Abstract
Background
Venous thromboembolism (VTE) is a common and potentially fatal complication of arthroplasty.
Methods
We reviewed randomized trials to determine which anticoagulant has the best safety and efficacy in hip/knee arthroplasty patients. We searched PubMed, MEDLINE, and EMBASE through January 2016.
Results
Compared to enoxaparin (most commonly dosed 40 mg once daily), the relative risk (RR) of VTE was lowest for edoxaban 30 mg once daily (0.49, 95% CI 0.32–0.75), fondaparinux 2.5 mg once daily (0.53, 95% CI 0.45–0.63), and rivaroxaban 10 mg once daily (0.55, 95% CI 0.46–0.66), and highest for dabigatran 150 mg once daily (1.19, 95% CI 0.98–1.44). The RR of major/clinically relevant bleeding was lowest for apixaban 2.5 mg twice daily (0.84, 95% CI 0.70–0.99), and highest for rivaroxaban (1.27, 95% CI 1.01–1.59) and fondaparinux (1.64, 95% CI 0.24–11.35). Fondaparinux was the only agent that was more effective than enoxaparin 30 mg twice daily (VTE RR = 0.58, 95% CI 0.43–0.76).
Conclusion
With the possible exception of apixaban, newer anticoagulants that lower the risk of post-operative VTE increase bleeding.
Keywords: arthroplasty, anticoagulant, bleed, deep vein thrombosis, meta-analysis, thromboembolism
Introduction
In the United States alone, venous thromboemboli (VTEs) cause 600,000 hospitalizations and 60,000 deaths each year.1 Even with thromboprophylaxis, VTE rates exceed 10% in studies that have screened patients for VTE after hip or knee arthroplasty.2 As the baby boomers age and the prevalence of obesity rises, VTE rates may also rise.3
These high rates of post-operative VTE have inspired the development of convenient alternatives to warfarin and low-molecular-weight heparins (LMWHs), the traditional thromboprophylaxis in arthroplasty patients. Like LMWHs, fondaparinux, apixaban, rivaroxaban, and edoxaban prevent clotting by inhibiting clotting factor Xa. Fondaparinux is administered subcutaneously, while apixaban, rivaroxaban, and edoxaban are administered orally. Dabigatran is another new oral anticoagulant with a different mechanism of action: it directly inhibits thrombin (factor IIa). None of these new agents require therapeutic monitoring, creating convenient alternatives for VTE prophylaxis.
These novel oral anticoagulants have varying degrees of approval. Apixaban, fondaparinux, and rivaroxaban are approved by the United States Food and Drug Administration (FDA) for VTE prophylaxis in patients undergoing hip or knee arthroplasty. Dabigatran is approved for both hip and knee arthroplasty in Europe, Australia, and Canada, but only for hip arthroplasty in the US. Edoxaban is approved for arthroplasty in Japan, but in Europe and the US, edoxaban is approved for indications other than arthroplasty.
The varied acceptance and usage of these newer agents for VTE prophylaxis brings up a salient clinical issue: which of these anticoagulants has the highest level of efficacy and safety in the hip and knee arthroplasty population? To answer this question, we conducted meta-analyses of the new anticoagulants. The first meta-analysis focused on efficacy, with the endpoint being the incidence of VTE. The second focused on safety, with two endpoints: the composite of major and/or clinically relevant bleeding and major bleeding alone. Based on the original trial designs, we were able to compare each of the novel anticoagulants to enoxaparin.
Methods
Data sources and searches
We searched PubMed, MEDLINE (through PubMed), and EMBASE through January 2016 using the following keywords: (apixaban OR dabigatran OR rivaroxaban OR fondaparinux OR edoxaban) AND (hip OR knee) AND arthroplasty. Additionally, references of included studies were reviewed as potential candidate trials. No betrixaban or darexaban trials met our inclusion criteria.
Study selection
Inclusion criteria were: double-blinded, randomized controlled trials that enrolled adult patients within 48 hours of surgery, prescribed anticoagulants for VTE prophylaxis after hip or knee surgery, dosed the experimental and control arms within 30 hours of each other, and confirmed VTE. VTE was defined in all trials as the presence of an objectively confirmed deep vein thrombosis (DVT) or an objectively confirmed pulmonary embolism. Exclusion criteria were the lack of a standard treatment arm (enoxaparin) or use of a dose not approved by the US Food and Drug Administration (FDA), the European Medicines Agency, or the Pharmaceutical and Food Safety Bureau of Japan.
Data extraction and quality assessment
Two researchers collected and assessed the eligibility of over 400 trials by viewing the title, abstract, and entire paper, in that order. Most trials were eliminated after viewing either the title or abstract based on the inclusion/exclusion criteria. Once trials that met the inclusion criteria were identified, two researchers assessed quality using the Jadad Criteria.4 All trials scored either a 4 or 5 on the Jadad scale, indicating high-quality trials. Once quality was established, independent data extraction was performed by at least two researchers using a standardized extraction form and comparing their findings to ensure data accuracy.
Outcome measures
Our efficacy outcome was the incidence of VTE. Our primary safety outcome was the composite of major/clinically relevant bleeding, and our secondary safety outcome was major bleeding alone. Outcomes were obtained from each study’s treatment period, which varied from 5 to 39 days. Major bleeding was defined similarly in all trials with one exception.5 The apixaban, dabigatran, and rivaroxaban trials defined major bleeding as the transfusion of 2 or more units of packed red blood cells or bleeding into a critical organ (including bleeding into the operated joint, if surgical intervention was needed), whereas the edoxaban trials defined major bleeding as the transfusion of four or more units. The definition of clinically relevant bleeding was not consistent throughout all trials but was similar. Clinically relevant bleeding was not available from the fondaparinux trials.
Statistical analysis
We calculated the relative risk (RR) for each trial compared to enoxaparin, weighed them using the inverse variance method, and calculated pooled RRs for each anticoagulant using the classic random-effect approach.6 In the analysis of major bleeds, we excluded one trial because it had no major bleeds.7 We tested for heterogeneity between trials using Cochran’s Q statistic. If heterogeneity was found, we performed subgroup analyses that focused on different doses of the anticoagulants.
Results
Initial searches located 435 trials. After applying inclusion/exclusion criteria (Figure 1), 4 apixaban trials, 4 dabigatran trials, 4 fondaparinux trials, 4 rivaroxaban trials, and 2 edoxaban trials were included. All 18 trials were sponsored by the manufacturers. The control in every trial was enoxaparin (given subcutaneously). Although the enoxaparin regimen varied across trials, its consistent use enabled us to compare the safety and efficacy of each new anticoagulant against enoxaparin. With the exception of fondaparinux, there were no more than 5 VTE-related deaths per treatment arm for each trial and no differences between treatment groups. Because of this very low event rate and lack of difference, we did not include these numbers in the results below.
Figure 1.
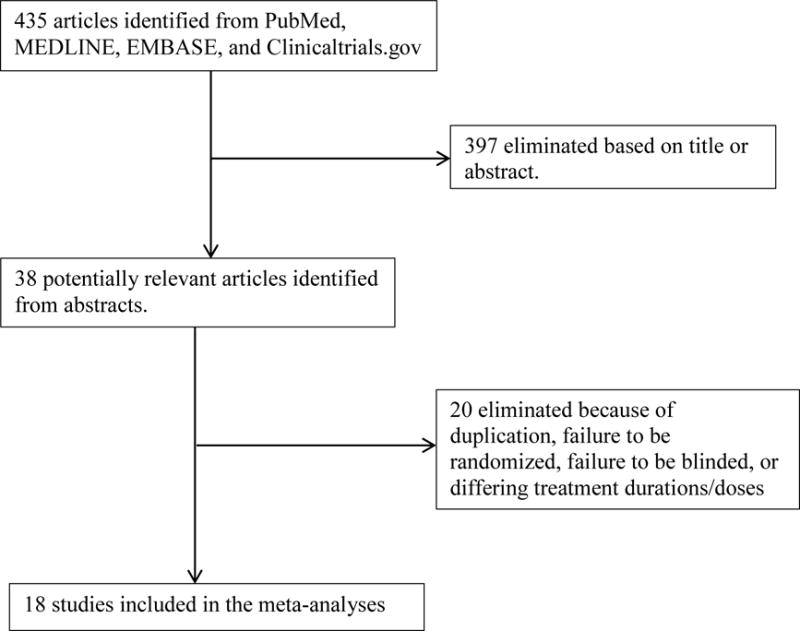
Selection process for trials included in meta-analyses
Apixaban (Eliquis)
Four trials comparing apixaban and enoxaparin were identified: APROPOS, ADVANCE-1, ADVANCE-2, and ADVANCE-3.7,8,9,10 Apixaban 2.5 mg twice per day was compared to enoxaparin 40 mg once per day in the first two trials and compared to enoxaparin 30 mg twice per day in the last two trials (Table 1), respectively. On average, apixaban reduced VTE by 29% (RR=0.71, 95% CI 0.52–0.96; p = 0.026). It failed the homogeneity test (Cochran’s Q = 9.7; I2 = 9.3%), reflecting differences in efficacy among trials: compared to enoxaparin 40 mg once daily, apixaban had greater efficacy in preventing VTE (RR = 0.57, 95% CI 0.46–0.72; p < 0.001).9,10 Alternatively, compared to enoxaparin 30 mg twice daily, apixaban did not prevent VTE (RR = 0.98, 95% CI 0.68–1.42).7,8 Apixaban significantly reduced major/clinically relevant bleeding by 16% (RR=0.84, 95% CI 0.70–0.99; p = 0.043), but had no effect on major bleeding (RR=0.85, 95% CI 0.53–1.34; p = 0.48). Bleeding analyses passed the homogeneity test.
Table 1.
Meta-analysis of Apixaban Trials
| Trial | Year | Regimen | No. of Patients Randomized | Treatment Duration | No. of VTEs | RR VTE* | No. of Major Bleeds | RR Major Bleeding* | No. of Major/Clinically Relevant Bleeds | RR Major/Clinically Relevant Bleeding* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| APROPOS | 2007 | Apixaban 2.5 mg BID | 153 | 12 days | 10 | 0.58 (0.28–1.20) | 0 | NA | 0 | 0.20 (.01–4.07) |
| Enoxaparin 30 mg BID | 152 | 17 | – | 0 | 0 | |||||
|
| ||||||||||
| ADVANCE-1 | 2009 | Apixaban 2.5 mg BID | 1599 | 10–14 days | 103 | 1.04 (0.80–1.35) | 11 | 0.50 (0.24–1.02) | 46 | 0.66 (0.46–0.96) |
| Enoxaparin 30 mg BID | 1596 | 97 | – | 22 | – | 69 | – | |||
|
| ||||||||||
| ADVANCE-2 | 2010 | Apixaban 2.5 mg BID | 1528 | 10–14 days | 146 | 0.61 (0.51–0.74) | 9 | 0.65 (0.28–1.49) | 53 | 0.74 (0.52–1.05) |
| Enoxaparin 40 mg QD | 1529 | 243 | – | 14 | – | 72 | – | |||
|
| ||||||||||
| ADVANCE-3 | 2010 | Apixaban 2.5 mg BID | 2708 | 35 days | 25 | 0.34 (0.21–.53) | 22 | 1.22 (0.65–2.26) | 129 | 0.96 (0.76–1.21) |
| Enoxaparin 40 mg QD | 2699 | 73 | – | 18 | – | 134 | – | |||
|
| ||||||||||
| Meta-analysis | 2007–2010 | Apixaban 2.5 mg BID | 5988 | 10–35 days | 284 | 0.71 (0.52–0.96) | 42 | 0.85 (0.53–1.34) | 228 | 0.84 (0.70–0.99) |
| Enoxaparin variable dose | 5976 | 430 | – | 54 | – | 275 | – | |||
RR compared to enoxaparin. Values in parentheses denote a 95% confidence interval.
Abbreviations: RR, relative risk; VTE, venous thromboembolism; NA: Not available
Dabigatran (Pradaxa)
Four trials comparing dabigatran and enoxaparin were identified: RE-MODEL, RE-MOBILIZE, RE-NOVATE, and RE-NOVATE II.11,12,13,14 RE-MOBILIZE compared subcutaneous enoxaparin 30 mg twice daily to dabigatran 150 and 220 mg orally once daily. The other three trials compared subcutaneous enoxaparin 40 mg once daily to dabigatran 150 mg or 220 mg once daily (Table 2).
Table 2.
Meta-analysis of Dabigatran Trials
| Trial | Year | Regimen | No. of Patients Randomized | Treatment Duration | No. of VTEs | RR VTE* | No. of Major Bleeds | RR major bleeding* | No. of Major/Clinically Relevant Bleeds | RR Major/Clinically Relevant Bleeding* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| RE-MODEL | 2007 | Dabigatran 150 mg QD | 708 | 6–10 days | 212 | 1.07 (0.92–1.25) | 9 | 0.99 (0.39–2.47) | 57 | 1.22 (0.84–1.78) |
| Dabigatran 220 mg QD | 694 | 182 | 0.96 (0.82–1.13) | 10 | 1.14 (0.46–2.78) | 50 | 1.11 (0.76–1.63) | |||
| Enoxaparin 40 mg QD | 699 | 193 | – | 9 | – | 46 | – | |||
|
| ||||||||||
| RE-MOBILIZE | 2009 | Dabigatran 150 mg QD | 877 | 12–15 days | 218 | 1.33 (1.11–1.60) | 5 | 0.42 (0.15–1.17) | 27 | 0.82 (0.49–1.34) |
| Dabigatran 220 mg QD | 862 | 187 | 1.22 (1.02–1.46) | 5 | 0.42 (0.15–1.19) | 28 | 0.86 (0.52–1.41) | |||
| Enoxaparin 30 mg BID | 876 | 163 | – | 12 | – | 33 | – | |||
|
| ||||||||||
| RE-NOVATE | 2007 | Dabigatran 150 mg QD | 1174 | 28–35 days | 74 | 1.27 (0.91–1.76) | 15 | 0.83 (0.42–1.63) | 70 | 1.20 (0.85–1.68) |
| Dabigatran 220 mg QD | 1157 | 51 | 0.87 (0.60–1.24) | 23 | 1.29 (0.70–2.37) | 71 | 1.23 (0.88–1.73) | |||
| Enoxaparin 40 mg QD | 1162 | 60 | – | 18 | – | 58 | – | |||
|
| ||||||||||
| RE-NOVATE | 2011 | Dabigatran 220 mg QD | 1036 | 28–35 days | 61 | 0.88 (0.63–1.22) | 14 | 1.54 (0.67–3.55) | 37 | 1.27 (0.79–2.04) |
| II | Enoxaparin 40 mg QD | 1019 | 69 | – | 9 | – | 29 | – | ||
|
| ||||||||||
| Meta-analysis | 2007–2011 | Dabigatran 150 mg QD | 2759 | 6–35 days | 504 | 1.19 (0.98–1.44) | 29 | 0.78 (0.48–1.27) | 154 | 1.13 (0.90–1.41) |
| Enoxaparin variable dose (compared to dabigatran 150 mg) | 2737 | 416 | – | 39 | – | 137 | – | |||
| Dabigatran 220 mg QD | 3749 | 481 | 1.04 (0.87–1.24) | 52 | 1.19 (0.80–1.77) | 186 | 1.14 (0.93–1.40) | |||
| Enoxaparin variable dose (compared to dabigatran 220 mg) | 3756 | 485 | – | 48 | – | 166 | – | |||
RR compared to enoxaparin. Values in parentheses denote a 95% confidence interval. Abbreviations: RR, relative risk; VTE, venous thromboembolism
Efficacy and safety of dabigatran were not significantly different from enoxaparin. Dabigatran 150 mg once per day tended to increase VTE compared to enoxaparin (RR=1.19, 95% CI 0.98–1.44; p = 0.072) yet had no significant effect on major/clinically relevant bleeding (RR=1.22, 95% CI 0.89–1.67; p = 0.22) or major bleeds (RR=0.78, 95% CI 0.48–1.27; p = 0.32). Dabigatran 150 mg passed homogeneity tests for all outcomes.
Dabigatran 220 mg per day also had no effect on VTE (RR=1.04, 95% CI 0.87–1.24; p = 0.68) when compared to enoxaparin. Similarly, rates of major/clinically relevant bleeding (RR=1.14, 95% CI 0.93–1.4; p = 0.20) and major bleeding (RR=1.19, 95% CI 0.80–1.77; p = 0.40) were equivalent between enoxaparin and dabigatran 220 mg, which passed homogeneity tests for all outcomes as well.
Fondaparinux (Arixtra)
Four trials comparing fondaparinux and enoxaparin were identified: PENTAMAKS, PENTHIFRA, PENTATHLON 2000, and EPHESUS.15,16,17,18 Subcutaneous fondaparinux 2.5 mg once daily was compared to subcutaneous enoxaparin 30 mg twice daily in PENTAMAKS and PENTATHLON 2000; the other two trials compared the same fondaparinux dose to enoxaparin 40 mg once daily (Table 3).
Table 3.
Meta-analysis of Fondaparinux Trials
| Trial | Year | Regimen | No. of Patients Randomized | Treatment Duration | No. of VTEs | RR VTE* | No. of Major Bleeds | RR major bleeding* | No. of Major/Clinically Relevant Bleeds | RR Major/Clinically Relevant Bleeding* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| PENTAMAKS | 2001 | Fondaparinux 2.5 mg QD | 526 | 5–9 days | 45 | 0.45 (0.33–0.62) | 11 | 11.00 (1.43–84.90) | 11 | NA |
| Enoxaparin 30 mg BID | 523 | 101 | – | 1 | 1 | – | ||||
|
| ||||||||||
| PENTHIFRA | 2001 | Fondaparinux 2.5 mg QD | 849 | 5–9 days | 52 | 0.52 (0.38–0.70) | 18 | 0.96 (0.51–1.82) | 18 | NA |
| Enoxaparin 40 mg QD | 862 | 119 | – | 19 | – | 19 | – | |||
|
| ||||||||||
| PENTATHLON | 2002 | Fondaparinux 2.5 mg QD | 1138 | 5–9 days | 48 | 0.74 (0.51–1.05) | 20 | 1.82 (0.88–3.78) | 20 | NA |
| 2000 | Enoxaparin 30 mg BID | 1137 | 66 | – | 11 | – | 11 | – | ||
|
| ||||||||||
| EPHESUS | 2002 | Fondaparinux 2.5 mg QD | 1155 | 5–9 days | 37 | 0.44 (0.30–0.64) | 47 | 1.46 (0.94–2.27) | 47 | NA |
| Enoxaparin 40 mg QD | 1154 | 85 | – | 32 | – | 32 | – | |||
|
| ||||||||||
| Meta-analysis | 2001–2002 | Fondaparinux 2.5 mg QD | 3668 | 5–9 days | 182 | 0.53 (0.45–0.63) | 96 | 1.64 (0.24–11.35) | 96 | NA |
| Enoxaparin variable dose | 3676 | 371 | – | 63 | – | 63 | – | |||
RR compared to enoxaparin. Values in parentheses denote a 95% confidence interval.
Abbreviations: RR, relative risk; VTE, venous thromboembolism
Compared to enoxaparin, fondaparinux decreased VTE by 47% (RR=0.53, 95% CI 0.45–0.63; p < 0.001). Compared to enoxaparin 30 mg twice daily, fondaparinux decreased VTE (RR = 0.58, 95% CI 0.43–0.76; p < 0.001). However, fondaparinux tended to increase major bleeds (RR=1.64, 95% CI 0.24–11.3; p = 0.62). Fondaparinux passed homogeneity testing for VTE while failing homogeneity testing for major bleeds, due to the 11-fold RR of bleeding in one trial.15 No fondaparinux data were available for clinically relevant bleeding.
Rivaroxaban (Xarelto)
Four trials comparing rivaroxaban and enoxaparin were identified: ODIXa-HIP, RECORD-1, RECORD-3, and RECORD-4; we excluded RECORD-2 because 31–39 days of rivaroxaban were compared to 10–14 days of enoxaparin.19,20,21,22,23 Rivaroxaban 10 mg once daily was compared to subcutaneous enoxaparin 40 mg once daily in all trials except RECORD-4, which compared rivaroxaban to enoxaparin 30 mg twice daily (Table 4).
Table 4.
Meta-analysis of Rivaroxaban Trials
| Trial | Year | Regimen | No. of Patients Randomized | Treatment Duration | No. of VTEs | RR VTE* | No. of Major Bleeds | RR major bleeding* | No. of Major/Clinically Relevant Bleeds | RR Major/Clinically Relevant Bleeding* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ODIXa-HIP | 2006 | Rivaroxaban 10 mg QD | 147 | 5–9 days | 12 | 0.42 (0.22–0.79) | 1 | 0.37 (0.04–3.50) | 4 | 0.55 (0.17–1.80) |
| Enoxaparin 40 mg QD | 160 | 27 | – | 3 | – | 8 | – | |||
|
| ||||||||||
| RECORD-1 | 2008 | Rivaroxaban 10 mg QD | 2266 | 31–39 days | 16 | 0.28 (0.16–0.49) | 6 | 3.02 (0.61–14.95) | 71 | 1.28 (0.90–1.80) |
| Enoxaparin 40 mg QD | 2275 | 55 | – | 2 | – | 56 | – | |||
|
| ||||||||||
| RECORD-3 | 2008 | Rivaroxaban 10 mg QD | 1254 | 10–14 days | 79 | 0.51 (0.40–0.66) | 7 | 1.18 (0.40–3.52) | 40 | 1.19 (0.76–1.87) |
| Enoxaparin 40 mg QD | 1277 | 164 | – | 6 | – | 34 | – | |||
|
| ||||||||||
| RECORD-4 | 2009 | Rivaroxaban 10 mg QD | 1584 | 10–14 days | 57 | 0.73 (0.53–1.02) | 10 | 2.47 (0.78–7.86) | 49 | 1.42 (0.92–2.19) |
| Enoxaparin 30 mg BID | 1564 | 79 | – | 4 | – | 34 | – | |||
|
| ||||||||||
| Meta-analysis | 2007–2010 | Rivaroxaban 10 mg QD | 5251 | 5–39 days | 164 | 0.55 (0.46–0.66) | 24 | 1.88 (0.67–5.29) | 164 | 1.27 (1.01–1.59) |
| Enoxaparin variable dose | 5276 | 325 | – | 15 | – | 132 | – | |||
RR compared to enoxaparin. Values in parentheses denote a 95% confidence interval.
Abbreviations: RR, relative risk; VTE, venous thromboembolism
Rivaroxaban decreased VTE by 45% (RR=0.55, 95% CI 0.46–0.66; p < 0.001). It increased major/clinically relevant bleeds by 27% (RR=1.27, 95% CI 1.01–1.59; p =0.039) but did not significantly increase major bleeds (RR=1.88, 95% CI 0.67–5.29; p = 0.23). Rivaroxaban passed homogeneity testing for all outcomes.
Edoxaban (Savaysa)
Two trials comparing edoxaban and enoxaparin were identified: STARS E-3 and STARS J-V, which were conducted in Japan and Taiwan.24,25 Both trials compared oral edoxaban 30 mg once daily to subcutaneous enoxaparin 20 mg twice daily (Table 5), the standard dose in Asian populations. Likewise, the average weight of participants in these trials was only 60 kg. Compared to enoxaparin 20 mg twice daily, edoxaban nearly halved VTE risk (RR=0.49, 95% CI 0.32–0.75; p = 0.001), yet did not significantly increase major/clinically relevant bleeds (RR=1.33, 95% CI 0.64–2.76; p = 0.44) or major bleeds (RR=1.58, 95% CI 0.05–54.38; p =0.65). Edoxaban passed homogeneity tests for VTE and major/clinically relevant bleeds, but not for major bleeds (Q = 6.9; I2 = 6.7%).
Table 5.
Meta-analysis of Edoxaban Trials
| Trial | Year | Regimen | No. of Patients Randomized | Treatment Duration | No. of VTEs | RR VTE* | No. of Major Bleeds | RR major bleeding* | No. of Major/Clinically Relevant Bleeds | RR Major/Clinically Relevant Bleeding* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| STARS E-3 | 2014 | Edoxaban 30 mg QD | 360 | 11–14 days | 22 | 0.53 (0.32–0.87) | 4 | 3.94 (0.44–35.11) | 22 | 1.67 (0.85–3.26) |
| Enoxaparin 20 mg BID | 365 | 41 | – | 1 | – | 13 | – | |||
|
| ||||||||||
| STARS J-V | 2015 | Edoxaban 30 mg QD | 307 | 11–14 days | 6 | 0.34 (0.14–0.86) | 2 | 0.33 (0.07–1.63) | 8 | 0.72 (0.29–1.77) |
| Enoxaparin 20 mg BID | 303 | 17 | – | 6 | – | 11 | – | |||
|
| ||||||||||
| Meta-analysis | 2014–2015 | Edoxaban 30 mg QD | 667 | 11–14 days | 28 | 0.49 (0.32–0.75) | 6 | 1.58 (0.05–54.38) | 30 | 1.33 (0.53–3.34) |
| Enoxaparin 20 mg BID | 668 | 58 | – | 7 | – | 24 | – | |||
RR compared to enoxaparin. Values in parentheses denote a 95% confidence interval.
Abbreviations: RR, relative risk; VTE, venous thromboembolism
Pooled Result
Overall, fondaparinux, rivaroxaban, and edoxaban had the highest efficacy in preventing VTE (Figure 2), but they also had the greatest risk of bleeding (Figure 3). Apixaban and dabigatran 150 mg had the lowest risk of bleeding yet apixaban was more effective. Dabigatran 220 mg was not inferior to enoxaparin, however it was neither safer nor more effective (Figure 4).
Figure 2.
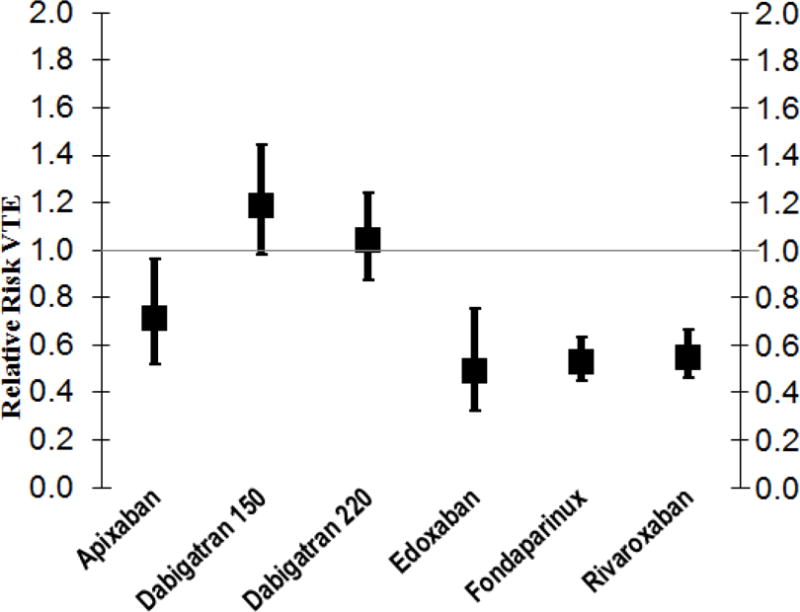
Pooled Relative Risks of VTE (Venous Thromboembolism) with Newer Anticoagulants Compared to Enoxaparin
Abbreviations: RR, relative risk; VTE, venous thromboembolism
Figure 3.
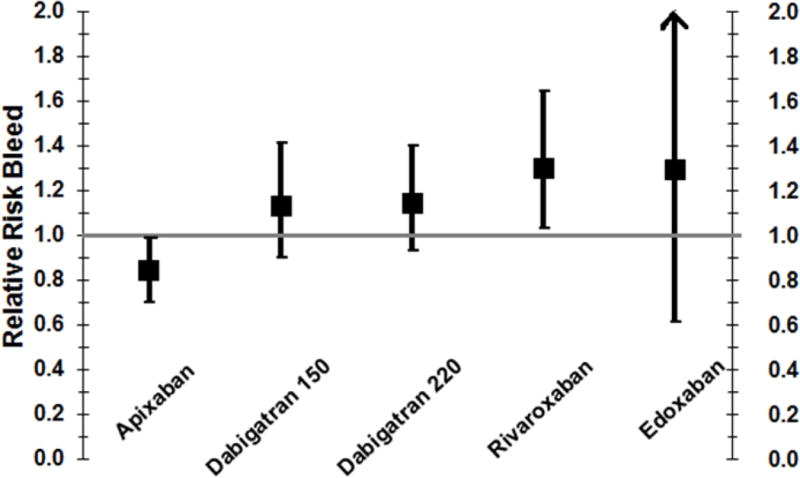
Pooled RR of Major/Clinically Relevant Bleeding for Newer Anticoagulants Compared to Enoxaparin
Figure 4.
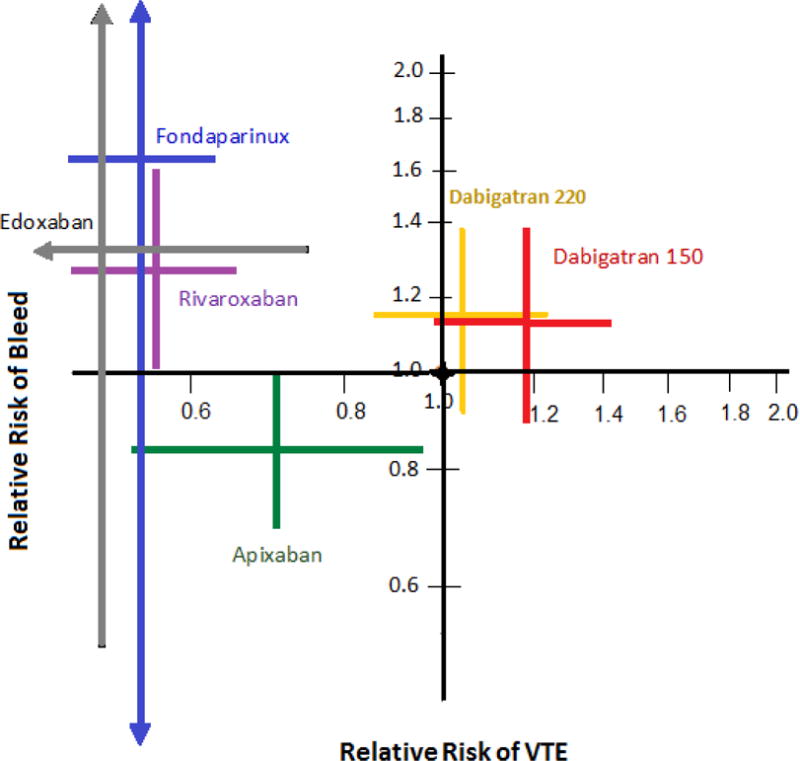
Relative risk of bleeding vs. relative risk of VTE
*The black circle at the origin (1.0, 1.0) shows enoxaparin, the referent therapy.
The relative risk of bleeding—either major or non-major clinical relevant bleeding (vertical axis) and the relative risk of venous thromboembolism (VTE) (horizontal axis). Each cross shows the 95% confidence intervals of the relative risk from a meta-analysis.
Discussion
As compared to subcutaneous enoxaparin, four newer anticoagulants reduced the rate of VTE after arthroplasty (Figure 2). Their RR (95% CI) were: apixaban 0.71 (0.52–0.96), rivaroxaban 0.55 (0.46–0.66), fondaparinux 0.53 (0.45–0.63), and edoxaban 0.49 (0.32–0.75). Apixaban also protected against major/clinically relevant bleeding: RR (95% CI) of 0.84 (0.70–0.99). In contrast, rivaroxaban increased major/clinically relevant bleeds (RR=1.27, 95% CI 1.01–1.59). The effect of fondaparinux and edoxaban on major/clinically relevant bleeding was not precise because the fondaparinux trials did not report non-major bleeds and only two edoxaban trials met inclusion criteria.
On average, oral apixaban 2.5 mg twice daily reduced the risks of both major/clinically relevant bleeding and of VTE. However, compared specifically with enoxaparin 30 mg twice daily, apixaban did not affect VTE rate (RR = 0.98); compared to enoxaparin 40 mg once daily, apixaban had greater efficacy.9, 10 In a prior analysis of the ADVANCE-2 and 3 trials, Raskob et al. reached a similar conclusion: arthroplasty patients randomized to apixaban had half as many VTEs as patients randomized to enoxaparin 40 mg daily.26 The FDA and EU have approved apixaban 2.5 mg twice daily beginning 12–24 hours post-operatively for approximately 32–38 days after hip arthroplasty and for 10–14 days after knee arthroplasty.27 Based primarily on its lower risk of bleeding, apixaban is an excellent alternative to enoxaparin for arthroplasty patients.
Like apixaban, rivaroxaban was significantly more effective than enoxaparin at preventing VTE. Unlike apixaban, oral rivaroxaban 10 mg once daily increased the RR (95% CI) of major/clinically relevant bleeding by 1.27 (1.01–1.59) (Figure 3). The decreased safety of rivaroxaban may reflect the timing of administration: in the RECORD trials, rivaroxaban was started 6–8 hours after arthroplasty, whereas in the ADVANCE trials apixaban was initiated 12–24 hours after arthroplasty. Rivaroxaban administered 6–8 hours after arthroplasty may be appropriate for patients at high risk of VTE, but suboptimal for patients at high risk of bleeding.
Compared to twice daily apixaban, the once daily dosing of rivaroxaban results in higher peak anti-Xa activity, which may also contribute to rivaroxaban’s increased bleeding.28 Our conclusion contrasts to that of Lassen et al. who concluded that bleeding events “occurred at similar rates in the rivaroxaban and enoxaparin groups.”29 Specifically, they reported that rivaroxaban had a RR (95% CI) for major/clinically relevant bleeding of 1.21 (0.99 to 1.48). However, we note that Lassen et al. included RECORD-2, while we excluded that study because of the different duration of thromboprophylaxis in the two study arms.
Edoxaban 30 mg once daily halved the rate of VTE (RR = 0.49; 95% CI 0.32–0.75). It did not increase the RR (95% CI) of bleeding significantly: for major bleeding the RR was 1.58 (0.05–54.38); for major/clinically relevant bleeding the RR was 1.33 (0.64–2.76). However, with only 2 eligible trials, the effect of edoxaban on bleeding was not precise. In both trials, edoxaban was compared to enoxaparin 20 mg twice daily, the standard dose in Japan and Taiwan where the STARS E-3 and STARS J-V trials were conducted. Thus, how edoxaban compares to enoxaparin 30 mg twice daily is unknown.
Dabigatran (at either 150 or 220 mg/d) had efficacy and safety that was not significantly different than enoxaparin. Dabigatran 150 mg/d trended toward a higher VTE rate (RR =1.19; 95% CI 0.98–1.44) than enoxaparin and had a similar bleed risk, thus we found no advantage to dabigatran in the arthroplasty population.
Fondaparinux significantly reduced the rate of VTE (by 47%). It was the only agent that was more effective than enoxaparin 30 mg twice daily (VTE RR = 0.58, 95% CI 0.43–0.76). However, because of its subcutaneous administration and trend (RR 1.64) for more major bleeding, we would recommend it only for arthroplasty patients at high risk for VTE and low risk for bleeding.
A clinical prediction rule for post-operative VTE would allow orthopedists to select thromboprophylaxis based on VTE. A classic clinical prediction rule can predict post-operative VTE overall, but classifies all arthroplasty patients as high risk.30 However, Kulshrestha et al. found that nearly half of TKA patients could be identified prospectively as having a low enough VTE risk that they could be treated with post-operative aspirin, rather than an anticoagulant.31 Although this approach could reduce the risk of post-operative hemorrhage, aspirin is only modestly effective at preventing VTE after arthroplasty.32, 33 Thus, the future treatment for low VTE risk arthroplasty patients may be the combination of aspirin plus a mobile compression device continuing after hospital discharge.34, 35
Variations in endpoints and methods explain the differences between our analysis and prior meta-analyses. The primary endpoint in the meta-analysis by Gómez-Outes et al. was symptomatic VTE.36 They concluded that rivaroxaban halved the risk of symptomatic VTEs (RR = 0.48). Although the RR we calculated for rivaroxaban was similar (0.55), because we included all VTEs, our 95% CI was more precise (0.46–0.66). In another meta-analysis, Neumann et al. reported that per 1000 patients, factor Xa inhibitors (as a class) prevented 4 symptomatic DVT and 0 pulmonary emboli and caused 2 major bleeds as compared with enoxaparin.37 We too found reductions in VTEs with Factor Xa inhibitors when compared to enoxaparin. Loke and colleagues’ findings are consistent with ours: They found rivaroxaban to be superior to enoxaparin for VTE prevention (RR 0.56), but with an increased risk of hemorrhage (RR 1.26).38 They also found dabigatran to be equivalent in safety (RR 1.10) and efficacy (RR 1.12) to enoxaparin. Indirectly, they suggested that rivaroxaban was more efficacious than dabigatran, but with more bleeding. An important difference between our study and prior meta-analyses is that we included fondaparinux and edoxaban.
There were limitations to our meta-analyses. Although the risk of VTE after arthroplasty persists for months, the trials had incongruent treatment periods, sometimes for less than 30 days.39 Second, in clinical practice, objective DVT screening is not done routinely, and screening in the trials might have prevented some DVTs from becoming symptomatic. Third, all trials were sponsored by the manufacturer of the newer drug. Furthermore, none of the studies were powered to detect reductions in symptomatic VTEs or death. Finally, the individual studies excluded patients at a higher risk of bleeding, suggesting that rates of bleeding may be greater in clinical practice than in the trials.
Our meta-analyses also had several important strengths. All of the included trials objectively confirmed DVTs with venography and were randomized controlled and double-blind, thereby minimizing bias. Finally, we included both efficacy and safety, thereby quantifying relevant tradeoffs. The tradeoffs could be used in future guidelines to favor more potent anticoagulants in the arthroplasty subpopulation at highest risk of VTE and lowest risk of bleeding.
There would be additional advantages to substituting the new anticoagulants for enoxaparin or fondaparinux: cost and route of administration. Enoxaparin and fondaparinux require subcutaneous administration while the newer anticoagulants are taken orally. Patients prefer oral administration, and subcutaneous administration can decrease compliance.40 At a cost of $14.32 for 30 mg twice daily or $9.58 for 40 mg once daily (plus nursing time to administer the injection), enoxaparin is also more expensive than the newer anticoagulants. In the US, the wholesale prices are $13.30 for rivaroxaban (10 mg once daily), $13.34 for dabigatran (150 mg once daily), $13.34 for apixaban (2.5 mg twice daily), and $11.65 for edoxaban (30mg once daily). These anticoagulants are cheaper in other countries.41 Additionally, an antidote for dabigatran (idarucizumab) is currently available and an antidote for Xa inhibitors (andexanet alfa) will likely be available soon.42
In summary, compared to enoxaparin 40 mg daily, apixaban, rivaroxaban, fondaparinux, and edoxaban reduced the rate of VTE after arthroplasty. Only fondaparinux proved superior to enoxaparin 30 mg twice daily. With the exception of apixaban, which reduced major/clinically relevant bleeding, the newer anticoagulants that lowered the risk of post-operative VTE increased bleeding.
Supplementary Material
Acknowledgments
Funding for this research was provided by the NIH grant HL R01 HL097036, UL1 RR024992 and TL1 RR024995.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A poster of the abstract and preliminary results were presented at the 2012 American College of Clinical Pharmacy in Hollywood, Florida.
References
- 1.Witt DM, Nutescu EA, Haines ST. Chapter 26. Venous Thromboembolism. In: Talbert RL, DiPiro JT, Matzke GR, Posey LM, Wells BG, Yee GC, editors. Pharmacotherapy: A Pathophysiologic Approach. 8th. New York: McGraw-Hill; 2011. [Google Scholar]
- 2.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW, Jr, American College of Chest Physicians Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e278S–325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009 Oct;467(10):2606–12. doi: 10.1007/s11999-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 5.Committee for Proprietary Medicinal Products. Points to Consider on Clinical Investigation of Medicinal Products for Prophylaxis of Intra and Post-Operative Venous Thromboembolic Risk. London: The European Agency for the Evaluation of Medicinal Products; 2007. (Guideline no. CPMP/EWP/707/98 Rev.1 corr.). [Google Scholar]
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 7.Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost. 2007 Dec;5(12):2368–75. doi: 10.1111/j.1538-7836.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 8.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 9.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–15. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 10.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the REMODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 12.RE-MOBILIZE Writing Committee. Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR, RE-NOVATE Study Group Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007 Sep 15;370(9591):949–56. doi: 10.1016/S0140-6736(07)61445-7. Erratum in: Lancet. 2007 Dec 15;370(9604):2004. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ, RE-NOVATE II Study Group Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011 Apr;105(4):721–9. doi: 10.1160/TH10-10-0679. [DOI] [PubMed] [Google Scholar]
- 15.Bauer KA, Eriksson BI, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Major Knee Surgery Study Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345(18):1305–1. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345(18):1298–304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 17.Turpie AG, Bauer KA, Eriksson BI, Lassen MR, PENTATHALON 2000 Study Steering Committee Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002 May 18;359(9319):1721–6. doi: 10.1016/S0140-6736(02)08648-8. Erratum in: Lancet 2002;360(9339):1102. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Bauer KA, Eriksson BI, Turpie AG, European Pentasaccharide Elective Surgery Study (EPHESUS) Steering Committee Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002;359(9319):1715–20. doi: 10.1016/S0140-6736(02)08652-X. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Muehlhofer E, Dierig C, Misselwitz F, Kälebo P, ODIXa-HIP Study Investigators A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation. 2006 Nov 28;114(22):2374–81. doi: 10.1161/CIRCULATIONAHA.106.642074. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, RECORD1 Study Group Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008 Jun 26;358(26):2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 21.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG, RECORD3 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008 Jun 26;358(26):2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 22.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD, RECORD4 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009 May 16;373(9676):1673–80. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S, RECORD2 Investigators Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008 Jul 5;372(9632):31–9. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 24.Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T, Ibusuki K, Ushida H, Abe K, Tachibana S. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res. 2014 Dec;134(6):1198–204. doi: 10.1016/j.thromres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Fuji T, Fujita S, Kawai Y, Nakamura M, Kimura T, Fukuzawa M, Abe K, Tachibana S. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V. Thromb J. 2015 Aug 12;13:27. doi: 10.1186/s12959-015-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Wright RT, Lassen MR. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br. 2012 Feb;94(2):257–64. doi: 10.1302/0301-620X.94B2.27850. [DOI] [PubMed] [Google Scholar]
- 27.Hughes Sue. Apixaban approved in Europe for use after hip/knee surgery. http://www.theheart.org/article/1229117.do (accessed 23 April 2016)
- 28.Frost C, Song Y, Barrett YC, Wang J, Pursley J, Boyd RA, LaCreta F. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014 Nov 13;6:179–87. doi: 10.2147/CPAA.S61131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassen MR, Gent M, Kakkar AK, Eriksson BI, Homering M, Berkowitz SD, Turpie AG. The effects of rivaroxaban on the complications of surgery after total hip or knee replacement: results from the RECORD programme. J Bone Joint Surg Br. 2012 Nov;94(11):1573–8. doi: 10.1302/0301-620X.94B11.28955. [DOI] [PubMed] [Google Scholar]
- 30.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38:12–9. doi: 10.1016/s0037-1963(01)90094-0. [DOI] [PubMed] [Google Scholar]
- 31.Kulshrestha V, Kumar S. DVT prophylaxis after TKA: routine anticoagulation vs risk screening approach - a randomized study. The Journal of arthroplasty. 2013;28:1868–73. doi: 10.1016/j.arth.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Drescher FS, Sirovich BE, Lee A, Morrison DH, Chiang WH, Larson RJ. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta-analysis. J Hosp Med. 2014 Sep;9(9):579–85. doi: 10.1002/jhm.2224. [DOI] [PubMed] [Google Scholar]
- 33.Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty. 2012 Jan;27(1):1–9.e2. doi: 10.1016/j.arth.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Hardwick ME, Pulido PA, Colwell CW., Jr A mobile compression device compared with low-molecular-weight heparin for prevention of venous thromboembolism in total hip arthroplasty. Orthop Nurs. 2011 Sep-Oct;30(5):312–6. doi: 10.1097/NOR.0b013e31822c5c28. [DOI] [PubMed] [Google Scholar]
- 35.Colwell CW, Jr, Froimson MI, Anseth SD, et al. A mobile compression device for thrombosis prevention in hip and knee arthroplasty. The Journal of bone and joint surgery American volume. 2014;96:177–83. doi: 10.2106/JBJS.L.01031. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Outes A, Terleira-Fernández AI, Suárez-Gea ML, Vargas-Castrillón E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ. 2012;344:e3675. doi: 10.1136/bmj.e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann I, Rada G, Claro JC, Carrasco-Labra A, Thorlund K, Akl EA, Bates SM, Guyatt GH. Oral direct Factor Xa inhibitors versus low-molecular-weight heparin to prevent venous thromboembolism in patients undergoing total hip or knee replacement: a systematic review and meta-analysis. Ann Intern Med. 2012 May 15;156(10):710–9. doi: 10.7326/0003-4819-156-10-201205150-00421. [DOI] [PubMed] [Google Scholar]
- 38.Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism–systematic review and adjusted indirect comparison. J Clin Pharm Ther. 2011 Feb;36(1):111–24. doi: 10.1111/j.1365-2710.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen AB, Johnsen SP, Sørensen HT. Increased one-year risk of symptomatic venous thromboembolism following total hip replacement: A nationwide cohort study. J Bone Joint Surg Br. 2012;94(12):1598–603. doi: 10.1302/0301-620X.94B12.29358. [DOI] [PubMed] [Google Scholar]
- 40.Wilke T. Patient preferences for an oral anticoagulant after major orthopedic surgery: results of a german survey. Patient. 2009 Mar 1;2(1):39–49. doi: 10.2165/01312067-200902010-00005. [DOI] [PubMed] [Google Scholar]
- 41.National Institute for Health and Clinical Excellence (NICE) Rivaroxaban for the prevention of venous thromboembolism after total hip or total knee replacement in adults. London (UK): National Institute for Health and Clinical Excellence (NICE); 2009. Apr, p. 23. ((Technology appraisal guidance; no. 170).). [Google Scholar]
- 42.Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, Luan P, Hutchaleelaha A, Inagaki M, Conley PB, Phillips DR, Sinha U. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013 Apr;19(4):446–51. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


