Abstract
While localizing sensory and motor deficits is one of the cornerstones of clinical neurology, behavioral and cognitive deficits in psychiatry remain impervious to this approach. In psychiatry, major challenges include the relative subtlety by which neural circuits are perturbed, and the limited understanding of how basic circuit functions relate to thought and behavior. Neurodevelopmental disorders offer a window to addressing the first challenge given their strong genetic underpinnings, which can be linked to biological mechanisms. Such links have benefited from genetic modeling in the mouse, and in this review we highlight how this small mammal is now allowing us to crack neural circuits as well. We review recent studies of mouse thalamus, discussing how they revealed general principles that may underlie human perception and attention. Controlling the magnitude (gain) of thalamic sensory responses is a mechanism of attention, and the mouse has enabled its functional dissection at an unprecedented resolution. Further, modeling human genetic neurodevelopmental disease in the mouse has shown how diminished thalamic gain control can lead to attention deficits. This breaks new ground in how we untangle the complexity of psychiatric diseases; by making thalamic circuits accessible to mechanistic dissection, the mouse has not only taught us how they fundamentally work, but also how their dysfunction can be precisely mapped onto behavioral and cognitive deficits. Future studies promise even more progress, with the hope that principled targeting of identified thalamic circuits can be uniquely therapeutic.
Introduction
The thalamus is an evolutionarily ancient region (1), traditionally divided into multiple nuclei that project to the mammalian cortex, or its counterpart, the dorsal pallium in reptiles, amphibians and birds (2). In mammals, the thalamus receives reciprocal inputs from the cortex, giving rise to the thalamo-cortical system and its associated large-scale neural dynamics (3-5). Because neural activity within the thalamo-cortical system appears to be most closely tied to perception (6), action (7) and mentation (8), understanding the thalamus is crucial for delineating general principles of sensorimotor and cognitive function. Aside from the insight it provides to basic human psychology, this understanding is required for linking identified thalamic deficits to specific phenotypes in a number of human disorders (9, 10). Given that the human brain is inaccessible to cellular-resolution functional circuit dissection; such links will have to be made in animals. In this review, we will explain how the mouse provides a unique model for deriving general principles of thalamic function and how it has already begun to provide links between thalamic dysfunction and specific symptoms in neurodevelopmental disorders. Future studies using similar approaches are likely to reveal deeper mechanistic links, and will undoubtedly provide novel venues for targeting perceptual and cognitive deficits in these devastating disorders.
All thalamic nuclei (except for the reticular nucleus, discussed later) are largely composed of cortically-projecting glutamatergic cells known as thalamocortical (TC) neurons. These neurons operate in a feedforward manner as they do not establish monosynaptic connections among themselves (11). Each thalamic nucleus receives a variety of inputs that determines its overall behavioral engagement and function (12). While several organizational schemes have been proposed for thalamic nuclei (specific vs. nonspecific (13); core vs. matrix (14); first order vs. higher order (15)), they all converge on the idea that some operate largely as cortical relays from the senses, and others as coordination hubs for cortico-cortical interaction. Despite differences in scale and specialization, this basic organization of thalamic function is conserved across all mammalian species studied, including humans.
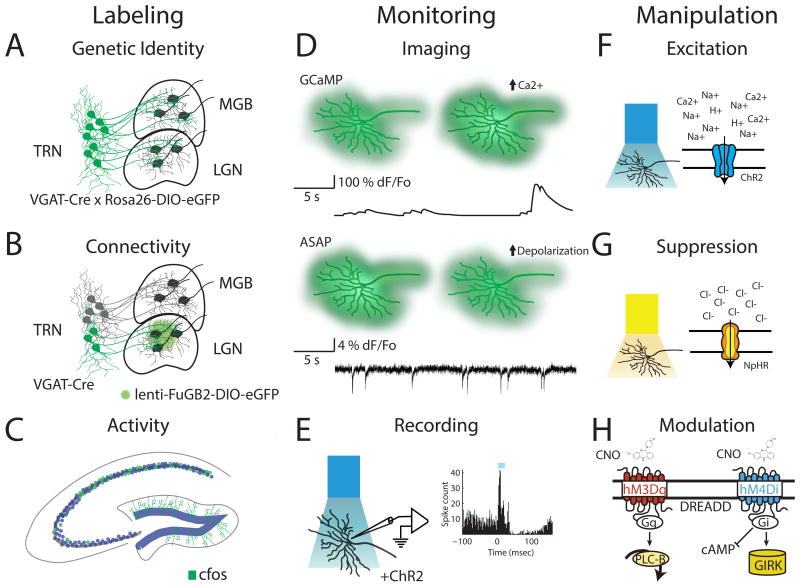
Due to its genetic accessibility, the mouse offers an unparalleled mammalian system for understanding evolutionarily conserved brain functions. There are now hundreds of available mouse lines that allow for targeting genetically-identified neuronal types (16), including those in different thalamic and associated cortical structures (Figure 1A). Further specificity is enabled by cross-sectional strategies that additionally target neuronal types based on their connectivity (Figure 1B) (17) or activity patterns (Figure 1C) (18). Such genetic labeling methods, when combined with tools to visualize (Figure 1D), electrically record (Figure 1E), or manipulate individual neural elements within intact circuits (Figure 1F-H) (19-21), make the mouse brain an extremely powerful and unique platform for functional circuit dissection. A subset of these insights will be directly applicable to understanding basic human brain function and its malfunction in disease.
Figure 1. Tools for functional dissection of intact circuits in mice.
Schematic diagram illustrating available approaches to study circuits in the mouse include labeling, monitoring and manipulation. Labeling: Neuronal subpopulations can be marked based on their genetic identity (A), connectivity or both (B). In this example, inhibitory neurons in the thalamic reticular nucleus (TRN) are labelled either based on their expression of VGAT in a cre- reporter line alone (A) or also based on their projections identified using a retrograde lentiviral vector (B). In addition, elevated activity can be determined based on immediate early genes expression (e.g. cfos, panel C). Monitoring: Activity can be monitored in genetically defined populations through (D) genetically encoded fluorescent calcium (e.g. GCaMP) and voltage (e.g. ASAP) sensors (top panel) or by (E) identification of optogenetically tagged neurons expressing ChR2 in extracellular single-unit recordings (bottom panel). Manipulation: Stimulation (F) or inhibition (G) of neurons via light-sensitive cation channels (e.g. ChR2, top panel) or inhibitory pumps (e.g. NpHR, middle panel) provides fast, cell-type specific modulation of activity while engineered G-protein coupled receptors (Designer Receptors Activated by Designer Drugs; DREADDs, panel H) can be used to modulate activity on a longer timescale.
Why do we think that thalamic function can be appropriately studied in the mouse? Mice exhibit natural states and behaviors that engage thalamic function in a manner that appears to be analogous to that of mammals with larger brains, including primates. For example, sleep rhythms appear to functionally engage thalamic (and cortical) circuits in a manner that is conserved across mammals (22-25). In addition, with recent success in developing quantitative mouse behavioral tasks, it appears that thalamic engagement is also conserved in processes such as sensory expectation (26) and attention (27-29). Using the mouse's functional circuit dissection tools, which are uniquely capable of revealing how neural computations are implemented by individual circuit elements (BOX 1), it is possible to derive general circuit principles applicable to human perception, action and cognition. In addition, the ability to mimic genetic vulnerabilities identified in human neurodevelopmental disorders makes the mouse a particularly useful translational model. In the rest of this review, we will provide evidence for this notion, while also clarifying areas of limitation and uncertainty. It is important to note that mice do not have a primate's behavioral repertoire, and that certain types of visual or abstract processing cannot be derived from mouse studies since required circuits are either absent or rudimentary. For perceptual and behavioral processes that are conserved, the degree to which mice implement associated computations through conserved or divergent circuits will be critical to determine. This will likely need to be examined on a case-by-case basis, but as we explain in the next section, certain thalamic functions provide examples where conserved implementation has been found. For example, the frontal cortex of the primate is substantially expanded in comparison to the mouse, which would most likely result in expanded interactions with its associated thalamic nuclei in relation to what would be observed in the mouse. Nonetheless, our thesis is that the mouse would still be useful in deriving general principles that may be shared within these two systems. Overall, as we highlight the usefulness of the mouse, we do not in any way discount the need for comparative studies across mammalian brains or the unique contribution of primate research to understanding human brain function.
BOX1. Comprehensive description of circuit dysfunction in neurodevelopmental disorders following David Marr's three levels of analysis.
Connecting the complex and variable symptoms of many neurodevelopmental disorders to their underlying causes remains a major scientific challenge. This challenge calls for an approach which can link the diverse genetic and environmental insults involved in these disorders to well characterized changes in brain function. An influential framework for connecting these different parts of the overall problem was proposed by one of the founders of computational neuroscience, David Marr in his “tri-level” approach to the analysis of information processing by the nervous system (74). In his seminal work “Vision”, Marr proposed that there are three inter-related levels of description needed to understand brain function: 1. The functional or computational level describing the information processing problems which the brain solves 2. The representational level describing the abstract processes or algorithms by which it solves these problems and 3. The implementation level describing the instantiation of these algorithms in neuronal, circuit and network properties. In the context of disease, this approach calls for investigation of how symptoms, which can be considered deviations from normal behaviors (computational level) relate to underlying deficits in processing (representational level) and finally of how these deficits result from changes in circuit anatomy and/or physiology (implementation level). Although mice will only re-capitulate a subset of human computations, they may offer a simplified model to examine all three levels for computations that are conserved. Throughout this review, we refer to computations, implementations and circuits within this framework
Thalamic organization and evidence for conservation of circuit functions
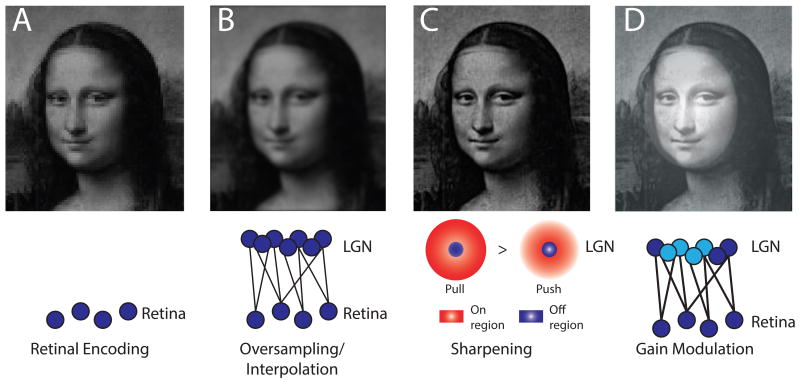
As mentioned in the introduction, the thalamus is traditionally divided into distinct nuclei. Each thalamic nucleus is defined by a unique set of inputs and outputs, which ultimately dictates its function. In general, the best studied thalamic nuclei are ones that receive peripheral sensory inputs and project to primary sensory cortical regions. In the visual system, this would be the lateral geniculate nucleus (LGN), which receives inputs from the retina and transmits outputs to primary visual cortex (V1). Since it carries sensory information to the cortex, the LGN is commonly referred to as a first-order, specific, or core thalamic nucleus. The LGN establishes topographic and reciprocal connections with V1, where its TC neurons project to cortical layer IV, and receive inputs from layer VI (30, 31). Similar organizational schemes are observed across the auditory and somatosensory systems. Even though the LGN transmits visual information from the retina to the cortex, it is far from a simple relay. The retinal signal is transformed by LGN circuits in at least three distinct ways. First, there are many more LGN neurons than there are retinal outputs, enabling the brain to resample and interpolate retinal inputs, enhancing visual acuity above what would be predicted by available photodetectors(32) (Figure 2A-B). Such interpolation however introduces image blur, which requires sharpening through a special type of spatial filtering called local contrast enhancement (LCE) (Figure 2C). This computational function is subserved by local LGN interneurons, each providing an input of opposite polarity to its neighboring TC neuron, in a push-pull manner (32, 33). Importantly, both interpolation (implemented through retina-LGN convergence) and LCE (implemented through local interneurons) appear to be evolutionary conserved (34). This computational conservation may be surprising, as the LGN of the primate is a layered structure, containing independent channels of information with distinct contrast and color content, a feature that does not appear to exist in the mouse (35).
Figure 2. Image processing operations through the visual thalamus.
(A-D) Schematic illustration of the effect of changes in sensory representations in the lateral geniculate nucleus following initial encoding by the retina (A). (B) Convergent input from the retina onto relay cells produces interpolation and smoothing of the image. (C) The spatial extent of the pull operation (suppressive response to positive contrast between the center and the periphery) in the geniculate cell's receptive field relative to the push operation (excitatory response to positive contrast between the center and the periphery) leads to local contrast enhancement. (D) Finally, Gain control can increase responses to weak inputs or suppress responses to prevent saturation. Top-down control of this process in a spatially-selective manner can generate a spotlight of enhanced perception (as shown by McAlonan et al.(29))
A third LGN computation is gain control, which allows for visual signal amplification without alteration of content (Figure 2D). As we describe in the next section, LGN gain modulation has been observed during attentional processing in humans (28), macaques (29) and mice (27) suggesting a broadly conserved circuit mechanism. While several circuit candidates exist for this function, experimental evidence point to the thalamic reticular nucleus (TRN) as the most likely source. The TRN is a shell of inhibitory neurons that surround thalamic nuclei and provide a powerful source of inhibitory control (36). The TRN is composed of individual subnetworks, each reciprocally and topographically connected to a particular thalamic nucleus (26). Studies in both monkeys and mice have shown that visual TRN neurons control LGN sensory responses based on how attention is directed. This confluence is critical, as it provides a clear example of a conserved functional implementation (BOX 1), further bolstering the usefulness of the mouse as a thalamic model. Additionally, these studies reinforce the idea that the LGN is not a simple relay, but a tunable filter that can be controlled based on behavioral demands. Because thalamic gain control is a computational function that likely extends beyond vision, understanding its circuit mechanisms in greater detail will be of translational value as it will explain how their failure contributes to perceptual, attentional and cognitive deficits in neurodevelopmental disorders.
Thalamic gain control as a mechanism for attentional selection
Attention is the process by which the brain augments relevant inputs and suppresses distractors (37). As humans, we have executive control over how attention is allocated, allowing us to flexibly attend to particular locations, features or objects (38). While the role of the prefrontal cortex (PFC) has been well-established in executive function, its interactions with sensory cortical regions had been assumed to be the primary mechanism of attentional selection (39). Nonetheless, several studies in humans and macaques have shown evidence for thalamic modulation in attentional tasks, raising the possibility that at least under certain conditions (e.g. when the attentional spotlight is spatially-broad), the thalamus can be the locus of attentional selection.
One of the earliest and most compelling pieces of evidence for thalamic attentional modulation came from studying the LGN during visuospatial attention with functional magnetic resonance imaging (fMRI) (28). Subjects performed a visuospatial attentional task in the fMRI scanner, which consisted of several individual trials. Trials were of two types, ones in which subjects were instructed to covertly attend to luminance changes along a checkboard arc, and others in which they were instructed to count letter at the fixation point instead. This design allowed for dissociating the effect of spatial allocation of attention and the visual-evoked response associated with the two stimulus types, because visual stimuli did not differ between the two trial types, only the instruction did (Figure 3A). In doing so, it was possible to precisely estimate the attentional modulation of the evoked blood oxygenation level dependent (BOLD) signal across the visual pathway. Strikingly, under these conditions, LGN attentional modulation was larger than that in V1 and was comparable to modulation of higher visual cortical regions often assumed to be the locus of attentional selection (40) (but also see (41)). This finding was the first to suggest that LGN gain modulation may be a mechanism for attentional allocation and was also supported by the fact that the BOLD response was enhanced for both the baseline and visual-evoked periods. A more recent study using voxel-based fMRI decoding found attention to selectively enhance orientation-related BOLD responses in the human LGN (42). This finding shows that attentional control of thalamic gain is not only spatially-specific but can also be feature-specific.
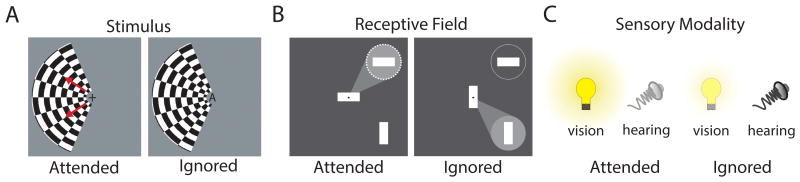
Figure 3. Experimental designs that have revealed attentional modulation of thalamic responses.
(A) Experiment by O'Connor et al. (28), in which subjects attended to either the checkerboard arc (left) or instead counted letter at fixation (right). (B) Experiment by McAlonan et al. (29), in which monkeys attended to stimulus within the receptive field of an LGN neuron (left) or outside of it (right). Note that the instruction to attend is based on the match between object at fixation and that in one of the two eccentric locations. (C) Experiment by Wimmer et al. (27), in which mice attended to either a visual stimulus or an auditory stimulus based on an instruction presented 500-700msec prior to target stimulus presentation.
In agreement with these human functional neuroimaging findings, neurophysiological studies in the macaque also show LGN spatial attentional modulation. McAlonan et al. trained monkeys to covertly attend to one of two spatial locations, where they had to make a perceptual judgement on luminance change (29). In each session, one of the spatial locations was chosen based on the spatial receptive field of an LGN neuron that was being recorded, while the other location was chosen to be of the same eccentricity (distance from the fovea in visual space), but in the opposing quadrant of the same hemifield. On each trial, the monkey was presented with one rectangle at fixation, and two rectangles at the two eccentric spatial locations; one rectangle matched the one at fixation and the other was orthogonal (Figure 3B). The monkey was trained to covertly attend to the matching rectangle, performing an eye movement towards it if it changed luminance. Because both rectangles changed luminance with a 50% probability independently, this task demanded selective attention to one spatial location. Under these conditions, LGN neurons showed an enhanced visual response when its receptive field coincided with the attended location compared to the trials in which it did not. These results are highly consistent with the human anticipatory attentional effects discussed earlier (28). To delineate the mechanisms of these physiological observations, the authors tested the hypothesis that spatial attentional effects are mediated by changes in synaptic inhibition. Therefore, they recorded from nearby visual TRN neurons while the monkeys performed the same task. Strikingly, TRN neurons exhibited an opposite-sign attentional modulation to LGN neurons that shared similar spatial receptive fields; they responded less vigorously to a visual stimulus if it was attended, and more vigorously if it was the distractor. Further analysis showed that attentional TRN modulation preceded that in the LGN, introducing the idea that the TRN can control sensory thalamic gain during attentional processing.
While not explicitly investigated by McAlonan et al., their task involved trial-by-trial changing of instruction that required matching the object at fixation with the object in one of the two ‘target’ spatial locations. Tasks of similar structures have been shown to engage prefrontal mechanisms thought to encode an abstract ‘rule’ (43). As such, one important idea that remained to be tested was whether top-down prefrontal executive control is responsible for attentional modulation of thalamic gain. Because the PFC projects broadly to the TRN in the primate brain, this idea had been previously suggested solely based on anatomical evidence (44).
In a recent study, we directly tested whether the TRN can be under PFC top-down control (27). To that end, we used the mouse as it offered a unique opportunity not only to test whether such process can be general across mammals, but also to interrogate its microcircuit details. When we started our investigation, it was unclear whether mice would be able to perform attention tasks that involved a trial-by-trial rule change. Fortunately, we were able to train mice to do exactly that; based on a contextual cue (high or low pass white noise), animals were instructed to select between conflicting visual (light flash) and auditory (frequency sweep) stimuli on a trial-by-trial basis (Figure 3C). Taking advantage of the high-throughput nature of mouse studies, we were able to explore the optimal training strategy to achieve performance of 70-80% correct responses across the cohort used in this study. Using multiple circuit dissection techniques including optogenetic manipulation and recording from genetically and regionally defined neuronal populations in mice performing this behavior, we found that the PFC did in fact control sensory thalamic gain via the TRN. On trials in which the mouse attended to vision, firing of visual TRN (visTRN) neurons decreased in a stimulus-anticipatory manner, while these same neurons increased firing when attention shifted to audition. These changes in firing rate translated to enhanced LGN responses in visual trials and reduced responses in auditory trials. Both TRN and LGN differential responses were PFC dependent, as optogenetic suppression of PFC eliminated them.
Combined with the aforementioned human and monkey studies, our findings strongly suggest that TRN-dependent gain control is a general mechanism for attentional allocation which is conserved across mammals (Figure 4). Since abnormal executive control of attention is a common feature of many neurodevelopmental disorders (45-47), it is now crucial to determine whether a subset of these disorders might be explained by failure of thalamic control mechanisms. As we show in the next section, by modeling thalamic genetic vulnerabilities in the mouse, unique opportunities can emerge to address this question with unparalleled mechanistic resolution.
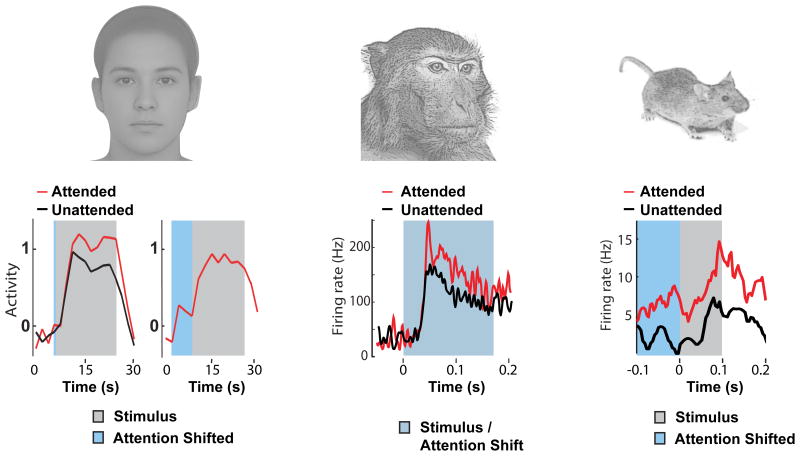
Figure 4. Thalamic gain control is evolutionarily conserved.
(A) Measurement of the BOLD response in human LGN during a visual attention task (28). Both evoked response (left panel, grey) and baseline activity in the absence of stimulus (right panel, blue) increase when attention is covertly directed to stimulus location. (B) Evoked responses of macaque LGN neurons were increases when attention was directed to the neuron's receptive field (29). (C) Attending to vision increases both baseline firing (blue portion) and evoked response (grey) of LGN neurons in mice performing a cross-modal attention task (27).
Thalamic gain control as a substrate for sensory and attentional dysfunction in neurodevelopmental disease
Untangling the complex changes in neurodevelopmental disorders presents a major challenge to translational research. As mentioned, advances in cell-type and region specific manipulation of disease related genes in mouse models provide a means to address this challenge, allowing particular symptoms to be linked to their underlying circuit substrates. Taking advantage of these tools, we recently discovered that perturbed TRN-mediated thalamic gain control underlies perceptual and attentional deficits in a monogenic form of autism spectrum disorder (ASD) and intellectual disability (ID).
Mutations in PTCHD1 are estimated to occur in ∼1% of patients diagnosed with ID and ASD and deletions of this gene produce a non-syndromic neurodevelopmental disorder characterized by attention deficits, ASD and ID as well as a range of other symptoms such as sleep disruption, hypotonia and aggression (48, 49). During early development, PTCHD1 is selectively expressed in the TRN and is enriched in this structure throughout adulthood (50). Given the critical role of TRN in thalamic regulation, preferential TRN expression suggested that some of the symptoms associated with PTCHD1 deletion might be due to thalamic dysfunction. Consistent with this idea, TRN neurons in PTCHD1 knockout (KO) mice showed abnormal biophysical properties including reduced high frequency bursting. TRN bursting depends on the interaction of T-type Ca2+ channels with small conductance, calcium-activated K+ (SK) channels (51) and the reduction in bursting in PTCHD1 KO was explained by reduction in SK channel activity. Because TRN bursting is classically associated with the generation of sleep spindles, changes in bursting were predicted to impact these dynamics and their hypothesized function in maintaining stable sleep architecture. Indeed, electroencephalographic recordings showed reduced sleep spindles and video monitoring showed sleep fragmentation in PTCHD1 KO mice. Through their recruitment of T-type Ca2+ currents, SK channels can generate long duration, calcium dependent spike bursts (52) transiently increasing firing rate and producing larger amplitude IPSPs. As such, diminished SK function could impact overall inhibitory output during sensory stimulation. Using a novel technique that we developed, known as inhibitory photometry to optically measure thalamic inhibition in the freely behaving mouse, we found that PTCHD1 KO mice indeed showed reduced sensory-evoked thalamic inhibitory transients. Because our earlier study showed a role for thalamic inhibition in suppressing distractor stimuli (as did McAlonan et al.), we wondered whether reduced thalamic inhibition would result in increased distractibility. Consistent with this prediction, we found that PTCHD1 KO mice were distractible, but they also showed other deficits seen in the human condition, such as aggression and learning deficits. It was logical then to ask which of these symptoms were explained by the TRN deficits, and whether distractibility could be mapped specifically to reduced thalamic inhibition.
To answer these questions, we took advantage of a selective overlap in the expression of the neuropeptide somatostatin and PTCHD1. Because this overlap is essentially limited to the TRN, crossing a somatostatin cre- line in combination with floxed PTCHD1 line resulted in a selective deletion of PTCHD1 in the TRN. These TRN selective KOs showed the predicted distractibility, along with an associated hyperactivity mapping the full gamut of ADHD symptoms onto the TRN for the first time. In addition, TRN-specific KO mice showed sleep fragmentation, strengthening the link between TRN function across sleep and attention (53). In contrast, aggression and learning deficits were not seen in these circuit-specific deletion mice. These experiments, to our knowledge, where the first to map disease-related phenotypes onto underlying circuits in a human neurodevelopmental disease model. In agreement with this conclusion, augmenting SK channel function in the full PTCHD1 KO selectively mitigated ADHD symptoms without impacting learning or aggression. These findings not only further strengthen the symptom-to-circuit mapping, but also provide a novel pathway for intervention.
In light of the findings outlined in previous sections, a plausible explanation for PTCHD1 KO distractibility is that the loss of sensory TRN gain control allows irrelevant inputs traveling through the thalamus to become more distracting. Loss of normal TRN function would therefore create a ‘leaky thalamus’, broadly increasing sensitivity and diminishing the ability to bias processing towards behaviorally relevant stimuli. Although in the PTCHD1 KO, disrupted TRN function appears to be due to abnormal function of SK channels, similar effects could be produced through a variety of mechanisms which either directly impair TRN function or which prevent engagement of this circuit to control thalamic gain. An imbalance between excitation and inhibition (E/I imbalance) has been suggested as a common feature underlying cognitive and sensory deficits in ASD as well as other neurodevelopmental disorders (54). Although signs of such an imbalance have been observed in multiple mouse models of ASD (55, 56) understanding of how it might produce specific symptoms is unclear at this stage. Our findings in PTCHD1 KO mice suggest that impaired thalamic E/I balance might be a locus for perceptual and attentional deficits in this form of human ASD, and perhaps others where TRN control is compromised due to changes in intrinsic properties or connectivity (BOX2).
BOX 2. Speculations on the computational function of TRN in sensory processing.
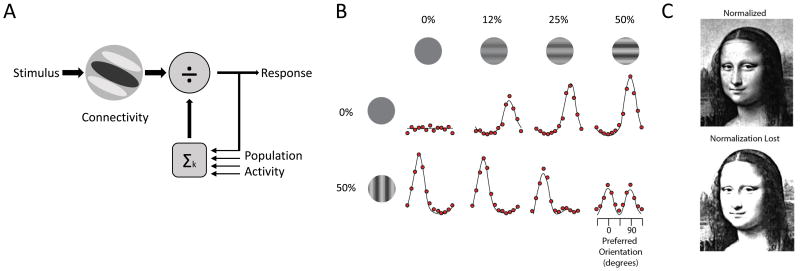
Changes in cortical E/I balance have been linked to perceptual deficits in ASD through altering a canonical computation known as divisive normalization (75). In this computation, the responses of individual neurons are scaled based on the ratio of their activity versus the integrated responses of a pool of neurons(76) (Figure 5A), as formalized in the following equation:
Where Rj is the output (response) of an individual neuron within a network, which is dependent on its individual drive (Dj) scaled by the pooled inputs of other neurons (ΣDk). The constants γ, σ and n are free parameters fit to empirical measurements: γ corresponds to overall responsiveness, σ affects how responses saturate with increasing driving input, and n is the exponent that amplifies the individual inputs, which can be different for different drivers.
Normalization was originally proposed to explain nonlinear responses of the primate's visual cortical neurons when their preferred stimulus was presented along with an orthogonal stimulus (77). Subsequent studies identified normalization across other sensory systems in flies, mice and humans, where responses are scaled nonlinearly based on the combination of stimuli presented (78-81) (Figure 5B). Perhaps the simplest example of such a computation is the contrast normalization observed in the retina in which the response to local contrast is scaled by the size of the grating presented and hence to the pooled inputs. Subsequent studies have also shown that normalization models effectively describe nonlinearities in population activity in higher sensory systems which show “winner-take-all” responses to opposing stimuli (82, 83) . While also observed in higher order and non-sensory systems (82, 83), a key benefit of normalization in sensory systems is that it adjusts the net gain of the neural responses to improve each neuron's dynamic range, maximizing responses to changes across a wide range of inputs and improving contrast sensitivity through a suppressive field proportional to net activity in the circuit (84) (Figure 5C). Loss of this suppressive field in the visual system has been proposed as an explanation for improved performance of ASD patients in psychophysical tasks using simple, high contrast stimuli (85), and although this characteristic can be beneficial in certain limited contexts, it may result in impaired processing of complex stimuli and discrimination among multiple similar inputs. While reduced normalization in ASD will undoubtedly explain some behavioral characteristics among patients, the circuit mechanisms responsible for implementing this computation are poorly understood.
Could the TRN provide a normalizing function to thalamic sensory responses, and therefore its dysfunction result in aberrant thalamic normalization? The highly complex receptive fields of TRN neurons (86, 87) are consistent with each receiving inputs from multiple thalamic neurons and therefore integrating changes in activity across a thalamic population, rather than a single cell. Such features are observed in inhibitory neurons that provide normalizing functions across several systems (78, 81, 88). In addition, sensory TRN neurons target thalamic dendrites providing both hyperpolarizing and shunting inhibition (89). Therefore, it is conceivable that the TRN may control thalamic gain through normalization. In this model the upper limit for signal amplification would be set by feedforward excitation, but relative gain would be established by TRN-mediated normalization. Loss of such function in ASD and perhaps other disorders would not only be expected to result in abnormal primary sensory responses, but would likely lead to abnormal development of sensory responses later in the sensory hierarchy. Could such aberrantly normalized cortical inputs result in the highly variable sensory responses observed in ASD studies? (90) This tantalizing possibility will require further studies, and we are confident that the mouse will continue to shed light onto these disease-relevant basic computations and their circuit implementation.
In addition to the LGN, the visual system contains the Pulvinar, a ‘higher-order’ thalamic nucleus. In contrast to the LGN, the Pulvinar does not receive its main driving input from the retina, but instead from visual cortical regions (57, 58). Also, the Pulvinar projects broadly across the visual cortical hierarchy, and makes connections across multiple layers in these different regions (57). Therefore, while the LGN can be described as a tunable filter, the Pulvinar operates like a central hub, coordinating interactions across multiple cortical regions. This notion gained substantial momentum through a landmark paper by Yuri Saalmann, Sabine Kastner and colleagues who showed that the Pulvinar coordinates rhythmic interactions between multiple visual cortical areas in a visuospatial attention task (59). Specifically, coherent sustained activity in the alpha range (10-15Hz) between higher order visual cortex (V4) and the temporo-occipital area (TEO) during the cued-delay period of the flanker task is coordinated by the Pulvinar.
Given the relevance of this rhythmic process to vision specifically and perception more generally, it will be important to dissect its underlying circuit mechanisms. In this case as in LGN gain control, the mouse might provide an appropriate model system. Clearly, this will require mouse behavioral tasks that will engage the Pulvinar and higher order visual regions in analogous functional interactions. There are reasons to be quite optimistic about this prospect, as studying the Pulvinar in mice performing visually-guided behavior has been with met with crucial initial success (60). Specifically, calcium imaging of Pulvinar and LGN terminals in visual cortex showed that the Pulvinar provides inputs that integrate contextual and motor information along with vision, consistent with its role in coordinating multiple cortical functions.
Understanding the role of Pulvinar in coordinating cortico-cortical interactions may shed light on thalamic involvement in other cognitive functions such as executive control. Frontally-connected thalamic nuclei, such as the mediodorsal thalamus (MD), exhibit a Pulvinar-like connectivity scheme, and may therefore coordinate interactions among frontal cortical areas during executive control (61). Such engagement would clarify why lesioning the MD in animals results in executive dysfunction, and how diminished MD function (62) and MD-PFC hypo-connectivity in schizophrenia (63) relate to the associated executive dysfunction. More generally, further understanding the thalamus will shed light on number of disorders associated with thalamic dysfunction in addition to schizophrenia (64), such as ADHD (65).
Similar to the LGN, the Pulvinar receives inputs from the TRN (66). Pioneering studies in primates have established that TRN neurons that project to the LGN and those that project to the Pulvinar occupy separate locations within this nucleus (67). The TRN may therefore provide a similar gain control function for the Pulvinar. Another, non-mutually exclusive possibility is temporal control; the TRN is known to engage in rhythmic dynamics in sleep, known as spindles (68, 69), and we have recently observed that the degree of engagement of connected TRN neurons in spindles during sleep is highly correlated with their engagement in alpha during wake on a cell-by-cell basis (53). Because alpha and spindles are spectrally overlapping oscillations (10-15Hz and 7-15Hz respectively), it is reasonable to assume that they could recruit similar circuits, and therefore TRN engagement in alpha oscillations is not totally surprising. Our observations were made in TRN neurons that project to the LGN, and therefore the TRN may contribute to the generation of alpha oscillations in the early visual pathway. What remains unclear, however, is whether the alpha observed in the early visual pathway is mechanistically related to that observed in higher visual circuits during visuospatial attention task. Answering this question will be important for clarifying if and how the TRN contributes to the hub-like function of the Pulvinar and perhaps other thalamic nuclei of similar connectivity patterns.
Discussion
The mouse has been an invaluable model in neuroscience, providing many insights into the genetic and cellular substrates of brain functions (70). With the recent explosion of tools to monitor and manipulate neurons, the mouse has also become an important model in systems neuroscience (71). The ability to label individual neurons with opsins based on genetic identity and connectivity has not only allowed for their manipulation in the context of behavior, but has also enabled monitoring their activity via ‘optical tagging’ (26, 27, 72). This has transformed our ability to connect physiological patterns of neural activity to function, as several studies have shown that genetically or projection-identified neurons exhibit unique activity patterns in different behaviors. With these approaches in hand, we can move beyond describing neurons strictly based on their physiological activity and instead directly connect physiology to cellular properties and network architecture.
We make the case that studying the thalamus has benefited tremendously from these technical advances and, given how evolutionarily conserved basic thalamic organization is; we suggest that studying the mouse thalamus will reveal principles applicable to human perception, action and cognition. These principles include homologous functions spanning the computational, algorithmic and implementational levels (Box 1). For example, sensory transmission through the mouse's lateral geniculate nucleus appears to undergo a similar transformation on its way to the cortex as it does in carnivores and primates. Such conservation of function also extends to how thalamic sensory responses are amplified as function of attention (27-29), including involvement of TRN. Through inhibitory control, the TRN can determine the magnitude and timing of thalamo-cortical transmission in a modality, spatial and perhaps, feature-specific manner. Further understanding of such behaviorally relevant thalamic modulation will be critical both to understand basic sensory processing and to refine our model of attention itself.
Finally, the mouse has enabled linking circuit dysfunction to specific behavioral abnormalities in neurodevelopmental disease, providing hope that psychiatric diseases can indeed be subjected to the mapping tradition of clinical neurology. Our work using the PTCHD1 mouse is an example of how this approach has allowed us to infer a TRN-circuit locus for the attention deficits and hyperactivity in the PTCHD1 deletion mouse, and presumably the human condition itself. We think that this model represents the first example of a ‘leaky thalamus’, where irrelevant inputs become much more distracting. Hyperactivity may be the motor counterpart of distractibility, but this conjecture will require much more detailed testing of motor thalamus function in the PTCHD1 KO. Of broad interest is to understand how common a leaky thalamus is across neurodevelopmental disorders, and whether the TRN can be targeted to mitigate specific symptoms. It is also important to note that various thalamic nuclei establish unique connectivity patterns with other subcortical structures such as the basal ganglia. As such, an even broader view of thalamic function beyond the thalamo-cortical system is its engagement in cortico-striatal-thalamo-cortical loops. Given that basal ganglia function can be perturbed in many neurodevelopmental disorders (e.g. ones involving Shank3 mutations (73)), it will be exciting to delineate the role of the thalamus in their manifestation and its potential utility as an interventional target. In all these efforts, the mouse will undoubtedly continue to reveal basic insights as well as translational leads of relevance to the human condition.
Figure 5. Normalization in sensory systems impacts population responses.
(A) Schematic diagram showing a typical implementation of the normalization operation. The qualitative response of an individual neuron to sensory input (receptive field; response filter) is based on connectivity. Quantitatively, this response is normalized by the overall population activity which is integrated to produce feedback suppression. (B) Normalization results in winner-take all competition across the population of sensory neurons. The population response (red dots) is maximal for one orientation when presented alone but equalized when a mask with opposite orientation is presented simultaneously (figure from Carandini and Heeger (76)). (C) Normalization maximizes sensitivity, thereby adjusting gain based on the average stimulus intensity to produce an effective dynamic range. Loss of this function would make perception highly sensitive to changes in contrast.
Acknowledgments
We thank Dr. Laszlo Acsady for Dr. Sabine Kastner for their comments. We also thank members of the Halassa lab for constructive discussion and feedback. This work has been funded by grants from the NIH (NIMH, NINDS), Brain and Behavior, Feldstein, Klingestein, Sloan, and Simons Foundations.
Footnotes
Conflict of interest: We declare no conflict of interest.
References
- 1.Butler AB. The evolution of the dorsal thalamus of jawed vertebrates, including mammals: cladistic analysis and a new hypothesis. Brain research Brain research reviews. 1994;19:29–65. doi: 10.1016/0165-0173(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 2.Mueller T. What is the Thalamus in Zebrafish? Frontiers in neuroscience. 2012;6:64. doi: 10.3389/fnins.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baars BJ, Franklin S, Ramsoy TZ. Global workspace dynamics: cortical “binding and propagation” enables conscious contents. Frontiers in psychology. 2013;4:200. doi: 10.3389/fpsyg.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victor JD, Drover JD, Conte MM, Schiff ND. Mean-field modeling of thalamocortical dynamics and a model-driven approach to EEG analysis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 3):15631–8. doi: 10.1073/pnas.1012168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3593–8. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Progress in brain research. 2005;150:127–42. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- 7.Yuste R, MacLean JN, Smith J, Lansner A. The cortex as a central pattern generator. Nature reviews Neuroscience. 2005;6:477–83. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- 8.Stratton P, Wiles J. Global segregation of cortical activity and metastable dynamics. Frontiers in systems neuroscience. 2015;9:119. doi: 10.3389/fnsys.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. The American journal of psychiatry. 2010;167:1339–48. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–55. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones EG. The anatomy of sensory relay functions in the thalamus. Progress in brain research. 1991;87:29–52. doi: 10.1016/s0079-6123(08)63046-0. [DOI] [PubMed] [Google Scholar]
- 12.Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Annals of the New York Academy of Sciences. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- 13.Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends in neurosciences. 1994;17:52–7. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 14.Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–45. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nature neuroscience. 2016;16:533–41. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–83. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ZJ, Zeng H. Genetic approaches to neural circuits in the mouse. Annual review of neuroscience. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Frontiers in neural circuits. 2014;8:37. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alivisatos AP, Andrews AM, Boyden ES, Chun M, Church GM, Deisseroth K, et al. Nanotools for neuroscience and brain activity mapping. ACS nano. 2013;7:1850–66. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. The Journal of neuroscience. 2006;26:10380–6. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deisseroth K, Schnitzer MJ. Engineering approaches to illuminating brain structure and dynamics. Neuron. 2013;80:568–77. doi: 10.1016/j.neuron.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of the thalamocortical system. The Journal of neuroscience. 2014;34:8875–93. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. Journal of neurophysiology. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 24.David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, et al. Essential thalamic contribution to slow waves of natural sleep. The Journal of neuroscience. 2013;33:19599–610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halassa MM. Thalamocortical dynamics of sleep: roles of purinergic neuromodulation. Seminars in cell & developmental biology. 2011;22:245–51. doi: 10.1016/j.semcdb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, et al. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–21. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526:705–9. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature neuroscience. 2002;5:1203–9. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 29.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–4. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffries AM, Killian NJ, Pezaris JS. Mapping the primate lateral geniculate nucleus: a review of experiments and methods. Journal of physiology, Paris. 2014;108:3–10. doi: 10.1016/j.jphysparis.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contreras D, Denman DJ. On Parallel Streams through the Mouse Dorsal Lateral Geniculate Nucleus. Frontiers in neural circuits. 2016 doi: 10.3389/fncir.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez LM, Molano-Mazon M, Wang X, Sommer FT, Hirsch JA. Statistical wiring of thalamic receptive fields optimizes spatial sampling of the retinal image. Neuron. 2014;81:943–56. doi: 10.1016/j.neuron.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Vaingankar V, Soto Sanchez C, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nature neuroscience. 2011;14:224–31. doi: 10.1038/nn.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch JA, Wang X, Sommer FT, Martinez LM. How inhibitory circuits in the thalamus serve vision. Annual review of neuroscience. 2015;38:309–29. doi: 10.1146/annurev-neuro-071013-014229. [DOI] [PubMed] [Google Scholar]
- 35.Usrey WM, Alitto HJ. Visual Functions of the Thalamus. Annu Rev Vis Sci 2015. 2015;1:351–71. doi: 10.1146/annurev-vision-082114-035920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain research Brain research reviews. 2004 Aug;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Buschman TJ, Kastner S. From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron. 2015;88:127–44. doi: 10.1016/j.neuron.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert CD, Li W. Top-down influences on visual processing. Nature reviews Neuroscience. 2013;14:350–63. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–14. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 41.Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–7. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling S, Pratte MS, Tong F. Attention alters orientation processing in the human lateral geniculate nucleus. Nature neuroscience. 2015;18:496–8. doi: 10.1038/nn.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–6. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 44.Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. The Journal of neuroscience. 2012;32:5338–50. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsabbagh M, Holmboe K, Gliga T, Mercure E, Hudry K, Charman T, et al. Social and attention factors during infancy and the later emergence of autism characteristics. Progress in brain research. 2011;189:195–207. doi: 10.1016/B978-0-444-53884-0.00025-7. [DOI] [PubMed] [Google Scholar]
- 46.Dajani DR, Uddin LQ. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in neurosciences. 2015;38:571–8. doi: 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Current topics in behavioral neurosciences. 2010;4:373–90. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhry A, Noor A, Degagne B, Baker K, Bok LA, Brady AF, et al. Phenotypic spectrum associated with PTCHD1 deletions and truncating mutations includes intellectual disability and autism spectrum disorder. Clinical genetics. 2014 doi: 10.1111/cge.12482. [DOI] [PubMed] [Google Scholar]
- 49.Noor A, Whibley A, Marshall CR, Gianakopoulos PJ, Piton A, Carson AR, et al. Disruption at the PTCHD1 Locus on Xp22.11 in Autism spectrum disorder and intellectual disability. Science translational medicine. 2010;2 doi: 10.1126/scitranslmed.3001267. 49ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM. Thalamic reticular impairment underlies attention deficit in Ptchd1 mice. Nature. 2016 doi: 10.1038/nature17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nature neuroscience. 2008;11:683–92. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 52.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. Journal of neurophysiology. 1992;68:1373–83. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Wimmer RD, Wilson MA, Halassa MM. Thalamic Circuit Mechanisms Link Sensory Processing in Sleep and Attention. Frontiers in neural circuits. 2015;9:83. doi: 10.3389/fncir.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Current molecular medicine. 2015;15:146–67. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of neurodevelopmental disorders. 2009;1:172–81. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–7. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bridge H, Leopold DA, Bourne JA. Adaptive Pulvinar Circuitry Supports Visual Cognition. Trends in cognitive sciences. 2016;20:146–57. doi: 10.1016/j.tics.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends in neurosciences. 2000;23:35–9. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 59.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–6. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature neuroscience. 2016;19:299–307. doi: 10.1038/nn.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saalmann YB. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Frontiers in systems neuroscience. 2014;8:83. doi: 10.3389/fnsys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. The Journal of neuroscience. 2014;34:15340–6. doi: 10.1523/JNEUROSCI.3289-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9276–80. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American journal of psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lakatos P, O'Connell MN, Barczak A. Pondering the Pulvinar. Neuron. 2016;89:5–7. doi: 10.1016/j.neuron.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conley M, Diamond IT. Organization of the Visual Sector of the Thalamic Reticular Nucleus in Galago. The European journal of neuroscience. 1990;2:211–26. doi: 10.1111/j.1460-9568.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 68.Bartho P, Slezia A, Matyas F, Faradzs-Zade L, Ulbert I, Harris KD, et al. Ongoing network state controls the length of sleep spindles via inhibitory activity. Neuron. 2014;82:1367–79. doi: 10.1016/j.neuron.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature neuroscience. 2011;14:1118–20. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 71.Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends in neurosciences. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–21. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peebles D, Cooper RP. Thirty Years After Marr's Vision: Levels of Analysis in Cognitive Science. Topics in cognitive science. 2015;7:187–90. doi: 10.1111/tops.12137. [DOI] [PubMed] [Google Scholar]
- 75.Rosenberg A, Patterson JS, Angelaki DE. A computational perspective on autism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9158–65. doi: 10.1073/pnas.1510583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nature reviews Neuroscience. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heeger DJ. Normalization of cell responses in cat striate cortex. Visual neuroscience. 1992;9:181–97. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- 78.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–99. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beaudoin DL, Borghuis BG, Demb JB. Cellular basis for contrast gain control over the receptive field center of mammalian retinal ganglion cells. The Journal of neuroscience. 2007;27:2636–45. doi: 10.1523/JNEUROSCI.4610-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouwer GJ, Heeger DJ. Cross-orientation suppression in human visual cortex. Journal of neurophysiology. 2011;106:2108–19. doi: 10.1152/jn.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nature neuroscience. 2009;12:1577–85. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- 82.Louie K, Khaw MW, Glimcher PW. Normalization is a general neural mechanism for context-dependent decision making. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6139–44. doi: 10.1073/pnas.1217854110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: divisive normalization in parietal cortex. The Journal of neuroscience. 2011;31:10627–39. doi: 10.1523/JNEUROSCI.1237-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Busse L, Wade AR, Carandini M. Representation of concurrent stimuli by population activity in visual cortex. Neuron. 2009;64:931–42. doi: 10.1016/j.neuron.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. The Journal of neuroscience. 2013;33:8243–9. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaingankar V, Soto-Sanchez C, Wang X, Sommer FT, Hirsch JA. Neurons in the thalamic reticular nucleus are selective for diverse and complex visual features. Frontiers in integrative neuroscience. 2012;6:118. doi: 10.3389/fnint.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simm GM, de Ribaupierre F, de Ribaupierre Y, Rouiller EM. Discharge properties of single units in auditory part of reticular nucleus of thalamus in cat. Journal of neurophysiology. 1990;63:1010–21. doi: 10.1152/jn.1990.63.5.1010. [DOI] [PubMed] [Google Scholar]
- 88.Katzner S, Busse L, Carandini M. GABAA inhibition controls response gain in visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5931–41. doi: 10.1523/JNEUROSCI.5753-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cox CL, Huguenard JR, Prince DA. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences of the United States of America. 1995;94:8854–9. doi: 10.1073/pnas.94.16.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann M. Cortical variability in the sensory-evoked response in autism. Journal of autism and developmental disorders. 2015;45:1176–90. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]