esp Cardiol. 2011;64(7):599–605. doi: 10.1016/j.recesp.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–7. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 11.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131(12):927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 17.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2(3):e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HJ, Zhang X, Dyck R. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med Res Methodol. 2010;10:97. doi: 10.1186/1471-2288-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 22.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 23.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Holt TA, Hunter TD, Gunnarsson C. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract. 2012;62(603):e710–7. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monette J, Gurwitz JH, Rochon PA, et al. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45(9):1060–5. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 26.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 27.January CT, Wann LS, Alpert JS, et al. American College of Cardiology/American Heart Association Task Force on Practice, 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih CJ, Ou SM, Chao PW, et al. Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: A competing risk analysis of a nationwide cohort. Circulation. 2016;133(3):265–272. doi: 10.1161/CIRCULATIONAHA.115.018294. [DOI] [PubMed] [Google Scholar]
- 30.The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med. 1997;157(11):1237–40. [PubMed] [Google Scholar]
- 31.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 32.Connolly SJ, Eikelboom J, Joyner C. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]


