Abstract
Loss-of-function of KIND1, a cytoskeletal protein involved in β1-integrin function, causes Kindler Syndrome (KS), a genetic disease characterized by skin fragility, photosensitivity and increased risk of squamous cell carcinoma (SCC). Dysregulation of β1-integrin underlies KS skin fragility. However, the mechanisms underlying SCC susceptibility are unclear. Here, we demonstrate that gene silencing of KIND1 decreased keratinocyte proliferation and increased apoptosis in vitro and in skin grafts regenerated on mice, which was correlated with reduced cyclinB1. In addition, KIND1-loss sensitized keratinocytes to cytokine and UV-induced NF-κB and JNK activation and upregulation of CXCL10 and TNFα. Moreover, KIND1-loss impaired DNA-repair, as indicated by the increased detection of γH2AX and cyclobutane pyrimidine dimers (CPD) 24 hours post UVB-radiation. Genetic or pharmacological JNK-inhibition and NF-κB-inhibition markedly reduced CPD-positive cells. Further, we show that KIND1 was regulated by JunB at the transcriptional level and, like JunB, it was downregulated in human SCC cells. Together, these results indicate that KIND1 is important not only for keratinocyte proliferation but also for the suppression of UV-induced inflammation and DNA-damage. These latter findings support a tumor suppressor function for KIND1, and identified JNK and NF-κB as potential therapeutic targets for prevention of SCC in KS patients.
Keywords: KIND1, inflammation, CXCL10, cell proliferation, DNA-damage
INTRODUCTION
KIND1 (also known as FERMT1) is a cytoskeletal protein important for integrin activation and focal adhesion (Ashton, 2004, Herz et al., 2006, Meves et al., 2009). It is predominantly expressed in epithelium of the skin and intestine. KIND1 loss-of-function mutation is linked to Kindler syndrome (KS, OMIM173650), an autosomal recessive disorder characterized by skin fragility with progressive atrophy, poikiloderma, photosensitivity and chronic mucosal inflammation (Has et al., 2011). KS-driver mutations (>70) consist of deletions, insertions, nonsense, splice-site and missense mutations that result in expression of a nonfunctional mutant KIND1 or at a reduced level (Arita et al., 2007, Fuchs-Telem et al., 2014, Has, Castiglia, 2011, Has et al., 2015, Lai-Cheong et al., 2009, Sadler et al., 2006, Wada et al., 2012). In addition to skin fragility, KS keratinocytes display premature senescence (Piccinni et al., 2013). These data indicate that KIND1 plays a key role in epithelial cell growth and tissue integrity.
Paradoxically, despite the apparent defects of keratinocyte growth, KS patients show an increased risk of squamous cell carcinoma (SCC) of the skin and the gastrointestinal tract (Emanuel et al., 2006, Lotem et al., 2001, Mizutani et al., 2012). Similarly, mice with epidermis targeted deletion of Kind1 are sensitive to DMBA/TPA induction of skin cancer, which is linked to elevated Wnt and decreased TGFβ signaling and consequently unrestrained hair follicle stem cell proliferation (Rognoni et al., 2014). Nevertheless, tumors chemically induced in Kind1 null mice are primarily basal cell carcinomas (BCC), while those of KS patients are mostly SCCs (Emanuel, Rudikoff, 2006, Lotem, Raben, 2001, Mizutani, Masuda, 2012), suggesting that SCCs linked to KIND1 deficiency in humans involve different molecular mechanisms.
The primary risk factor for human SCC is UV radiation, especially UVB (de Gruijl, 1999, Pleasance et al., 2010). UV predominantly induces cyclobutane pyrimidine dimers (CPDs) and pyrimidine [6–4] pyrimidone photoproducts ([6–4]PPs) (Freeman et al., 1989). The majority of these DNA lesions are repaired within 24-hour after induction (Courdavault et al., 2005). Inefficient DNA-repair results in gene mutations and consequently cell transformation or apoptosis. CPDs are repaired less efficiently than [6–4]PPs, and are responsible for a majority of UV-induced gene mutations (You et al., 2001). In response to UV radiation, the histone variant H2AX undergoes phosphorylation on serine 139 (pH2AX, also referred as γH2AX), which initiates the recruitment of DNA-repair proteins. γH2AX is frequently used as a marker for DNA-damage (Podhorecka et al., 2010). Additionally, UV induces inflammatory responses that unequivocally involve keratinocytes and immune cells. Inflammation is on the one hand important for the elimination of the terminally damaged cells, but on the other hand enhances DNA-damage and tumor development (Kidane et al., 2014, Mukhtar and Elmets, 1996).
In this study, we demonstrate that KIND1 targeted gene silencing impaired human keratinocyte cell growth in vitro and in regenerated human skin grafts. In addition, we found that KIND1-loss sensitized UV-induced DNA-damage, which was associated with increased activation NF-κB and JNK inflammatory signaling pathways and upregulation of TNFα and CXCL10. JNK-inhibition pharmacologically and genetically restored DNA-repair in KIND1-deficient cells. We further show that KIND1 is a direct target of JunB transcription factor, and is downregulated in human SCC. Together, our findings indicate that KIND1 regulates keratinocyte proliferation and resistance to UV-induced inflammation and DNA-damage.
RESULTS
KIND1-loss impairs cell proliferation and survival
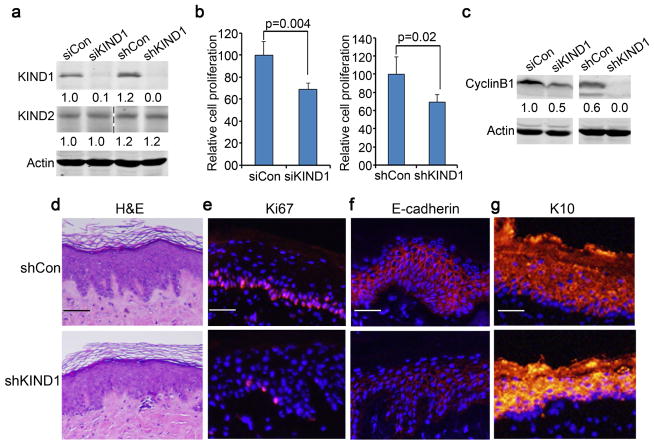
Keratinocytes derived from KS patients show defects in adhesion, migration and cell proliferation compared to normal keratinocytes (Herz, Aumailley, 2006). To determine whether KIND1 deficiency induces these defects, we performed KIND1 targeted gene silencing with siRNA oligonucleotides (siKIND1) and shRNA (shKIND1) in normal human keratinocytes. Efficiency and specificity of KIND1 targeted gene silencing was confirmed by immunoblotting (Figure 1a). siKIND1 and shKIND1 significantly reduced keratinocyte proliferation as compared to respective non-silencing controls (siCon and shCon) (Figure 1b, Supplementary Figure S1a). Further cell cycle analysis revealed that KIND1 gene silencing induced a significant increase of cells in the sub-G0 and G0/G1-phases and a reduction in the M-phase (Supplementary Figure S1b–c). Consistently, CyclinB1, a key S-to-M promoter, was markedly reduced in shKIND1 cells and increased in cells transduced to overexpress KIND1 (Figure 1c, Supplementary Figure S1d). These results indicate that KIND1 plays a key role in keratinocyte cell cycle progression.
Figure 1. KIND1 gene silencing reduces epidermal cell proliferation both in vitro and in vivo.
(a) Immunoblotting. Protein lysates were isolated from keratinocytes 48 hours after transfection with siRNA oligonucleotides or transduction with shRNA lentivirus. Note, KIND1, but not KIND2, was reduced by siKIND1 or shKIND1. (b) Cell proliferation. Cells in triplicate wells were counted 3 days after gene silencing. Graphs represent averages of relative cell numbers + SD. P-values <0.05 were obtained via student T-test. (c) Immunoblotting for CyclinB1 and Actin with protein lysates isolated in (a). (d) H&E staining of 6-week old skin grafts regenerated on immunodeficient mice with keratinocytes transduced as described in (a). (e–g) Immunostaining of skin grafts for Ki67, E-cadherin and K10 [orange], nuclei [blue, Hoechst]. Scale bars: 50 μm.
To confirm the effects of KIND1-loss on keratinocyte growth in vivo, we generated skin grafts in immunodeficient mice using human keratinocytes transduced to express shCon or shKIND1. As expected, KIND1, but not KIND2, was reduced in shKIND1 epidermis (Supplementary Figure S2a–b). Further histological analysis of the 6-week old skin grafts revealed that the epidermal thickness was similar between the two groups (Figure 1d), however, shKIND1 epidermis was less proliferative as indicated by the reduced number of Ki-67-positive cells (Figure 1e, Supplementary Figure S2c). In addition, shKIND1 epidermis expressed a reduced level of the epithelial cell marker E-cadherin and an increased level of the differentiation marker cytokeratin 10 (K10) (Figure 1f, g). To verify the effects on E-cadherin, we performed RT-PCR, and found that E-cadherin mRNA level was significantly decreased by KIND1 gene silencing, and increased by KIND1 overexpression (Supplementary Figure S3). These results indicate that KIND1 is essential for epidermal cell proliferation and homeostasis.
KIND1-loss sensitizes keratinocytes to UVB-induced inflammatory response
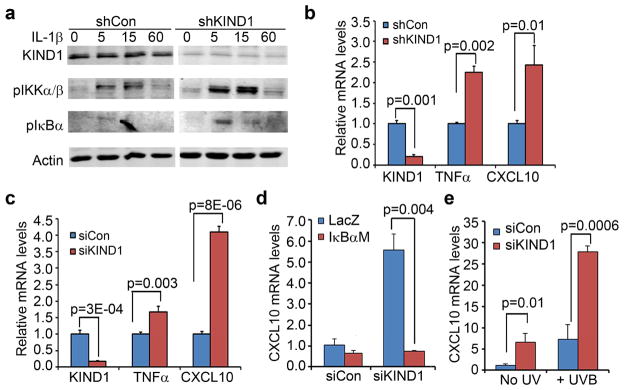
KS patient skins are hypersensitive to inflammation (Jobard et al., 2003). We asked whether KIND1 insufficiency is inherently associated with a proinflammatory response. To address this question, we first tested whether KIND1 gene silencing affected NF-κB signaling pathway, a known player in inflammation (Karin and Greten, 2005). By immunoblotting, we found that both pIKKα/β and pIκBα were expressed at elevated levels in shKIND1 cells treated with or without IL-1β (Figure 2a). To verify the increase of NF-κB activity, we next examined the expression levels of TNFα and CXCL10, which are induced by NF-κB in keratinocytes (Zhang et al., 2015). By real-time RT-PCR, we found that both siKIND1 and shKIND1 significantly increased mRNA levels of TNFα and CXCL10 (Figure 2b–c). NF-κB inhibition via overexpression of a non-degradable IκBα mutant (IκBαM) prevented such an increase (Figure 2d). These results indicate that KIND1 deficiency leads to increased NF-κB activation and expression of NF-κB target genes.
Figure 2. KIND1 gene silencing sensitizes keratinocytes to inflammatory responses.
(a) Immunoblotting for pIKKα/β, pIκBα, KIND1 and Actin. (b–c) Quantitative RT-PCR of KIND1, TNFα and CXCL10. Total RNA was isolated from primary human keratinocytes 48 hours after (b) transfection with siCon or siKIND1 oligonucleotides or (c) gene transduction with shCon or shKIND1 lentiviruses. (d–e) Quantitative RT-PCR of CXCL10. Total RNA was isolated from human keratinocytes transfected with siCon or siKIND1 oligonucleotides followed by (d) retroviral gene transduction for expression of LacZ or IκBαM or (e) treatment with 6.8 mJ/cm2 UVB. Graphs represent averages of relative mRNA levels + SD with 18S ribosomal RNA used as an internal control. P-values of <0.05 were obtained with student T-test.
UV radiation induces an array of inflammatory molecules (Sesto et al., 2002). We asked whether KIND1-loss affects UV-induced inflammation. To do this, we treated keratinocytes with UVB at 24-hour after transfection with siRNA oligonucleotides, and then isolated total RNA 24 hours later. Subsequent RT-PCR showed that UVB and shKIND1 each induced a 7-fold increase of CXCL10, while their combination induced a 27-fold increase (Figure 2e), indicating that KIND1-deficiency sensitizes keratinocytes to UV-induction of inflammatory molecules.
KIND1-loss impairs DNA-repair following UVB-induction
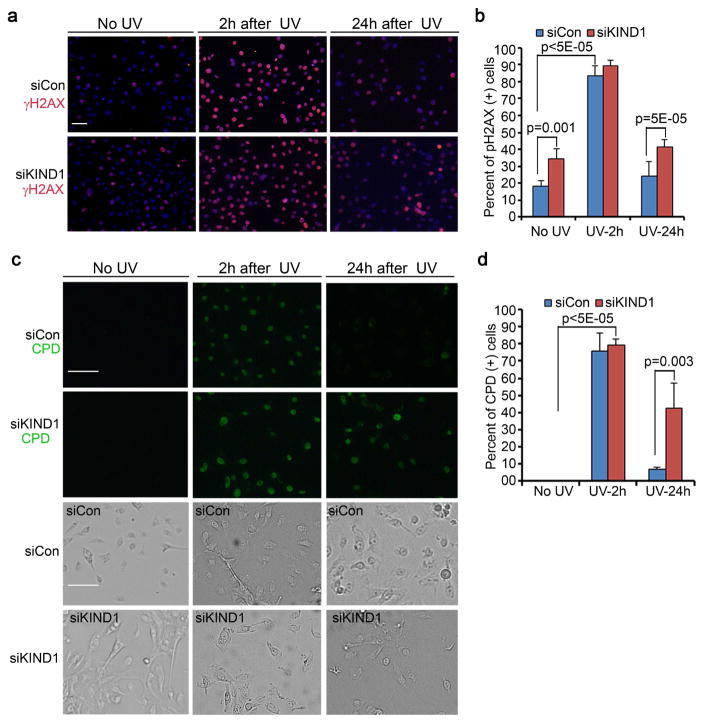
UVB induces DNA-damages, and is a predominant risk factor for non-melanoma skin cancer (de Gruijl, 1999, Pleasance, Cheetham, 2010). We predicted that KIND1 might be involved in the regulation of DNA-repair. To test this idea, we treated keratinocytes with UVB, and then performed immunostaining at the 0, 2 and 24-hour time-points for γH2AX, a marker for DNA-damage response (Podhorecka, Skladanowski, 2010). As anticipated, γH2AX displayed a robust increase in both control and shKIND1 cells 2 hours after UVB-exposure (Figure 3a–b, Supplementary Figure S4). By the 24-hour time-point, γH2AX returned to near basal levels in control cells but remained high in shKIND1 cells (Figure 3a–b), implicating that shKIND1 cells has a defect in DNA-repair. To verify this possibility, we performed immunostaining for CPD, a common UVB-induced DNA-lesion (Freeman, Hacham, 1989). We found that CPD-positive cells were evidently increased in both cell types 2 hours after UVB-exposure (Figure 3c–d). By the 24-hour time-point, CPD-lesions had markedly reduced in control cells, but remained readily detectable in shKIND1 cells (Figure 3c–d). These data indicate that KIND1-loss impairs DNA-repair.
Figure 3. KIND1-loss sensitizes keratinocytes to UVB-induced DNA-damage.
(a) Immunofluorescent staining of γH2AX. Human keratinocytes transfected with siCon or siKIND1 were treated with UVB for 5 seconds (6.8mJ/cm2), and then immunostained for γH2AX followed by detection with an Alexa-555-conjugated secondary antibody. γH2AX [orange], nuclei [blue, Hoechst]. (b) Quantification of γH2AX-positive cells. Graph represents average percentages of γH2AX-positive cells + SD. (c) Immunofluorescent staining with a FITC-conjugated antibody against CPD [green]. Bright field images were shown below each corresponding CPD image. (d) Quantification of CPD-positive cells. Graph represents average percentages of CPD-positive cells + SD. P-values of <0.05 were obtained with student T-test. Scale bars: 50 μm.
KIND1-loss augments UVB-induced JNK activation, and impairs DNA-repair
Cell death and transformation are two major consequences of inefficient DNA-repair (You, Lee, 2001), and JNK is responsible for UV-induced cell death (Katagiri et al., 2006, Li et al., 2001). We asked whether JNK contributes to the DNA-repair defects caused by KIND1-loss. To address this question, we first examined JNK phosphorylation (pJNK) by immunoblotting. We found that pJNK was markedly increased 2 hours after UVB-treatment in both shCon and shKIND1 cells. By 24 hours after UVB-irradiation, pJNK had noticeably decreased in control cells, but remained elevated in shKIND1 cells (Figure 4a). These data indicate that JNK activation is augmented and prolonged by KIND1-loss. Next, we examined effects of genetic and pharmacological JNK-inhibition on DNA-repair. Genetic inhibition was achieved via retrovirus-mediated overexpression of dominant negative mutants of JNK1 (DN-JNK1, also referred to JNK1(APF)) and c-Jun (DNc-Jun, also referred to TAM67) as described previously (Ke et al., 2010, Zhang et al., 2005). As expected, expression of these proteins resulted in decreased levels of phosphorylated c-Jun (pc-Jun), as shown by immunoblotting (Figure 4b). Immunofluorescent CPD staining revealed that expression of DN-JNK1 or DNc-Jun significantly reduced CPD-positive cells 24 hours after UVB-treatment (Figure 4c–d). Pharmacological JNK-inhibition was achieved via treatment of siKIND1 cells with SP600125 (Bennett et al., 2001), and verified by immunoblotting for pc-Jun (Supplementary Figure S5a). SP600125 markedly reduced the number of CPD-positive cells 24 hours after UVB-treatment (Supplementary Figure S5b–c). Interestingly, NF-κB inhibition genetically with IκBαM or pharmacologically with PDTC also decreased the percentage of CPD-positive cells though at a reduced efficiency compared to JNK-inhibition (Figure 4b–d and Supplementary Figure S5a–c). These data indicate that JNK along with NF-κB signaling is responsible for the ineffective DNA-repair in cells with KIND1-loss.
Figure 4. JNK/c-Jun and NF-κB inhibitions reduce UV-induced DNA-damage in cells with KIND1 gene silencing.
(a) Immunoblotting for pJNK, JNK and Actin with protein lysates isolated from human keratinocytes 0, 2 and 24 hours after UVB-treatment. (b) Verification of JNK/c-Jun inhibition by immunoblotting. Protein lysates were collected 24 hours after UVB-treatment (6.8mJ/cm2) of keratinocytes that had been transduced for expression of LacZ control, DN-JNK1, IκBαM or DNc-Jun, and transfected with siKIND1. (c) Immunostaining for CPD [green]. Cells were fixed 24 hours after UVB-treatment. Bright field images were shown below each corresponding CPD image. Scale bars: 50 μm. (d) CPD quantification. Graph represents average percentages of CPD-positive cells quantified from 5–6 images of each condition + SD.
KIND1 is a JunB target, and is reduced in squamous cell carcinoma
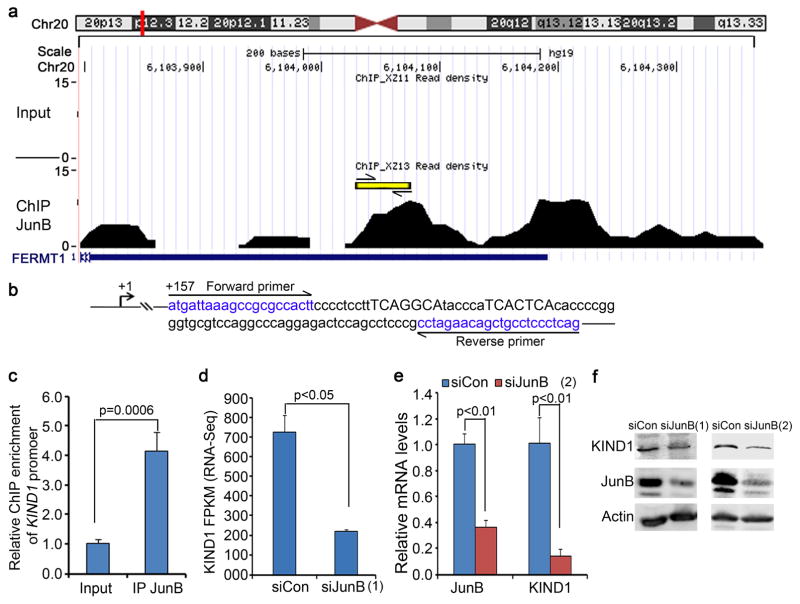
Our recent studies have shown that JunB, an AP1 family transcription factor, suppresses keratinocyte inflammation and tumorigenesis (Jin et al., 2011, Zhang, Jin, 2015). Analysis of JunB ChIP-seq data (access# GSE63080) using UCSC genome browser (hg19) revealed two sequence peaks inside KIND1 gene located on chromosome 20 (Figure 5a). Embedded within the peak at around 200 bp from KIND1 transcription start site were two putative AP-1 response elements (TGAGGCA and TCACTCA) (Figure 5b). To verify that JunB directly interacts with KIND1, we performed real-time ChIP-PCR with primers flanking those putative AP-1 response elements, and observed that JunB ChIP achieved an over 4-fold enrichment of KIND1 sequence (Figure 5c). Further analysis of the RNA-seq data (NCBI access# GSE63081) revealed that KIND1 mRNA was downregulated by over 3-fold in response to JunB gene silencing (Figure 5d). This downregulation was verified by real-time RT-PCR and immunoblotting (Figure 5e–f). These data indicate that KIND1 expression is upregulated by JunB at the transcriptional level.
Figure 5. KIND1 expression is regulated by JunB transcription factor.
(a) KIND1 ChIP-seq peaks enriched by JunB ChIP (NCBI Accession GSE63080). Locations of putative AP-1 response elements were marked by the yellow line. (b) Putative AP-1 response elements (capital letters) and ChIP-PCR primers (blue letters). (c) ChIP-PCR. Graph represents the average of relative fold enrichment of KIND1 over the input DNA by JunB ChIP + SD. (d) KIND1 FPKM in response to siJunB(1) (S7661) (NCBI accession GSE63081). (e) quantitative RT-PCR with RNA isolated from keratinocytes 48 hours after transfection with siJunB(2) (S7662). Graphs represent averages of relative KIND1 mRNA levels + SD. 18S RNA was used for internal control. (f) Immunoblotting. Protein lysates were isolated from keratinocytes 48 hours after transfection with siCon or siJunB (S7661 and S7662). P-values of <0.05 were obtained via student T-test.
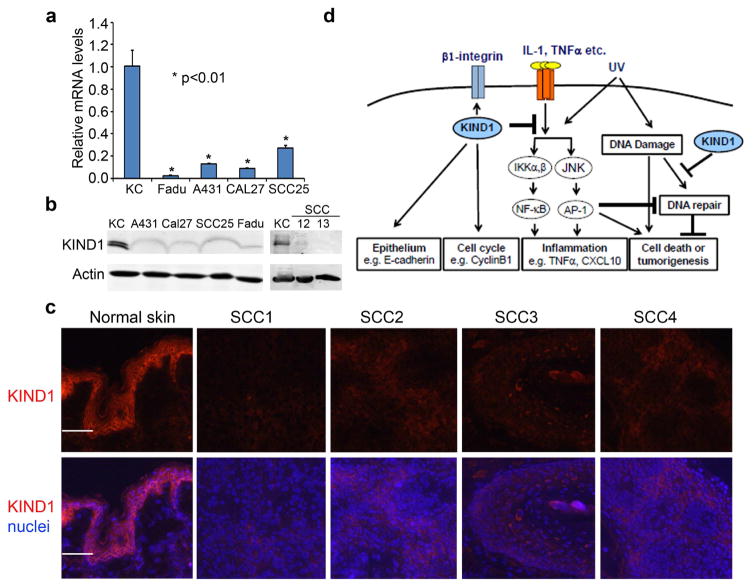
JunB is expressed at a reduced level in human SCC (Jin, Ke, 2011). Consistently, KIND1 mRNA was significantly reduced in human SCC cell lines (A431, Fadu, CAL27 and SCC25), compared to normal keratinocytes, as shown by quantitative RT-PCR (Figure 6a). Immunoblotting verified that KIND1 was downregulated in both epidermal (A431, SCC12 and SCC13) and head and neck (Fadu, CAL27 and SCC25) SCC cell lines (Figure 6b). Further immunostaining showed that KIND1 was markedly reduced in human skin SCC tissue samples as compared to normal skin (Figure 6c). These data indicate that KIND1 loss-of-function has a general relevance to human SCC.
Figure 6. KIND1 is reduced in SCC cells and Tissues.
(a) qRT-PCR of KIND1 with total RNA isolated from human keratinocytes and SCC cell lines. GAPDH was used as an internal control. P-values of <0.05 were obtained via student T-test. (b) Immunoblotting for KIND1 with protein lysates isolated from human keratinocytes and SCC cell lines. Actin was used as a loading control. (c) Immunostaining of frozen tissue sections of human skin and SCC samples for KIND1 followed by detection with an Alex555 dye-conjugated secondary antibody. KIND1 [orange], nuclei [Hoechst, blue]. Scale bars: 100 μm. (d) Working model depicting multi-functions of KIND1.
Taken together, our findings along with earlier reports support a working model that depicts multi-functions of KIND1 (Figure 6d), including 1) promotion of keratinocyte proliferation via CyclinB1 and β1-integrin (Ashton, 2004, Herz, Aumailley, 2006, Meves, Stremmel, 2009), 2) maintenance of epithelial cell characteristics through upregulation of E-cadherin, and 3) control inflammation and DNA-damage via inhibition of JNK and NF-κB signaling pathways. Specifically, the effects on DNA-repair and E-cadherin expression, along with the evidence of KIND1 downregulation in human SCC, underscores a tumor suppressor function for KIND1.
DISCUSSION
It has become increasingly clear that cytoskeletal proteins are not just inert rivets and scaffolds of the cell (Simpson et al., 2011). They actively participate in signal transduction to regulate tissue morphogenesis and function. In particular, the Kindlin family proteins, including KIND1, 2, and 3, are evolutionally evolved from Talin, another FERMT-domain containing cytoskeletal protein critical (Meller et al., 2015). In mammals, KIND1 is specific to epithelial cells, while Talin and KIND2 are ubiquitously expressed, and KIND3 is expressed predominantly in hematopoietic and endothelial cells. It is intriguing that skin grafts with KIND1-loss show downregulation of E-cadherin, a change commonly detected during epithelial-to-mesenchymal transition (Lamouille et al., 2014). Also like E-cadherin, KIND1 is critical for epithelial tissue integrity, and is reduced in human SCC. In agreement with a positive role of KIND1-loss in skin carcinogenesis, KS patients and Kind1−/− mice display an increased risk of SCC and BCC, respectively (Arita, Wessagowit, 2007, Ashton, 2004, Emanuel, Rudikoff, 2006, Has et al., 2006, Lai-Cheong, Tanaka, 2009, Lotem, Raben, 2001, Rognoni, Widmaier, 2014). In addition, KS patients tend to suffer from chronic mucosal inflammation and express elevated levels of inflammatory molecules in the skin (Has, Castiglia, 2011, Rognoni, Widmaier, 2014). By demonstrating that KIND1 gene silencing sensitizes cells to inflammation and DNA-damage, we identify molecular mechanisms underlying effects of cytoskeletal proteins on carcinogenesis. Further studies are required to understand whether KIND1 regulations of E-cadherin, CyclinB1, NF-κB and JNK signaling are interrelated to each other and to β1-integrin; and whether KIND1-loss alters the threshold of neoplastic transformation of epithelial cells by other genetic changes such as oncogenic Ras.
A role of JNK/c-Jun signaling pathway in skin cancer has been established in several genetic studies (Barthelman et al., 1998, Hanke et al., 2008, Jin, Ke, 2011, Ke, Harris, 2010). JNK is activated by UV, and relays UVB-induced CPD signals to transcriptional responses that interfere with the DNA-repair program (Boros et al., 2015, Hibi et al., 1993). Along with JNK, NF-κB is activated by UV radiation and other genotoxic agents (McCool and Miyamoto, 2012), and promotes skin carcinogenesis through inflammatory cytokines including TNFα (Chen and Goeddel, 2002, Picco and Pages, 2013, Vincek et al., 1993, Walsh, 1995). Conversely, NF-κB inhibition with anti-inflammatory agents, such as 4-hexyl-1,3-phenylenediol, blackberry extracts and curcumin, reduces UV-induced DNA-damage in skin equivalents (Aggarwal et al., 2013, Divya et al., 2015, Kaur et al., 2013). These data are in agreement with our findings demonstrating that genetic and pharmacological inhibition of JNK/c-Jun and NF-κB reduces DNA-damage in cells with reduced KIND1 expression. In normal cells, JNK and NF-κB are transiently induced by stress signals, and are quickly balanced by pro-repair signals. Such a balance is disrupted by the prolonged inflammatory responses in cells with KIND1 mutation. On another note, mitochondria stress is elevated in keratinocytes derived from KS patients (Zapatero-Solana et al., 2014). It will be interesting to determine whether and how NF-κB and JNK affect the DNA-repair machinery, and contribute to the mitochondria stress and vice versa.
In contrast to c-Jun, JunB suppresses epidermal tumorigenesis, and JunB loss-of-function is associated with increased inflammatory responses and epidermal tumorigenesis (Jin, Ke, 2011, Zhang, Jin, 2015). Presumably, KIND1 represents one of the key JunB downstream effectors that control keratinocyte inflammation and growth. It is intriguing to note that, contrary to the aforementioned tumor suppressive role, KIND1 is upregulated in multiple cancers including lung, colon, bladder, breast and pancreatic cancers (Mahawithitwong et al., 2013, Sin et al., 2011, Weinstein et al., 2003). KIND1 deletion decreases breast and pancreatic cancer cell motility and invasion (Mahawithitwong, Ohuchida, 2013, Sin, Bonin, 2011). In line with these data, we have recently shown that KIND1 regulates cutaneous and head and neck cancer cell motility (Zhang et al., 2016). Thus, it appears that KIND1 exhibits functional dichotomy. In one hand, it inhibits tumorigenesis through suppression of inflammation and promotion of DNA-repair and epithelial cell characteristics. In another hand, it promotes cell motility and tissue invasion through β1-integrin activation. Taken together, the role of KIND1 in cancer is dependent on tissue environment and cancer stage.
Of further interest, mice with epidermal deletion of Kind1 show elevated Wnt signaling and decreased TGFβ signaling (Rognoni, Widmaier, 2014). Interestingly, expressions of β-Catenin and pSmad2/3 were not apparently different between shCon and shKIND1 epidermis (Supplementary Fig. S6). Additionally, while KS patients generally have normal life span, mice with germline Kind1 deletion exhibit perinatal lethality accompanied with skin atrophy and intestinal epithelial erosion and inflammation (Ussar et al., 2008). It is unclear whether these phenotypic differences are attributed to species-specific effects or other compensatory mechanisms that may be launched in humans.
MATERIALS AND METHODS
Cell culture and gene transfer
Primary human keratinocytes were isolated from surgically discarded foreskin samples obtained from Duke Children’s Hospital in accordance to an institutionally approved IRB protocol. Patient consent for experiments was not required because French laws consider human tissue left over from surgery as discarded material. Cells were grown and passaged in keratinocyte serum free (KSF) media (Invitrogen, Grand Island, NY) at 37 °C with 5% CO2, and used at passage 2 or 3. Human SCC cell lines (A431, CAL27 and Fadu) cells were obtained from ATCC and cultured in 10%FBS/DMEM. SCC12 and SCC13 cells were kindly provided by M. Ramsey and JG. Rheinwald (Harvard University), and cultured as described (Hu et al., 1991). Stealth siRNA oligonucleotides targeting KIND1, JunB and non-silencing control were from Invitrogen and used at 100nM concentrations. The KIND1 and IκBαM expression constructs have been described previously (Margadant et al., 2013) (Zhang, Tao, 2005). The lentiviral shRNA constructs were obtained from Duke siRNA Core. siRNA transfection was performed with GeneMute following the manufacture’s protocols (SignaGen Laboratories, Gaithersburg, MD). Lentiviral and retroviral gene transduction was performed as described (Zhang, Jin, 2015). UVB-treatment was performed a hand-held 312 nm narrow band UVB lamp (Analtech, Inc. Newark, DE) (6.85 J/cm2). Cells were then immediately fed with fresh KSF media with DMSO solvent, 20 μM PDTC or 10 μM SP600125 (LC Laboratories Woburn, MA), and collected for RNA isolation or fixed with methanol for immunostaining. All in vitro experiments were repeated for at least 3 times. KIND1 siRNA and shRNA and JunB siRNA sequence information were listed in (Table S1).
ChIP-PCR and RT-PCR
Chromatin immunoprecipitation (ChIP) was performed with DNA extracted from primary human keratinocytes and an antibody against JunB as described (Zhang, Jin, 2015). ChIP-PCR was performed with primers flanking the putative AP-1 response elements. For RT-PCR, total RNA was isolated from keratinocytes at 48-hour after gene transfection or transduction using RNAeasy column (Qiagen, Germantown, MD). cDNA library was prepared via oligo-dT-directed reverse transcription. SYBR green-based real-time RT-PCR was performed in Bio-Rad iCycler with 18S RNA or GAPDH for internal control. PCR primers were listed in (Table S2).
Skin regeneration and histology
Skin regeneration was performed as described in our previous studies in accordance with protocols approved by the Duke Animal Care and Use Committee (Jin, Ke, 2011). Primary human keratinocytes transduced with shCon or shKIND1lentivirus were seeded at 5x105 cells per 0.8–1.2 cm2 devitalized split thickness human dermis. The graft recipient mice (NSG.SCID) were purchased from Duke Cancer Center Isolation Facility (n=3 per group). Six-week old skin grafts were collected, and were fixed with 10% formalin for H&E staining or embedded in OCT for cryostat tissue sectioning.
Protein analysis
For western blotting, 20 μg protein lysates of human keratinocytes collected 48–72 hours post transfection were separated on SDS-PAGE, and immunoblotted with primary antibodies (Table S3) followed by detection with IR-dye-conjugated secondary antibodies (Invitrogen). The blots were scanned on the Odyssey imaging system (Li-COR, Lincoln, NE). Densitometry was carried out in Photoshop and normalized to actin control and then to respective control samples. For immunostaining, 5 μm frozen tissue sections were fixed with cold methanol, incubated with primary antibodies, detected with Alexa 555-conjugated secondary antibodies (Invitrogen), and then counterstained with Hoechst 33342. CPD staining was performed following manufacture’s protocols (Cell Biolabs, Inc. San Diego, CA). Images were processed using Olympus BX41 microscopic imaging system. Human SCC samples were obtained from Duke Dermatology Mohs surgery unit in accordance to an exempt IRB protocol.
Supplementary Material
Acknowledgments
This work was supported by the grants from NIH/NIAMS (AR057746) to JZ and (AR066527) to RH, and Tianjin Medical University General Hospital Fund to SL. We thank A. Sonnenberg of Netherlands Cancer Institute for the KIND1 expression construct, S. Kim (Duke University) for the shKIND1 construct, L. Brancazio, A. Murtha, J. Cook and S. Ray (Duke University) for providing skin samples, and JY. Jin (Duke University) for technical assistance, as well as M. Ramsey and JG. Rheinwald (Harvard University) for providing SCC12 and SCC13 cell lines.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–92. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita K, Wessagowit V, Inamadar AC, Palit A, Fassihi H, Lai-Cheong JE, et al. Unusual molecular findings in Kindler syndrome. Br J Dermatol. 2007;157:1252–6. doi: 10.1111/j.1365-2133.2007.08159.x. [DOI] [PubMed] [Google Scholar]
- Ashton GH. Kindler syndrome. Clin Exp Dermatol. 2004;29:116–21. doi: 10.1111/j.1365-2230.2004.01465.x. [DOI] [PubMed] [Google Scholar]
- Barthelman M, Chen W, Gensler HL, Huang C, Dong Z, Bowden GT. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998;58:711–6. [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros G, Miko E, Muramatsu H, Weissman D, Emri E, van der Horst GT, et al. Identification of Cyclobutane Pyrimidine Dimer-Responsive Genes Using UVB-Irradiated Human Keratinocytes Transfected with In Vitro-Synthesized Photolyase mRNA. PLoS One. 2015;10:e0131141. doi: 10.1371/journal.pone.0131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Courdavault S, Baudouin C, Charveron M, Canguilhem B, Favier A, Cadet J, et al. Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair (Amst) 2005;4:836–44. doi: 10.1016/j.dnarep.2005.05.001. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35:2003–9. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- Divya SP, Wang X, Pratheeshkumar P, Son YO, Roy RV, Kim D, et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-kappaB signaling pathways in SKH-1 mice skin. Toxicol Appl Pharmacol. 2015;284:92–9. doi: 10.1016/j.taap.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel PO, Rudikoff D, Phelps RG. Aggressive squamous cell carcinoma in Kindler syndrome. Skinmed. 2006;5:305–7. doi: 10.1111/j.1540-9740.2006.05369.x. [DOI] [PubMed] [Google Scholar]
- Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci U S A. 1989;86:5605–9. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Telem D, Nousbeck J, Singer A, McGrath JA, Sarig O, Sprecher E. New intragenic and promoter region deletion mutations in FERMT1 underscore genetic homogeneity in Kindler syndrome. Clin Exp Dermatol. 2014;39:361–7. doi: 10.1111/ced.12222. [DOI] [PubMed] [Google Scholar]
- Hanke NT, Finch JS, Bowden GT. Loss of catalase increases malignant mouse keratinocyte cell growth through activation of the stress activated JNK pathway. Mol Carcinog. 2008;47:349–60. doi: 10.1002/mc.20391. [DOI] [PubMed] [Google Scholar]
- Has C, Castiglia D, del Rio M, Diez MG, Piccinni E, Kiritsi D, et al. Kindler syndrome: extension of FERMT1 mutational spectrum and natural history. Hum Mutat. 2011;32:1204–12. doi: 10.1002/humu.21576. [DOI] [PubMed] [Google Scholar]
- Has C, Chmel N, Levati L, Neri I, Sonnenwald T, Pigors M, et al. FERMT1 promoter mutations in patients with Kindler syndrome. Clin Genet. 2015;88(3):248–54. doi: 10.1111/cge.12490. [DOI] [PubMed] [Google Scholar]
- Has C, Wessagowit V, Pascucci M, Baer C, Didona B, Wilhelm C, et al. Molecular basis of Kindler syndrome in Italy: novel and recurrent Alu/Alu recombination, splice site, nonsense, and frameshift mutations in the KIND1 gene. J Invest Dermatol. 2006;126:1776–83. doi: 10.1038/sj.jid.5700339. [DOI] [PubMed] [Google Scholar]
- Herz C, Aumailley M, Schulte C, Schlotzer-Schrehardt U, Bruckner-Tuderman L, Has C. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem. 2006;281:36082–90. doi: 10.1074/jbc.M606259200. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–81. [PubMed] [Google Scholar]
- Jin JY, Ke H, Hall RP, Zhang JY. c-Jun promotes whereas JunB inhibits epidermal neoplasia. J Invest Dermatol. 2011;131:1149–58. doi: 10.1038/jid.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard F, Bouadjar B, Caux F, Hadj-Rabia S, Has C, Matsuda F, et al. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003;12:925–35. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Katagiri C, Nakanishi J, Kadoya K, Hibino T. Serpin squamous cell carcinoma antigen inhibits UV-induced apoptosis via suppression of c-JUN NH2-terminal kinase. J Cell Biol. 2006;172:983–90. doi: 10.1083/jcb.200508064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Oddos T, Tucker-Samaras S, Southall MD. Regulation of DNA Repair Process by the Pro-Inflammatory NF-κB Pathway. INTCH. 2013:215–27. [Google Scholar]
- Ke H, Harris R, Coloff JL, Jin JY, Leshin B, Miliani de Marval P, et al. The c-Jun NH2-terminal kinase 2 plays a dominant role in human epidermal neoplasia. Cancer Res. 2010;70:3080–8. doi: 10.1158/0008-5472.CAN-09-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49:116–39. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Tanaka A, Hawche G, Emanuel P, Maari C, Taskesen M, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233–42. doi: 10.1111/j.1365-2133.2008.08976.x. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Turi TG, Schuck A, Freedberg IM, Khitrov G, Blumenberg M. Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. FASEB J. 2001;15:2533–5. doi: 10.1096/fj.01-0172fje. [DOI] [PubMed] [Google Scholar]
- Lotem M, Raben M, Zeltser R, Landau M, Sela M, Wygoda M, et al. Kindler syndrome complicated by squamous cell carcinoma of the hard palate: successful treatment with high-dose radiation therapy and granulocyte-macrophage colony-stimulating factor. Br J Dermatol. 2001;144:1284–6. doi: 10.1046/j.1365-2133.2001.04262.x. [DOI] [PubMed] [Google Scholar]
- Mahawithitwong P, Ohuchida K, Ikenaga N, Fujita H, Zhao M, Kozono S, et al. Kindlin-1 expression is involved in migration and invasion of pancreatic cancer. Int J Oncol. 2013;42:1360–6. doi: 10.3892/ijo.2013.1838. [DOI] [PubMed] [Google Scholar]
- Margadant C, Kreft M, Zambruno G, Sonnenberg A. Kindlin-1 regulates integrin dynamics and adhesion turnover. PLoS One. 2013;8:e65341. doi: 10.1371/journal.pone.0065341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool KW, Miyamoto S. DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol Rev. 2012;246:311–26. doi: 10.1111/j.1600-065X.2012.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller J, Rogozin IB, Poliakov E, Meller N, Bedanov-Pack M, Plow EF, et al. Emergence and subsequent functional specialization of kindlins during evolution of cell adhesiveness. Mol Biol Cell. 2015;26:786–96. doi: 10.1091/mbc.E14-08-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves A, Stremmel C, Gottschalk K, Fassler R. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 2009;19:504–13. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Masuda K, Nakamura N, Takenaka H, Tsuruta D, Katoh N. Cutaneous and laryngeal squamous cell carcinoma in mixed epidermolysis bullosa, kindler syndrome. Case Rep Dermatol. 2012;4:133–8. doi: 10.1159/000339619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:356–7. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Piccinni E, Di Zenzo G, Maurelli R, Dellambra E, Teson M, Has C, et al. Induction of senescence pathways in Kindler syndrome primary keratinocytes. Br J Dermatol. 2013;168:1019–26. doi: 10.1111/bjd.12184. [DOI] [PubMed] [Google Scholar]
- Picco V, Pages G. Linking JNK Activity to the DNA Damage Response. Genes Cancer. 2013;4:360–8. doi: 10.1177/1947601913486347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorecka M, Skladanowski A, Bozko P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. Journal of nucleic acids. 2010;2010:920161. doi: 10.4061/2010/920161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Widmaier M, Jakobson M, Ruppert R, Ussar S, Katsougkri D, et al. Kindlin-1 controls Wnt and TGF-beta availability to regulate cutaneous stem cell proliferation. Nat Med. 2014;20:350–9. doi: 10.1038/nm.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler E, Klausegger A, Muss W, Deinsberger U, Pohla-Gubo G, Laimer M, et al. Novel KIND1 gene mutation in Kindler syndrome with severe gastrointestinal tract involvement. Arch Dermatol. 2006;142:1619–24. doi: 10.1001/archderm.142.12.1619. [DOI] [PubMed] [Google Scholar]
- Sesto A, Navarro M, Burslem F, Jorcano JL. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2002;99:2965–70. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–80. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin S, Bonin F, Petit V, Meseure D, Lallemand F, Bieche I, et al. Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. J Natl Cancer Inst. 2011;103:1323–37. doi: 10.1093/jnci/djr290. [DOI] [PubMed] [Google Scholar]
- Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, et al. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincek V, Kurimoto I, Medema JP, Prieto E, Streilein JW. Tumor-Necrosis-Factor-Alpha Polymorphism Correlates with Deleterious Effects of Ultraviolet B Light on Cutaneous Immunity. Cancer Res. 1993;53:728–32. [PubMed] [Google Scholar]
- Wada M, Masuda K, Tsuruta D, Tamai K, Lai-Cheong JE, McGrath JA, et al. Case of Kindler syndrome resulting from mutation in the FERMT1 gene. J Dermatol. 2012;39:1057–8. doi: 10.1111/j.1346-8138.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- Walsh LJ. Ultraviolet-B Irradiation of Skin Induces Mast-Cell Degranulation and Release of Tumor-Necrosis-Factor-Alpha. Immunol Cell Biol. 1995;73:226–33. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- Weinstein EJ, Bourner M, Head R, Zakeri H, Bauer C, Mazzarella R. URP1: a member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Biochim Biophys Acta. 2003;1637:207–16. doi: 10.1016/s0925-4439(03)00035-8. [DOI] [PubMed] [Google Scholar]
- You YH, Lee DH, Yoon JH, Nakajima S, Yasui A, Pfeifer GP. Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J Biol Chem. 2001;276:44688–94. doi: 10.1074/jbc.M107696200. [DOI] [PubMed] [Google Scholar]
- Zapatero-Solana E, Garcia-Gimenez JL, Guerrero-Aspizua S, Garcia M, Toll A, Baselga E, et al. Oxidative stress and mitochondrial dysfunction in Kindler syndrome. Orphanet J Rare Dis. 2014;9:211. doi: 10.1186/s13023-014-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Tao S, Kimmel R, Khavari PA. CDK4 regulation by TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth control. J Cell Biol. 2005;168:561–6. doi: 10.1083/jcb.200411060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jin JY, Wu J, Qin X, Streilein R, Hall RP, et al. RNA-Seq and ChIP-Seq Reveal SQSTM1/p62 as a Key Mediator of JunB Suppression of NF-kappaB-Dependent Inflammation. J Invest Dermatol. 2015;135:1016–24. doi: 10.1038/jid.2014.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Luo S, Lechler T, Zhang JY. FRA1 promotes squamous cell carcinoma growth and metastasis through distinct AKT and c-Jun dependent mechanisms. Oncotarget. 2016;7:34371–83. doi: 10.18632/oncotarget.9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.