Abstract
Background and Aim
Monitoring mucosal inflammation in inflammatory bowel disease (IBD) is of major importance to prevent complications and improve long-term disease outcome. The correlation of clinical activity indices with endoscopic disease activity is, however, moderate. Fecal calprotectin (FC) is a better predictor of mucosal inflammation, but values between 100 and 250 µg/g are difficult to interpret in clinical practice. We aimed to evaluate the occurrence of indefinite FC levels in a real-life IBD cohort and study the additional value of a combination of biochemical markers and clinical activity indices.
Methods
In total, 148 Crohn’s disease (CD) and 80 ulcerative colitis (UC) patients visiting the outpatient clinic were enrolled. FC, clinical disease activity scored by the Harvey–Bradshaw index or Simple Clinical Colitis Activity Index, and C-reactive protein (CRP) were assessed. In a subset of patients, endoscopic activity was scored by the simple endoscopic score-Crohn’s disease and Mayo endoscopic subscore. Clinical activity index, CRP, and FC were integrated in a combination score and compared with endoscopy.
Results
Indefinite FC values were present in 24% of CD and 15% of UC. In the cohort of patients with endoscopy scores available, the combination score predicted endoscopic disease activity in CD with a sensitivity of 83% and specificity of 69% [positive predictive value (PPV) 58%, negative predictive value (NPV) 89%]. In UC, this was 88 and 75% (PPV 93%, NPV 60%).
Conclusions
A combination of FC with clinical activity indices or CRP may aid in classifying patients with indefinite disease activity according to FC alone.
Keywords: Calprotectin, Indefinite, Disease activity, Biomarkers, Combination
Introduction
The classical treatment goals in patients with inflammatory bowel diseases (IBDs) are induction and maintenance of steroid-free clinical remission and prevention of surgery [1, 2]. In IBD patients, chronic bowel inflammation, irreversible bowel damage and complications such as strictures or fistula, and surgery are frequent, and many patients still use steroids years after diagnosis [3]. To improve the long-term outcome, tight disease control and endoscopic remission have become important therapeutic goals in CD and UC. Endoscopic remission is associated with lower rates of hospitalization and surgery [4, 5]. Due to the relapsing character of IBD combined with a diagnosis often at young age, repeated evaluation of disease activity during lifetime is necessary. Ileocolonoscopy, however, is an invasive and expensive procedure with an inconvenient bowel preparation, and noninvasive follow-up tools are therefore warranted.
In order to standardize the assessment of remission, clinical disease activity indices, such as the Crohn’s disease activity index (CDAI) for Crohn’s disease (CD) and Truelove and Witts Severity Index for ulcerative colitis (UC), have been developed [6, 7]. These activity indices have been validated against expert physician global assessment and are now applied in numerous trials, but their use in daily practice can be a burden for patients [e.g., those (CDAI) requiring a 7-day patient diary]. Furthermore, a substantial set of the clinical scores is derived from subjective parameters that overlap with symptoms of irritable bowel syndrome, and not necessarily reflect active inflammation. Several recent studies did show that the indices are not reliable predictors of endoscopically active disease or remission [8–11].
In clinical practice, serological markers like C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are widely used. Both are nonspecific inflammatory markers, and a CRP response is absent in a large proportion of CD and UC patients with active disease [12–15].
Fecal calprotectin (FC) is a marker for intestinal inflammation that shows good correlation with endoscopy findings in both CD and UC [16, 17]. In an anti-TNFα-treated IBD population, median FC values were found to be significantly lower (50 vs. 288 µg/g) in patients with “deep” remission (i.e., both clinical and endoscopic remissions) compared to patients being only in clinical remission or having persistent active disease [18]. Several studies found a FC value above 250 µg/g to be indicative of active inflammation and ulcerations, using endoscopic disease activity as standard [17, 19]. Furthermore, a FC value <100 µg/g as cutoff point for remission is supported by two recent studies in an anti-TNFα-treated population [20, 21]. Consequently, FC levels in the normal range (<100 µg/g) or significantly elevated FC values (>250 µg/g) are reliable to interpret as remission or as active disease. However, FC values in the “intermediate or gray zone” ranging from 100 to 250 µg/g are difficult to classify. FC as a single marker seems therefore insufficient to provide an accurate prediction of mucosal inflammation in all IBD patients. Data on the frequency of these indefinite values in daily clinical practice are lacking.
As the current standard endoscopy is too invasive for patient monitoring, using a combination of FC with CRP or clinical scores may improve the diagnostic accuracy of FC. Recent studies suggest that combining clinical activity indices with markers like FC or CRP has better diagnostic accuracy for intestinal inflammation than a single marker, but results in CD patients are conflicting. In a study by Langhorst et al. [15], sensitivity of their comprehensive activity index in CD was not superior to FC alone, only in UC diagnostic accuracy increased.
The aim of our present study was (1) to evaluate the occurrence of FC values between 100 and 250 µg/g in a real-life IBD cohort and (2) to define a novel combination score of FC with CRP or clinical activity index and compare accuracy with endoscopic disease activity scores.
Materials and Methods
Patient Selection
Between September 2009 and March 2010, consecutive IBD patients were recruited during routine follow-up at the Gastroenterology outpatient clinic of the Maastricht University Medical Center+ (MUMC+) and participated in a prospective 1-year follow-up study. Baseline data of subjects with both clinical and bio-samples available (i.e., baseline cohort) and data from a subgroup undergoing endoscopy during follow-up (i.e., endoscopy cohort) were included in the present study. Patients with previous restorative proctocolectomy with ileal pouch-anal anastomosis were excluded from the analysis. The study was approved by the Medical Ethics Committee of the MUMC+, and written informed consent was obtained prior to participation from all patients.
All patients had an established diagnosis of IBD based on clinical, endoscopic, histological, or radiological criteria. Demographic and clinical data (e.g., disease duration and phenotype, history of bowel resection, and medication) were obtained using the MUMC+ computer-based medical registration database. Disease phenotype was determined using the Montreal classification [22].
Clinical Activity Indices and Biomarkers
The Harvey–Bradshaw index (HBI) was used for clinical evaluation of disease activity in CD (cutoff point for remission ≤4 points) [23] and the Simple Clinical Colitis Activity Index (SCCAI) in UC patients (cutoff point for remission <3 points) [24].
Feces, serum, and clinical activity indices were collected in the week before patients visited the outpatient clinic. Patients were instructed to collect a fecal sample at home in a small plastic container (Fisher Scientific, Landsmeer, The Netherlands) and to store the sample at 4 °C until delivery at the laboratory within 24 h. CRP and FC were analyzed routinely by the laboratory of clinical chemistry. FC < 100 µg/g was considered as indicative of remission and >250 µg/g as active disease. A CRP cutoff point of ≥5 mg/l was used for definition of active disease.
Combination Score
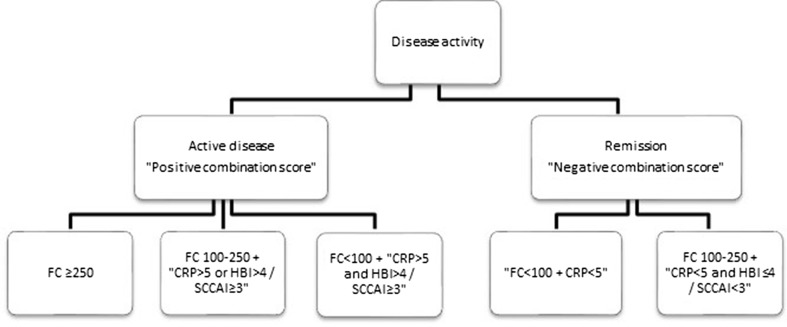
We defined a combination score based on FC, CRP, and clinical activity indices. A FC value >250 µg/g was considered as active disease (positive combination score), but in case of an indefinite FC value between 100 and 250 µg/g, a positive clinical activity index or CRP was added to determine active disease. A normal FC value (<100 µg/g) combined with both an elevated CRP and clinical activity index was considered as active disease. Remission (negative combination score) was defined as a normal FC and CRP, or an indefinite FC value combined with both normal CRP and negative clinical activity index (Fig. 1).
Fig. 1.
Definitions of active disease or remission based on new combination score
Endoscopic Evaluation
In a subset of patients, an endoscopy was performed during follow-up when indicated by the treating physician. In CD, the Simple Endoscopic Score for CD (SES-CD) was used to classify endoscopic findings [25]. Although validated cutoff points for remission or different levels of disease activity are lacking, the following cutoff points are generally used in the literature: remission (0–3 points), mild activity (4–10), moderate activity (11–19), and high activity (≥20 points) [16]. In UC, endoscopic activity was assessed by the Mayo (endoscopy sub)score, which describes the degree of inflammation on a 4-point scale (0–3) [26]. A SES-CD of ≥4 points and Mayo score of ≥1 were considered as active disease. In this endoscopy cohort, FC, CRP, and clinical activity indices were collected in the week before the endoscopy was performed.
Data Analyses and Statistics
First, the number of patients with FC values <100, >250 µg/g, and indefinite values was assessed in the baseline cohort. Secondly, the newly defined combination score was evaluated at a different time point in a subset of patients who underwent an endoscopy during the follow-up period (endoscopy cohort). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the clinical activity indices and FC alone as well as for the combination score for UC and CD separately. Finally, we checked how many (additional) patients of the baseline cohort could be classified as having active disease or remission according to the combination score compared to FC or clinical activity indices based on definition only.
Continuous variables were expressed as mean ± standard deviation (SD) or as medians with interquartile range (IQR), depending on the normality of distributions as tested by the Kolmogorov–Smirnov test. Correlations between clinical activity indices, CRP, and FC were made by the Spearman’s rank correlation coefficient. The Kruskal–Wallis H test was used to assess differences in fecal and serum markers between disease locations in CD.
A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using the software package PASW Statistics 22.
Results
Baseline Cohort
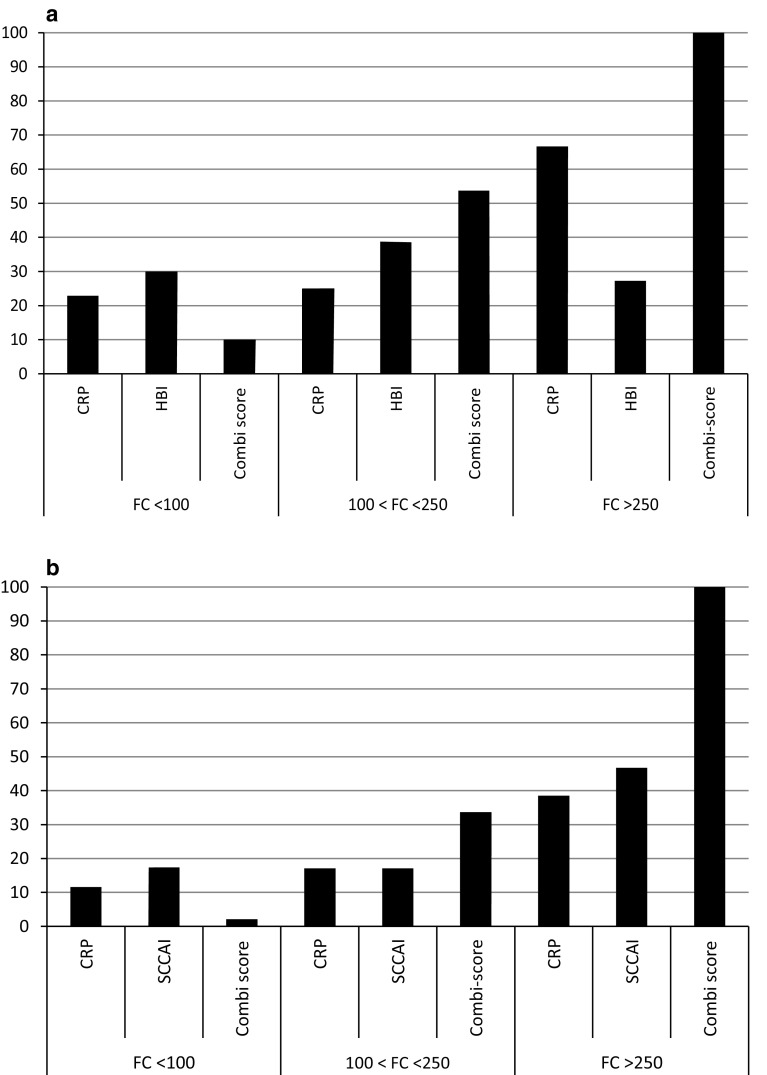
Two hundred and twenty-eight consecutive IBD patients (148 CD and 80 UC) were enrolled in the current study. Patient characteristics, disease phenotype, and medication at the time of enrollment are listed in Table 1. The percentage of patients with active disease and remission based on FC, CRP, and clinical activity indices are given in Table 2. Based on FC alone, 26% (39/148) of CD patients from the baseline cohort had active disease and 50% (73/148) was in remission. In UC, this was 33% (26/80) and 53% (42/80), respectively. In total, 24% (36/148) of CD and 15% (12/80) of UC patients had FC levels between 100 and 250 µg/g and were classified as indefinite. Fifty-eight percent (21/36) of CD patients with indefinite FC values had ileocolonic (L3) disease phenotype, 22% (8/36) had pure colonic (L2), and 19% (7/36) had pure ileal (L1) disease. In UC, 67% (8/12) of patients with indefinite FC values had left-sided colitis (E2), 25% (3/12) had extensive disease (E3), and 8% (1/12) had proctitis. The prevalence of L1, L2, or L3 disease location did not differ between CD patients with indefinite FC values and clearly defined (<100 or >250 µg/g) FC values (p = 0.13; 0.72, and 0.09, respectively). In UC, disease extent was not statistically different between patients with indefinite and definite FC values (p = 0.19, 0.75, and 0.68 for E1, E2, and E3, respectively). Figure 2 shows the percentage of CD and UC patients with a positive clinical activity index, CRP, or combination score in the different ranges of FC.
Table 1.
Baseline patient characteristics and disease phenotype according to the Montreal classification (baseline cohort)
| CD | UC | |
|---|---|---|
| N = 148 | N = 80 | |
| Female | 95 (64%) | 34 (43%) |
| Age (years: median + IQR) | 44 (32–55) | 50 (40–63) |
| Smoking | 42 (28%) | 3 (4%) |
| (IBD-related) surgery | 62 (42%) | 2 (3%) |
| Age at diagnosis | ||
| A1 <16 years | 7 (5%) | |
| A2 17–40 years | 101 (68%) | |
| A3 ≥41 years | 40 (27%) | |
| Disease phenotype | ||
| B1 Non-stricturing/non-penetrating | 101 (68%) | |
| B2 Stricturing | 29 (20%) | |
| B3 Penetrating | 21 (14%) | |
| Disease location | ||
| L1 Ileal | 41 (28%) | |
| L2 Colonic | 37 (25%) | |
| L3 Ileocolonic | 70 (47%) | |
| L4 Proximal | 8 (5%) | |
| Extent of disease | ||
| E1 Proctitis | 6 (8%) | |
| E2 Left-sided | 50 (62%) | |
| E3 Pancolitis | 24 (30%) | |
| Medication | ||
| Mesalazines | 21 (14%) | 33 (41%) |
| Corticosteroids | 17 (12%) | 3 (4%) |
| Thiopurines | 38 (26%) | 17 (21%) |
| Methotrexate | 6 (4%) | 3 (4%) |
| Biologicals | 65 (44%) | 20 (25%) |
| No medication | – | 4 (5%) |
Table 2.
Percentages of CD and UC patients in baseline cohort, with active disease and remission based on FC, CRP, and clinical activity index. Also, the percentages of patients with indefinite disease activity based on FC values are presented
| Active disease | Remission | Indefinite disease activity | |
|---|---|---|---|
| CD | |||
| FC | 26% (39/148) | 50% (73/148) | 24% (36/148) |
| CRP | 36% (53/148) | 64% (95/148) | – |
| HBI | 32% (47/148) | 68% (101/148) | – |
| UC | |||
| FC | 33% (26/80) | 53% (42/80) | 15% (12/80) |
| CRP | 21% (17/80) | 79% (63/80) | – |
| SCCAI | 26% (21/80) | 74% (59/80) | – |
Fig. 2.
Percentage of CD (a) and UC (b) patients with a positive CRP, clinical activity index, or combination score in different ranges of fecal calprotectin (FC)
Endoscopy Cohort
During the 1-year follow-up period, 84 endoscopies were performed in 50 CD patients [64% female; median age 39 (IQR 27–56) years] and 34 UC patients [27% female; median age 54 (IQR 47–60) years]. Thirty-eight percent (19/50) of CD patients and 82% (28/34) of UC patients had endoscopically active disease. Median SES-CD and Mayo endoscopic scores were 3.0 (IQR 0.0–6.0) and 1.0 (IQR 1.0–2.0), respectively. SES-CD showed a significant correlation with FC (r = 0.45; p ≤ 0.01) and CRP (r = 0.45; p ≤ 0.01), but not with the HBI scores (r = 0.07; p = 0.59). In UC, a significant correlation was found between the Mayo endoscopic score and FC (r = 0.67; p ≤ 0.01) and SCCAI (r = 0.55; p ≤ 0.01), but not with CRP (r = 0.35; p = 0.07). In CD, 48, 16, and 36% of patients had FC values <100, 100–250, and >250 µg/g, respectively. In UC, these percentages were 26, 12, and 62%, respectively.
In Table 3, the sensitivity, specificity, PPV, and NPV for defining active disease are given for FC, CRP, and clinical activity indices alone and the combination score, compared to endoscopy as standard. The combination score resulted in the highest sensitivity, being 83% for CD and 88% for UC. Accordingly, specificity, PPV, and NPV were 69, 58, and 89% for CD and 75, 93, and 60%, respectively, for UC.
Table 3.
Diagnostic accuracy of clinical activity index, calprotectin >250 µg/g, and combination score for prediction of active endoscopic disease or remission
| CD | UC | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Clinical activity index | 0.79 | 0.61 | 0.50 | 0.86 | 0.82 | 0.60 | 0.88 | 0.50 |
| Calprotectin >250 µg/g | 0.76 | 0.86 | 0.79 | 0.84 | 0.86 | 0.78 | 0.97 | 0.46 |
| CRP ≥5 mg/l | 0.56 | 0.65 | 0.32 | 0.83 | 0.50 | 0.65 | 0.39 | 0.74 |
| Combination score | 0.83 | 0.69 | 0.58 | 0.89 | 0.88 | 0.75 | 0.93 | 0.60 |
PPV positive predictive value, NPV negative predictive value
Application of Combination Score in Baseline Cohort
The combination score was composed of the 148 CD and 80 UC patients of the baseline cohort. In line with the definition, all 39 (26%) CD and 26 (33%) UC patients with FC > 250 µg/g had a positive combination score. Of those 73 (49%) CD and 42 (53%) UC patients with FC < 100 µg/g indicating remission, in 9 of the 73 CD (12%) and 3 of the 42 UC patients (7%), this could not be confirmed by the combination score. This was due to slightly elevated CRP values (>5 mg/l).
Indefinite FC values between 100 and 250 µg/g were present in 36 (24%) CD patients and in 12 (15%) UC patients at baseline. The combination score could be applied in all these patients, of whom 20 and 16 of the CD and 5 and 7 of the UC patients could be classified as having active disease or being in remission, respectively.
Discussion
In this real-life cohort of IBD patients, we evaluated the occurrence of indefinite FC values and studied the additional value of a combination of noninvasive markers to assess disease activity, especially in patients with indefinite FC values. In the baseline cohort, we found that 24% of patients had indefinite FC values, which appears not to be related to disease location in CD or disease extent in UC, though it should be stated that the number of patients in certain subgroups was limited. Our combination score predicted endoscopic disease activity with 83% sensitivity and 69% specificity in CD and 88 and 75%, respectively, in UC. Using this combination score, we were able to classify all patients with indefinite FC values as having active disease or remission.
During the last decade, many studies have been performed on FC as marker for disease activity in IBD, but there is ongoing discussion on the cutoff point for active disease or remission to be used in clinical practice. A recent meta-analysis showed an overall sensitivity and specificity of FC for endoscopically active disease of 87 and 67% in CD and 88 and 79% in UC, with large heterogeneity in cutoff points [27]. We evaluated a combination score of disease activity, aiming to better define indefinite FC values between 100 and 250 µg/g, and found that by adding the clinical activity score or CRP, sensitivity for prediction of endoscopic disease activity was higher than with FC, CRP, or clinical activity index alone. Though lower, specificity of the combination score was still acceptable with 69 and 75% in CD and UC, respectively, but specificity was slightly better for FC with a cutoff above 250 µg/g. However, sensitivity of a noninvasive disease activity marker is in our opinion more important than specificity, as identifying active inflammation will have more direct therapeutic consequences in the management of patient than exclusion of active inflammation.
Despite the fact that the FC ≥ 250 µg/g cutoff point showed good sensitivity and specificity for active disease in our endoscopy cohort, the reality in daily clinical practice is that a significant number of patients have “indefinite” FC values. All these patients in our baseline cohort could, however, be classified as having active disease or remission with the combination score.
To our knowledge, only few studies focused on a combination of noninvasive markers as surrogate for assessment of endoscopic inflammation. Langhorst et al. combined the three fecal markers [lactoferrin (Lf)/FC/polymorphonuclear neutrophil elastase] with CRP and clinical symptom indices in 85 consecutive IBD patients (42 UC/43 CD) undergoing diagnostic ileocolonoscopy. Their combination score was rated positive when two of the stool parameters and CRP or clinical activity index was positive. In UC, an overall diagnostic accuracy of 95% was found, and in CD diagnostic accuracy reached 77% (sensitivity 79%, specificity 70%) which was inferior to FC and Lf alone [15]. Sensitivity and specificity of our combination score in CD patients are in line with these results, but direct comparison between these combination scores is not feasible as endoscopic scoring and cutoff points of the markers used (e.g., FC > 48 µg/ml, CRP > 7 mg/l) differed from those in our study. Furthermore, a combination of three stool markers with CRP and clinical activity indices is probably more difficult to use in daily practice and leads to higher costs. In another study including only CD patients on anti-TNFα therapy, the combination of HBI and FC had the highest sensitivity (86%) and specificity (82%) for prediction of endoscopic remission, but the diagnostic accuracy did not differ from FC alone (sensitivity 84%, specificity 74%, FC cutoff 94 µg/g) [8].
Our data also show that the combination score and FC are both able to identify endoscopic active disease with sufficient sensitivity and specificity. The additional value of the combination score in relation to FC alone is most pronounced in the subset of patients with FC values between 100 and 250 µg/g, which was in our real-life cohort 24% of the total study population. By improving the diagnostic accuracy of the existing biomarkers in a combination score, a more accurate prediction of patients who need treatment optimization is possible, and avoidance of an invasive endoscopy even in single patients is considered a gain from a patient’s perspective but also with regard to healthcare costs.
We cannot exclude a selection bias in our endoscopy cohort as a large majority (82%) of UC patients had active disease, which makes firm conclusions on the results in the UC population difficult to draw. Furthermore, in the endoscopy cohort, the percentage of patients with indefinite FC values was lower than in the baseline cohort. Given the relatively limited number of endoscopies, stratification for disease location in CD or disease extent in UC was not possible, and therefore, validation of our results in a larger and independent CD and UC cohort with follow-up over time is needed. Notably, a substantial number of CD patients with FC values below 100 µg/g did have a positive HBI score (30%) or elevated CRP (23%). However, based on the preceding clinical course and the rather mildly elevated CRP values, it is unlikely that these patients would have active disease instead of remission. We have deliberately chosen to use a cohort representative for daily clinical practice and to study easy accessible disease activity markers which are widely used in daily clinical practice and available in most clinics. Treatment decisions in clinical practice are still partially based on clinical symptoms, but the use of more objective biomarkers reflecting mucosal inflammation more accurately should be stimulated.
In conclusion, in our real-life cohort of IBD patients, a substantial part of patients had indefinite FC values, which make active disease or remission difficult to predict. The combination of FC with clinical activity indices or CRP may improve the prediction of endoscopic active disease or remission in these patients. In daily clinical practice, our combination score instead of FC as single noninvasive marker could be a practical tool for gastroenterologists to select patients that need treatment optimization or re-evaluation of disease activity, given the large number of patients with FC “gray zone” results between 100 and 250 µg/g. Our combination score has shown potential as noninvasive disease activity marker, but needs further validation in a second independent cohort of CD and UC patients with endoscopic disease activity as gold standard.
Compliance with ethical standards
Conflict of interest
None.
References
- 1.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohn’s Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohn’s Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104:371–383. doi: 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 5.Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 6.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 7.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.af Bjorkesten CG, Nieminen U, Turunen U, et al. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 9.Regueiro M, Kip KE, Schraut W, et al. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–126. doi: 10.1002/ibd.21355. [DOI] [PubMed] [Google Scholar]
- 10.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 11.Keohane J, O’Mahony C, O’Mahony L, et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1789–1794. doi: 10.1038/ajg.2010.156. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 13.Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 14.Solem CA, Loftus EV, Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.MIB.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 15.Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 16.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 17.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 18.Molander P, Sipponen T, Kemppainen H, et al. Achievement of deep remission during scheduled maintenance therapy with TNFα-blocking agents in IBD. J Crohn’s Colitis. 2013;7:730–735. doi: 10.1016/j.crohns.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043–1052. doi: 10.1097/MIB.0b013e3182807577. [DOI] [PubMed] [Google Scholar]
- 20.Sipponen T, Savilahti E, Karkkainen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-α therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 21.Molander P, Farkkila M, Ristimaki A, et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohn’s Colitis. 2015;9:33–40. doi: 10.1016/j.crohns.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/S0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 27.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]