Abstract
Background
In 2009 the US Preventive Services Task Force (USPSTF) recommended that the decision to start screening mammography prior to age 50 should be individualized. We examined whether healthcare providers are communicating about mammography decision-making with women and if communication is associated with screening behavior.
Methods
Data were drawn from the 2011–2014 Health Information National Trends Survey (HINTS). We included 5,915 female respondents age 40 or older who responded to the following question: ‘Has a doctor or other health professional ever told you that you could choose whether or not to have a mammogram?’. We used logistic regression to generate odds ratios (OR) and 95% confidence intervals (CI) for predictors of provider communication and assessed whether provider communication was associated with mammography in the previous two years overall and stratified by age.
Results
Less than half of women reported provider communication on mammogram choice. Women who reported provider communication were not more likely to report no mammogram in the past two years (OR: 1.07, 95% CI: 0.87–1.31) compared to those who did not. When stratified by 10-year age group, provider communication was associated with higher likelihood of no mammogram only among women age ≥70 (OR: 1.64, 95% CI: 1.15–2.34) and was associated with lower likelihood of no mammogram only among women age 40–49 (OR: 0.63, 95% CI: 0.43–0.92).
Conclusions
Between 2011 and 2014 less than half of women received communication on mammogram choice despite USPSTF recommendations. Provider communication on mammogram choice can influence screening behavior, particularly for younger and older women.
Keywords: communication, decision-making, mammography, surveys and questionnaires
Condensed abstract
From 2011–2014 less than half of women received provider communication on mammogram choice in the study population despite USPSTF recommendations. Provider communication on mammogram choice can influence screening behavior, particularly for women ages 40–49 and ≥70.
Introduction
The introduction and uptake of screening mammography among US women is one of the most striking public health successes in the past 50 years. In 1987 approximately 30% of US women age 40 or older reported a screening mammogram in the past two years, by 2000 it was 70%.1 In the decades since mammography was introduced, the number of breast cancer diagnoses has nearly doubled in the US, with a steep rise in in-situ and early-stage invasive cancers.2 However, in recent years research has shifted from a focus on the benefits of mammography to the potential harms and how best to balance the two. Concerns about potential overdiagnosis, the diagnosis of a cancer that would not have become clinically apparent during a woman’s lifetime,3 are growing, particularly among older women with competing co-morbidities.4 Overdiagnosis can theoretically lead to the administration of unnecessary cancer treatments with potentially harmful side effects. Additionally, potential harms of mammography screening include radiation exposure, and anxiety associated with false positive or unequivocal screening results including psychological distress, additional medical visits, imaging and biopsies.5
In 2009 the US Preventive Services Task Force (USPSTF) released new breast cancer screening guidelines which state that “the decision to start regular, biennial screening mammography before age 50 should be an individual one” that takes into account the patient’s personal history and risks.6 This recommendation was upheld by the 2016 update to the USPSTF guidelines.7 However, many patients may be under-informed or misinformed regarding the facts surrounding breast cancer and mammography and thus remain unable to make informed decisions regarding breast cancer screening.8 For example, one study found that 94% of women in their cohort doubted even the existence of ductal carcinoma in-situ, a histology that may not progress to invasive cancer,9 while another study suggested that most women overestimated the benefits of mammography screening when they were making decisions.8, 10
Based on the USPSTF guidelines, women under 50 are asked to collaborate with healthcare providers to decide when to begin breast cancer screening and how often to screen. In order to make informed choices women must understand the complex relationships between the benefits and harms of mammography screening.11 While medical professionals may understand the complexities of breast cancer screening, the general public may need more guidance and communication from their healthcare providers in order to make educated decisions regarding screening. Patients can educate themselves about screening, however simply having knowledge of breast cancer has not been shown to be associated with patients taking an active role in their screening decisions.12 Multiple studies suggest that mammography utilization is largely unchanged since the change in guidelines,13–15 yet the reasons why remain unclear. We need to understand the extent to which healthcare providers communicate the choices available.
Using data from the Health Information National Trends Survey (HINTS) a nationally representative survey conducted by the National Cancer Institute, we examined whether women are having discussions about mammography with their healthcare providers and how that has changed over time. We hypothesized that communication would increase with time, particularly with the youngest and oldest women, given the emphasis of the USPSTF guidelines on shared decision-making. We further explored other demographic and medical characteristics associated with provider communication on mammography choice. Lastly, we investigated the association between provider communication and screening behavior, and whether this association differs by age.
Methods
Data
Data were obtained from NCI’s HINTS fourth iteration, Cycles 1–4 (HINTS 4, Cycle 1–4), a nationally representative cross-sectional survey of the U.S. adult, civilian, non-institutionalized population. Data were collected by questionnaires mailed from over four time periods: October 2011 through February 2012 (HINTS 4, Cycle 1; n=3,959); October 2012 through January 2013 (HINTS 4, Cycle 2; n=3,630); September through November 2013 (HINTS 4, Cycle 3; n=3,185); August through November 2014 (HINTS 4, Cycle 4; n=3,677). The sample design for each cycle was a two-stage, stratified sample. First, addresses were randomly selected from a U.S. Postal Service file of residential addresses, and then individual respondents were selected within each sampled household. Response rates in each cycle ranged from 40% (HINTS 4, Cycle 2) to 34.4% (HINTS 4, Cycle 4).
Exposure, Outcome and Covariate Assessment
In HINTS 4, Cycles 1–4, female participants were asked ‘A mammogram is an x-ray of each breast to look for cancer. Has a doctor ever told you that you could choose whether or not to have a mammogram?’. In HINTS 4, Cycle 3 only, participants were asked: ‘A mammogram is an x-ray of each breast to look for breast cancer. During the past 12 months, did a doctor, nurse, or other health professional advise you to get a mammogram?’. Response options for both questions were ‘yes’ or’ no’. In cycles 1–4 women were also asked: ‘When did you have your most recent mammogram to check for breast cancer?’. Response options were: ‘A year ago or less’, ‘more than one, up to two years ago’, ‘more than two, up to five years ago’, ‘more than five years ago’, and ‘I have never had a mammogram’. We combined the ‘a year ago or less’ and ‘more than one, up to two years ago’ categories to create an indicator of whether a woman had a mammogram in the previous two years. Because the majority of women reported a mammogram in the previous two years we model the odds of no mammogram.
Sociodemographic variables included in the analysis were: age (40–49, 50–59, 60–69, ≥70); education (less than high school, 12 years of school or completed high school, some college, college graduate or greater); race/ethnicity (Hispanic, non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, Non-Hispanic Other or Unknown race); household income (< $20,000, $20,000 to $34,999, $35,000 to $49,999, $50,000 to $74,999, ≥ $75,000); family history of any cancer (yes, no, not sure); health insurance status (yes, no); presence of regular health care provider (yes, no), and health status (excellent or very good, good, fair or poor). The survey instrument for each cycle can be found here: http://hints.cancer.gov/instrument.aspx.
Statistical Analysis
All analyses were conducted using SAS, version 9.4, to allow for appropriate weighting for the complex survey design of HINTS and to provide representative estimates of the U.S. population. A full-sample weight was used to calculate population estimates, and 200 replicate weights (50 for each cycle), calculated using the jackknife variance estimation method, were used to compute standard errors. These two weighting approaches ensured valid inferences from the sample to the U.S. population, correcting for non-response and non-coverage bias. We restricted our sample to females age 40 or older. Women with a personal history of breast cancer (N=265) or who did not respond to the question on provider communication on mammogram choice (N=105) were excluded, leaving an analytical sample of 5,915 women. An additional 22 women who did not respond to the question about their receipt of a mammogram were excluded from analyses where mammogram was the outcome.
We calculated weighted, unadjusted prevalence estimates for each HINTS 4 Cycle (1–4), in order to examine trends in the proportion of respondents that reported that a ‘doctor ever told you that you could choose whether or not to have a mammogram’. We report the proportion responding ‘yes’ in each year according to age group. Chi-square tests were used to compare differences in proportions within each cycle according to age and Cochran–Armitage tests for trend were used to assess time trends across the cycles within each age group. Multivariable logistic regression models were used to generate odds ratios (OR) and 95% confidence intervals (CI) for sociodemographic predictors of provider communication on mammogram choice. Next, we used weighted, multivariable logistic regression models to examine the association between reporting provider communication on mammogram choice with receipt of a mammogram in the past two years adjusting for potential confounders overall and stratified by age. We used likelihood ratio tests to evaluate interaction between age and provider communication on mammogram choice with respect to mammography utilization in the previous two years. Tests for statistical significance used alpha of 0.05. All p-values are two-sided. Missing categories were included in covariates to account for missing data.
Using data from HINTS 4, Cycle 3, we performed a secondary analysis to determine the relationship between provider communication on mammogram choice with whether a provider advised a woman to get a mammogram in the past 12 months and whether any observed association differed by age. We further investigated the association of provider mammogram advice with non-adherence to mammography screening.
Results
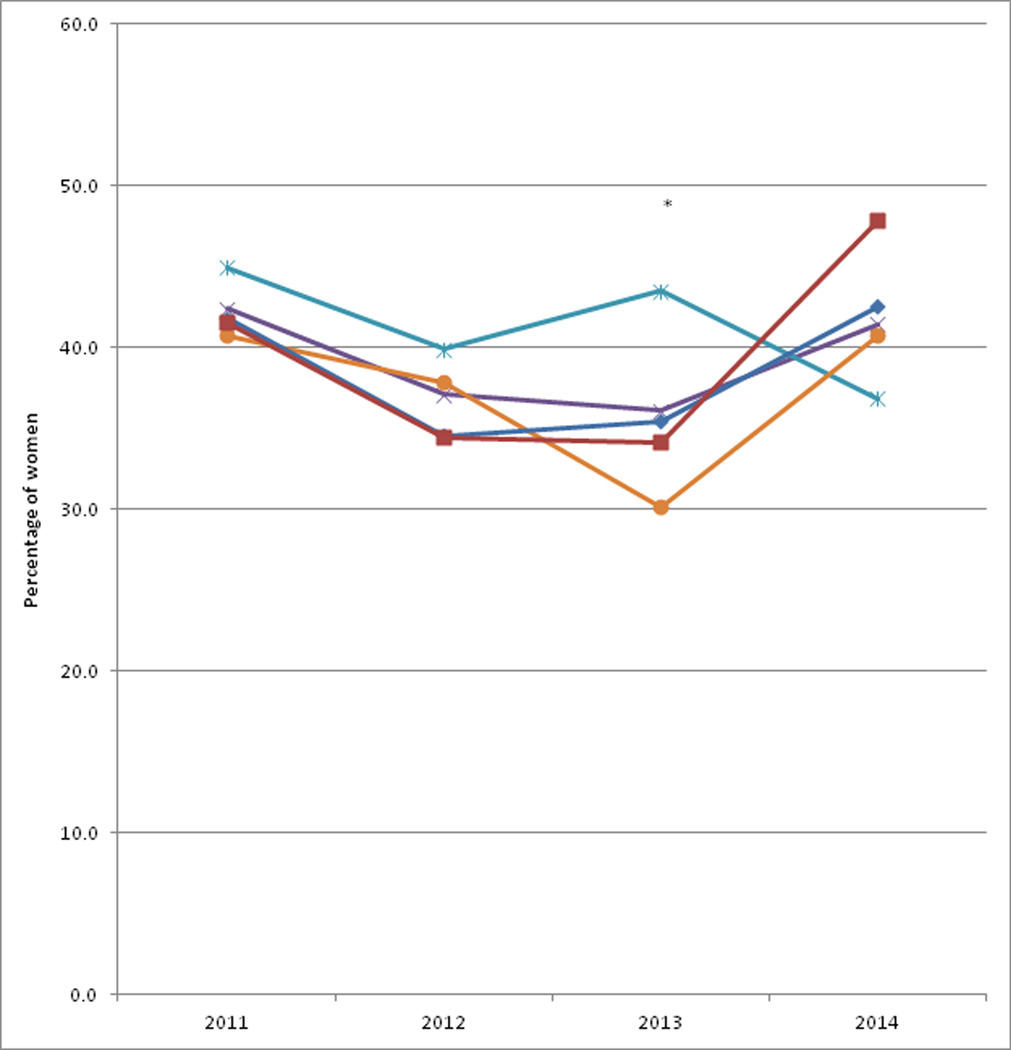
The characteristics of female HINTS 4 Cycle 1–4 respondents age 40 or older according to age group are displayed in Table 1. Less than half of the women reported provider communication on mammogram choice in each year, with the highest percentage in 2011 (42.4%) and lowest in 2013 (36.1%) (Figure 1). In every year except 2014, women age 40–49 were most likely to report provider communication, though this difference was only significant in 2013 (43.5% vs. 30.1%; p=0.04). There were no significant time trends in responses overall, or within each age group (p>0.10). Age-adjusted and multivariable models for receipt of provider communication on mammography choice were similar suggesting limited confounding (Table 2). In multivariable-adjusted models, women age 40–49 were 20% more likely to report provider communication (OR: 1.20, 95% CI: 0.97–1.48) compared to women age 50–59, though this did not reach statistical significance. Non-Hispanic black women were 34% less likely to report provider communication (OR: 0.66, 95% CI: 0.52–0.85) compared to non-Hispanic Whites. Non-Hispanic Asian women were 40% more likely to report provider communication on choice than non-Hispanic Whites, but this did not reach statistical significance (OR: 1.40, 95% CI: 0.90–2.19). Other factors such as health insurance status, Census region, and health status were not associated with provider communication on mammogram choice.
Table 1.
Characteristics of female HINTS 4 Cycle 1–4 respondents age 40 or older according to age group
| 40–49 years | 50–59 years | 60–69 years | ≥70 years | |||||
|---|---|---|---|---|---|---|---|---|
| N=1390 | N=1735 | N=1500 | N=1290 | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 44.7 | 0.07 | 54.4 | 0.05 | 64.0 | 0.06 | 78.7 | 0.12 |
| Race | N | % | N | % | N | % | N | % |
| Hispanic | 284 | 19.5 | 204 | 9.9 | 153 | 8.2 | 121 | 9.0 |
| Non-Hispanic White | 659 | 60.6 | 969 | 72.3 | 881 | 77.1 | 776 | 80.8 |
| Non-Hispanic Black | 255 | 12.8 | 327 | 11.9 | 238 | 9.8 | 128 | 7.2 |
| Non-Hispanic Asian | 66 | 4.8 | 51 | 4.1 | 29 | 2.2 | 24 | 1.8 |
| Non-Hispanic Other or Unknown | 52 | 2.3 | 51 | 1.7 | 54 | 2.8 | 19 | 1.2 |
| Education | ||||||||
| Less than high school | 123 | 13.6 | 126 | 10.6 | 129 | 12.4 | 227 | 22.8 |
| 12 years or completed high school | 207 | 15.8 | 367 | 21.7 | 360 | 26.3 | 428 | 33.8 |
| Some college | 386 | 32.5 | 543 | 33.9 | 489 | 33.3 | 353 | 28.1 |
| College graduate or higher | 661 | 38.1 | 686 | 33.7 | 507 | 28.0 | 254 | 15.3 |
| Household income | ||||||||
| < $20,000 | 285 | 18.5 | 366 | 17.5 | 339 | 21.4 | 437 | 40.0 |
| $20,000 to $34,999 | 163 | 10.2 | 214 | 11.8 | 225 | 15.5 | 216 | 22.8 |
| $35,000 to $49,999 | 178 | 15.0 | 226 | 14.1 | 198 | 15.3 | 148 | 16.0 |
| $50,000 to $74,999 | 205 | 15.8 | 270 | 17.2 | 233 | 20.7 | 103 | 10.5 |
| ≥ $75,000 | 469 | 40.5 | 508 | 39.5 | 298 | 27.1 | 96 | 10.7 |
| Has regular health care provider, % | 891 | 65.0 | 1236 | 71.7 | 1132 | 77.9 | 1025 | 84.8 |
| Has health insurance, % | 1137 | 79.8 | 1457 | 81.4 | 1355 | 90.4 | 1237 | 98.5 |
| No checkup in past two years, % | 234 | 18.3 | 272 | 17.5 | 202 | 12.5 | 122 | 9.5 |
| Region | ||||||||
| Northeast | 212 | 17.8 | 267 | 19.7 | 275 | 19.1 | 219 | 20.5 |
| Midwest | 240 | 20.1 | 363 | 24.1 | 303 | 24.8 | 240 | 20.2 |
| South | 597 | 37.6 | 720 | 34.3 | 617 | 38.4 | 530 | 36.9 |
| West | 341 | 24.5 | 385 | 21.9 | 305 | 17.7 | 301 | 22.3 |
| Family history of any cancer, % | ||||||||
| Yes | 901 | 66.8 | 1226 | 73.6 | 1032 | 73.4 | 891 | 72.7 |
| No | 353 | 27.1 | 349 | 21.1 | 323 | 21.9 | 279 | 21.2 |
| Not Sure | 87 | 6.1 | 92 | 5.3 | 87 | 4.7 | 87 | 6.1 |
| Health status, % | ||||||||
| Excellent or very good | 662 | 46.3 | 777 | 48.0 | 639 | 44.2 | 437 | 35.7 |
| Good | 460 | 35.9 | 605 | 35.5 | 550 | 37.6 | 541 | 45.2 |
| Fair or poor | 249 | 17.8 | 318 | 16.5 | 272 | 18.2 | 268 | 19.1 |
Figure 1.
Percentage of females reporting that physician or other health professional told them they could ‘choose whether or not to have a mammogram’ according to age group and year, HINTS 4 Cycle 1-Cycle 4 (2011–2014)
Table 2.
Odds ratios and 95% confidence intervals for predictors of reporting that physician or other health professional said they could ‘choose whether or not to have a mammogram’ among female HINTS 4 Cycles 1–4 respondents age 40 or older
| Total N |
Reported Provider Communication |
Age-Adjusted | Multivariable Adjusted |
|
|---|---|---|---|---|
| Age | OR (95% CI) | OR (95% CI) | ||
| 40–49 | 1390 | 559 | 1.18 (0.96, 1.45) | 1.20 (0.97, 1.48) |
| 50–59 | 1735 | 643 | 1.0 (reference) | 1.0 (reference) |
| 60–69 | 1500 | 526 | 1.06 (0.86, 1.29) | 1.04 (0.84, 1.28) |
| ≥70 | 1290 | 491 | 1.11 (0.91, 1.33) | 1.07 (0.86, 1.33) |
| Race | ||||
| Hispanic | 762 | 281 | 0.85 (0.67, 1.09) | 0.83 (0.63, 1.09) |
| Non-Hispanic White | 3285 | 1308 | 1.0 (reference) | 1.0 (reference) |
| Non-Hispanic Black | 948 | 285 | 0.71 (0.56, 0.88) | 0.66 (0.52, 0.85) |
| Non-Hispanic Asian | 170 | 79 | 1.39 (0.91, 2.13) | 1.40 (0.90, 2.19) |
| Non-Hispanic Other | 176 | 67 | 1.24 (0.66, 2.32) | 1.14 (0.62, 2.10) |
| Health insurance | ||||
| No | 655 | 183 | 0.95 (0.74, 1.21) | 0.80 (0.58, 1.11) |
| Yes | 5186 | 1738 | 1.0 (reference) | 1.0 (reference) |
| Checkup in past two years | ||||
| No | 830 | 263 | 0.68 (0.55, 0.84) | 0.63 (0.50, 0.81) |
| Yes | 5085 | 1956 | 1.0 (reference) | 1.0 (reference) |
| Regular health care provider | ||||
| No | 1514 | 552 | 1.10 (0.92, 1.30) | 0.95 (0.77, 1.17) |
| Yes | 4284 | 1630 | 1.0 (reference) | 1.0 (reference) |
| Family history of any cancer | ||||
| No | 1304 | 498 | 0.98 (0.82, 1.17) | 0.98 (0.83, 1.17) |
| Yes | 4050 | 1499 | 1.0 (reference) | 1.0 (reference) |
| Not sure | 353 | 141 | 1.30 (0.91, 1.85) | 1.30 (0.92, 1.85) |
| Hints 4 cycle | ||||
| 1 (10/11 – 2/12) | 1573 | 645 | 1.04 (0.85, 1.27) | 1.02 (0.83, 1.25) |
| 2 (10/12 – 1/13) | 1489 | 519 | 0.84 (0.67, 1.04) | 0.82 (0.66, 1.02) |
| 3 (9/13 – 11/13) | 1344 | 458 | 0.80 (0.63, 1.02) | 0.80 (0.63, 1.02) |
| 4 (8/13 – 11/14) | 1509 | 597 | 1.0 (reference) | 1.0 (reference) |
. adjusted for age only (except for age);
. mutually adjusted for all variables in table plus health status, educational attainment, household income, HINTS 4 cycle and Census region.
The youngest (40–49 years) and oldest (≥70 years) women were most likely to report no mammogram in the previous two years (Table 3). Compared to women age 50–59, those aged 40–49 were 77% (OR: 1.77, 95% CI: 1.33–2.34) more likely, and women aged ≥ 70 were 57% (OR: 1.57, 95% CI: 1.19–2.08) more likely, to report no mammogram in the past two years. Women who reported provider communication were 7% more likely to report no mammogram in the past two years (OR: 1.07, 95% CI: 0.87–1.31) compared to those who did not, though this was not statistically significant. When stratified by 10-year age group, provider communication was only associated with higher likelihood of no mammogram among women age 70 and older (OR: 1.64, 95% CI: 1.15–2.34) and was associated with lower likelihood of no mammogram only among women age 40–49 (OR: 0.63, 95% CI: 0.43–0.92) (Table 4).
Table 3.
Odds ratios and 95% confidence intervals for predictors not receiving a mammogram in the past two years among female HINTS 4 Cycles 1–4 respondents age 40 or older
| Total N |
No Mammogram in past two years |
Age-Adjusted | Multivariable Adjusted |
|
|---|---|---|---|---|
| Age | OR (95% CI)a | OR (95% CI)a | ||
| 40–49 | 1386 | 939 | 1.59 (1.25, 2.04) | 1.77 (1.33, 2.34) |
| 50–59 | 1729 | 1343 | 1.0 (reference) | 1.0 (reference) |
| 60–69 | 1496 | 1187 | 0.86 (0.68, 1.09) | 0.97 (0.76, 1.25) |
| ≥70 | 1282 | 915 | 1.28 (1.01, 1.62) | 1.57 (1.19, 2.08) |
|
Has a doctor or other health professional ever told you that you could choose whether or not to have a mammogram? |
||||
| No | 3680 | 2764 | 0.97 (0.81, 1.17) | 1.07 (0.87, 1.31) |
| Yes | 2213 | 1620 | 1.0 (reference) | 1.0 (reference) |
. adjusted for age only (except for age);
. mutually adjusted for variables in table plus race/ethnicity, household income, educational attainment, health insurance, regular healthcare provider, geographic region, family history of cancer, and self-reported health status.
Table 4.
Odds ratios and 95% confidence intervals for predictors not receiving a mammogram in the past two years among female HINTS 4 Cycles 1–4 respondents age 40 or older, according to 10-year age group
| 40–49 years | 50–59 years | 60–69 years | ≥70 years | |||||
|---|---|---|---|---|---|---|---|---|
| Has a doctor or other health professional ever told you that you could choose whether or not to have a mammogram? |
N | OR (95% CI)a | N | OR (95% CI) a | N | OR (95% CI) a | N | OR (95% CI) a |
| No | 939 | 1.0 (reference) | 1343 | 1.0 (reference) | 1187 | 1.0 (reference) | 915 | 1.0 (reference) |
| Yes | 447 | 0.63 (0.43, 0.92) | 386 | 1.11 (0.79, 1.56) | 309 | 0.88 (0.57, 1.37) | 367 | 1.64 (1.15, 2.34) |
Note: p-interaction=0.004
. age (continuous), race/ethnicity, household income, educational attainment, health insurance, regular healthcare provider, geographic region, family history of cancer, and self-reported health status
Among 1,335 respondents in HINTS 4, Cycle 3 the percentage of women advised by a healthcare provider to get a mammogram in the past 12 months differed by age (Supplementary Table 1). Nearly three-quarters of women age 40–49 (73.8%) reported being advised to get a mammogram compared to 81.3% of 50–59 year olds, 85.3% of 60–69 year olds and 69.4% of women aged 70 or older. Within each age, women whose providers told them they could choose whether or not to have a mammogram were more likely to be advised to have a mammogram than those whose providers did not discuss mammogram choice. This difference was greatest among women age 70 or older where 81.1% of those who reported provider communication on choice were advised to have a mammogram compared to 62.3% of those whose providers did not communicate about choice. Among women age 40–49, 80.0% of those whose providers communicated about mammogram choice were advised to have a mammogram compared to 72.9% of those whose providers did not communicate about choice. Women who were advised to get a mammogram were much less likely to have received a mammogram in the past two years (OR: 0.05, 95% CI: 0.03, 0.19; data not shown).
Discussion
This is one of the first studies to explore healthcare provider communication about mammography and the association with screening behavior in a nationally representative sample of women. Our results revealed less than half of women reported provider communication on mammogram choice overall. As expected based on guideline recommendations emphasizing provider communication, women age 40–49 were more likely to report communication overall, though this number remained under 50% each year, suggesting an important area of need. The youngest (40–49 years) and oldest (≥70 years) women were least likely to have received a mammogram in the prior two years, which is to be anticipated given the guidelines for mammography screening are most clear for women age 50–69 and shared decision-making is specifically recommended for women 40–49. The impact of provider communication on the youngest and oldest age groups was different, with provider communication associated with higher likelihood of no mammogram among women age ≥70 and lower likelihood of no mammogram among women age 40–49 in HINTS 4, Cycle 3. The reasons for this finding warrant further exploration given the potential for provider communication to impact mammogram utilization. While shared decision-making is not specifically recommended for women age 50–74, provider communication in general has the potential to improve adherence to mammography screening recommendations.
While women age 40–49 were most likely to receive provider communication on mammogram choice between 2011–2014 in this study, the majority of women in that age group did not receive such communication despite national recommendations emphasizing using a shared approach for this age group. The 2009 USPSTF recommendations,6 and the recent 2016 update,7 state the decision to start screening mammography prior to age 50 should be an individualized one. The 2015 guideline update from the American Cancer Society (ACS) states women should have the opportunity to begin screening between the ages of 40 and 44.16 According to the ACS clinicians “should acknowledge that different choices will be appropriate for different patients” and clinicians “must help each patient arrive at a management decision consistent with her or his values and preferences”. Given that many professional recommendations emphasize using a shared approach, our results are unexpected, though they may reflect the increasing time pressures faced by providers among other barriers.17 Our results are consistent with research findings suggesting patients experience minimal discussion of screening in medical encounters, despite much literature showing a desire among patients to be engaged in their health care decisions.18 Conversely, some patients do not desire shared decision-making in all situations and express a paternalism preference.19 Focus groups involving a total of 77 women held after the 2009 changes to the USPSTF guidelines showed many were suspicious the revised guidelines represented a cost-savings measure and most felt unprepared to participate in shared decision-making.12
Shared decision-making takes time and the same message cannot be given to all age groups. For example, while younger women are less likely to be diagnosed with breast cancer, the potential years of life lost due to breast cancer are greater.20 Co-morbidities and competing risk factors must also be weighed, all of which can be extremely difficult to accomplish during an appointment often filled with multiple agenda items. Describing the harms of screening is inherently difficult, especially in limited time. The term ‘harm’ can be highly charged and more specific language may be helpful. The most common immediate unwanted outcome from screening is recall for additional imaging evaluation. This can be both inconvenient and anxiety provoking, though it is challenging to study the balance of this with the beneficial reassurance of an ultimately negative screening report.21 There is also no validated way to individualize population estimates of breast cancer risk, especially given most breast cancers are sporadic and occur in women who would not be considered high risk using available predictive tools.22 Given these challenges, additional resources to aid communication, such as multimedia-based patient education tools, could be helpful to improve consistent communication surrounding screening mammography.
Non-Hispanic black women were 34% less likely to report provider communication compared to non-Hispanic Whites. The reasons for this are unclear and may reflect broader health disparities. There is data showing physicians are less likely to reference relevant scientific research in shared decision-making discussions with minority groups.23 Another plausible contributing explanation worthy of further research could be provider awareness that non-Hispanic black women have a greater risk of developing more aggressive disease phenotypes, including triple negative breast cancer.24–27 This could result in a more paternalistic approach to screening in this population and less reliance on shared decision-making, though it is unclear to what extent this accounts for the discrepancy observed. Other factors such as health insurance status, Census region, and health status were not associated with provider communication on mammogram choice in this study. When stratified by 10-year age group, provider communication was only associated with higher likelihood of no mammogram among women age 70 and older as noted. This could be due to the higher prevalence of co-morbid conditions among older women. However, an analysis using the DECISIONS study demonstrated that women in fair-poor health were as likely to discuss reasons to have vs. to not have a mammogram as women in excellent-good health.28 We also adjusted for health status in our analyses and it did not eliminate the effect of age. A recent study using data from HINTS 4 from 2011–2013 of 1,085 women younger than 50 found that being given a choice to undergo a mammogram by a provider was strongly associated with utilization in this age group, which is supported by our findings.29 The impact of communication on mammogram utilization is likely influenced by provider opinion on cancer screenings and the message conveyed. A provider can present an option to a patient while also making a recommendation by way of shared decision-making.30 Provider recommendations are known to play a critical role in the medical decisions made by patients.31 Further study of the way in which providers present the choice regarding mammogram screening to patients, particularly in regard to differences in guidelines among different organizations, is needed.
Strengths of the study include its use of a national representative sample and its relatively large size. However, the study is limited by the questions asked in the survey. The survey did not capture family history of breast cancer, only cancer in general. Additionally, the type of provider communication, when the discussion occurred, and whether or not providers were generally in favor or against screening, is unknown. Our data suggest that women who reported provider communication, albeit what this communication consisted of was unknown, were more likely to have been advised to get a mammogram in the past year with this difference being greatest among women age 70 or older. We did not have power to examine women age 70–74 separate from age 75 and older. According to the USPSTF, evidence is insufficient to recommend mammography for women 75 and older. Future work should gain further insight into distinctions in shared decision making among these oldest women. Furthermore, the survey does not distinguish if the provider that advised a mammogram is the same provider that discussed choice. Additionally, our study began two years after the guidelines were released, and whether or not patients had already started a screening regimen based on prior guidelines is not captured in the survey. There is evidence that patients may be unlikely to change practices.32 The survey also does not explore the issue of screening interval or intentions for future screening. Furthermore, analyses related to whether a woman reported a mammogram in the past two years were stratified by age at questionnaire completion rather than age at the time of mammogram due to the constraints of the survey, and this could result in bias as some survey participants would be expected to span two age groups during the two year period. More research is needed to explore why provider communication regarding screening mammography is not consistently happening and how this might be improved. Future analyses may be strengthened by considering both patient and provider reports of communication regarding screening mammography, as well as actual and perceived preferences for screening.
In conclusion, provider communication on mammogram choice can influence screening behavior. Our study demonstrates that despite national recommendations, less than 50% of patients received communication regarding mammogram choice. This includes the age groups (youngest and oldest) with the strongest recommendations for such communication. The call for patient-centered shared decision-making needs to be supported with validated communication tools, and healthcare provider training informed by evidence-based strategies is warranted.
Supplementary Material
Acknowledgments
Dr. Warner was supported by National Cancer Institute (NCI) grant 1K01CA188075-02. Dr. Spring was supported by NCI grant 5T32CA071345-19. The study sponsors had no role in study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication. Dr. Warner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work had no specific funding.
Footnotes
Conflict of interest disclosures: The authors have no conflicts of interest to report.
Author contributions: LMS, MRM, and ETW contributed to literature search, study design, data collection, data analysis, data interpretation, and writing. All authors contributed to critical revision of this report, and read and approved the final report.
References
- 1.Breen N, Gentleman JF, Schiller JS. Update on mammography trends: comparisons of rates in 2000, 2005, and 2008. Cancer. 2011;117:2209–2218. doi: 10.1002/cncr.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 3.Kalager M, Adami H-O, Bretthauer M, Tamimi RM. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Annals of Internal Medicine. 2012;156:491–499. doi: 10.7326/0003-4819-156-7-201204030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336–1347. doi: 10.1001/jama.2014.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164–165. doi: 10.1001/jama.2009.2007. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 7.Siu AL U. S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 8.Gigerenzer G, Mata J, Frank R. Public knowledge of benefits of breast and prostate cancer screening in Europe. Journal of the National Cancer Institute. 2009;101:1216–1220. doi: 10.1093/jnci/djp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz LM, Woloshin S, Sox HC, Fischhoff B, Welch HG. US women's attitudes to false positive mammography results and detection of ductal carcinoma in situ: cross sectional survey. BMJ (Clinical research ed.) 2000;320:1635–1640. doi: 10.1136/bmj.320.7250.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann TC, Del Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175:274–286. doi: 10.1001/jamainternmed.2014.6016. [DOI] [PubMed] [Google Scholar]
- 11.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. Jama. 2014;311:1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 12.Allen JD, Bluethmann SM, Sheets M, et al. Women's responses to changes in U.S. Preventive Task Force's mammography screening guidelines: results of focus groups with ethnically diverse women. BMC public health. 2013;13:1169–2458. 1113–1169. doi: 10.1186/1471-2458-13-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang AT, Fan J, Van Houten HK, et al. Impact of the 2009 US Preventive Services Task Force guidelines on screening mammography rates on women in their 40s. PloS one. 2014;9:e91399. doi: 10.1371/journal.pone.0091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer. 2013;119:2518–2523. doi: 10.1002/cncr.28105. [DOI] [PubMed] [Google Scholar]
- 15.Calvocoressi L, Sun A, Kasl SV, Claus EB, Jones BA. Mammography screening of women in their 40s: impact of changes in screening guidelines. Cancer. 2008;112:473–480. doi: 10.1002/cncr.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JS, Sprague BL, Klabunde CN, et al. Provider Attitudes and Screening Practices Following Changes in Breast and Cervical Cancer Screening Guidelines. J Gen Intern Med. 2016;31:52–59. doi: 10.1007/s11606-015-3449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum L. The Paternalism Preference--Choosing Unshared Decision Making. N Engl J Med. 2015;373:589–592. doi: 10.1056/NEJMp1508418. [DOI] [PubMed] [Google Scholar]
- 20.Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. AJR Am J Roentgenol. 2014;203:1379–1381. doi: 10.2214/AJR.14.12646. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite D, Walter LC, Izano M, Kerlikowske K. Benefits and Harms of Screening Mammography by Comorbidity and Age: A Qualitative Synthesis of Observational Studies and Decision Analyses. J Gen Intern Med. 2016 doi: 10.1007/s11606-015-3580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 23.Lin MY, Kressin NR. Race/ethnicity and Americans' experiences with treatment decision making. Patient Educ Couns. 2015 doi: 10.1016/j.pec.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Warner ET, Tamimi RM, Hughes ME, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol. 2015;33:2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 26.Batina NG, Trentham-Dietz A, Gangnon RE, et al. Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat. 2013;138:519–528. doi: 10.1007/s10549-013-2435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox J, Zikmund-Fisher BJ, Gross CP. Older patient experiences in the mammography decision-making process. Archives of Internal Medicine. 2012;172:62–64. doi: 10.1001/archinternmed.2011.601. discussion 64. [DOI] [PubMed] [Google Scholar]
- 29.Gunn CM, Soley-Bori M, Battaglia TA, Cabral H, Kazis L. Shared Decision Making and the Use of Screening Mammography in Women Younger Than 50 Years of Age. J Health Commun. 2015;20:1060–1066. doi: 10.1080/10810730.2015.1018628. [DOI] [PubMed] [Google Scholar]
- 30.Gillick MR. Re-engineering shared decision-making. J Med Ethics. 2015;41:785–788. doi: 10.1136/medethics-2014-102618. [DOI] [PubMed] [Google Scholar]
- 31.Gurmankin AD, Baron J, Hershey JC, Ubel PA. The role of physicians' recommendations in medical treatment decisions. Med Decis Making. 2002;22:262–271. doi: 10.1177/0272989X0202200314. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CL, Couper MP, Levin CA, Pignone MP, Zikmund-Fisher BJ. Plans to stop cancer screening tests among adults who recently considered screening. J Gen Intern Med. 2010;25:859–864. doi: 10.1007/s11606-010-1346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.