Abstract
Bile acids (BAs) play critical physiological functions in cholesterol homeostasis and deregulation of BA metabolism causes cholestatic liver injury. Maternally expressed gene 3 (MEG3) was recently shown as a potential tumor suppressor, however its basic hepatic function remains elusive. Using RNA pull-down with biotin-labeled sense or anti-sense MEG3RNA followed by mass spectrometry, we identified RNA binding protein polypyrimidine tract-binding protein 1 (PTBP1) as a MEG3 interaction protein and validated their interaction by RNA immunoprecipitation (RIP). Bioinformatics analysis revealed putative binding sites for PTBP1 within the coding region (CDS) of small heterodimer partner (SHP); a key repressor of BA biosynthesis. Forced expression of MEG3 in hepatocellular carcinoma (HCC) cells guided and facilitated PTBP1 binding to Shp CDS, resulting in Shp mRNA decay. Transient overexpression of MEG3RNA in vivo in mouse liver caused rapid Shp mRNA degradation and cholestatic liver injury, which was accompanied by the disruption of BA homeostasis, elevation of liver enzymes, and dysregulation of BA synthetic enzymes and metabolic genes. Interestingly, RNA-seq coupled with qPCR revealed a drastic induction Meg3RNA in Shp−/− liver. SHP inhibited MEG3 gene transcription by repressing cAMP response element-binding protein (CREB) transactivation of the MEG3 promoter. In addition, the expression of MEG3 and PTBP1 was activated in human fibrotic and NASH cirrhotic liver. Conclusion: MEG3 causes cholestasis by destabilizing Shp via serving as a guide RNA scaffold to recruit PTBP1 to Shp mRNA. SHP in turn represses CREB-mediated activation of MEG3 expression in a feedback regulatory fashion.
Keywords: lncRNA, RNA binding protein, nuclear receptor, cholestatic liver injury
Long noncoding RNA (lncRNAs) are defined as transcripts that are above 200 nucleotides in length and without proof of protein coding potential (1). lncRNAs mediate physiological and pathological processes and human diseases through regulating the expression of protein-coding genes both transcriptionally and post-transcriptionally (2). XIST(3), NEAT1 (4), and HOTAIR (5) can modulate chromatin status via recruiting chromatin-modifying complexes to affect gene transcription. lncRNA-p21 inhibits target mRNA translation via modulating RNA binding Protein HuR (6). MD1 and HULC are reported to act as miRNA sponges competing with mRNA for miRNA binding (7, 8). OIP5-As1 and GADD7 have been implicated to control mRNA stability through RNA bind proteins (RBPs) (9, 10).
Maternally expressed gene 3 (MEG3) is an imprinted gene maternally expressed from the Dlk1-Gtl2 imprinted locus located at chromosome 14q32.3 in humans (11). In mouse, MEG3 is referred to as gene trap locus2 (Gtl2) located at chromosome 12 (12). MEG3 has been implicated as a putative tumor suppressor because of its lost expression in several cancers and of its inhibition of cell proliferation (11). MEG3 regulates genes in TGF-beta signaling pathway via recruitment of polycomb repressive complex 2 (PRC2) in breast cancer cell lines (13). Although the roles of MEG3 in insulin resistance (14) and liver fibrosis (15) have been reported, the basic hepatic function of MEG3 remains largely unknown.
Bile acids (BAs) as physiological detergents synthesized in the liver facilitates intestinal digestion and absorption of dietary lipid, drugs and vitamins (16). The levels of BAs are tightly controlled by a series of enzymes and transporters that are regulated by nuclear receptors and excessive levels of BAs can cause hepatic cholestasis, liver fibrosis, and cancer (17). More recent major breakthroughs have included the discovery of the role of G-protein-coupled receptor (18), fibroblast growth factor (19), and sphingosine-1-phosphate receptor 2 (20) signaling in bile acid metabolism. Nuclear receptor small heterodimer partner (SHP) is a key inhibitor of BA synthesis (21) via farnesoid X receptor (FXR) (22) and other transcription factor-mediated pathways (23, 24). SHP exerts its effect by repressing BA synthetic enzymes cholesterol 7α-hydroxylase (Cyp7A1) and sterol 12α-hydroxylase (Cyp8B1) expression (25). Shp-deletion by gene targeting or its rapid degradation induced by Bcl2 result in elevation of serum BA levels (26) and cholestatic liver fibrosis (27), suggesting an important role of SHP to maintain normal liver function.
Polypyrimidine tract-binding protein 1 (PTBP1, also known as PTB, or hnRNP I) belongs to a subfamily of heterogeneous nuclear ribonucleoproteins (hnRNPs) that control almost all aspects of mRNA metabolism including alternative splicing, polyadenylation, IRES-mediated translation initiation and mRNA stability (28). More and more RNA-binding protein (RBP)-lncRNA interactions have been identified recently. Up to date, two lncRNAs, Pnky and TUNA, have been proven to recruit PTBP1 to regulate neuronal differentiation of neural stem cells (NSCs) (29) and embryonic stem cells (ESCs) (30). However, limited information is available regarding the function of PTBP1 in metabolic diseases.
In the present study, we identified a novel interaction between MEG3 and PTBP1 through which to modulate hepatic BA metabolism. We showed that MEG3 served as a guide RNA scaffold to recruit PTBP1 to Shp mRNA, which facilitated Shp mRNA decay. The rapid downregulation of SHP resulted in activation of Cyp7a1 and Cyp8b1, increased BA synthesis, and cholestatic liver injury. SHP in turn repressed CREB-mediated activation of MEG3 expression in a feedback regulatory fashion. The reactivation of MEG3 and PTBP1 in human fibrotic and cirrhotic livers highlighted the importance of both genes in human chronic liver diseases. To the best of our knowledge, this is the first study to elucidate the molecular basis and function of an lncRNA in BA metabolism.
Experimental Procedures
Mice
Shp+/+ (C57BL/6J, wild type [WT]), Shp−/− (C57BL/6J, single knockout) and their maintenance (31, 32), cholic acid (CA, 1%) and cholestyramine (Chol, 2%) feeding (33), serum and liver collection (34), serum enzymes (27), liver histology (21), hepatocyte isolation and culture (35), serum BA, BA pool size, BA excretion, and BA composition (27, 36) were performed as previously described. Serum C4 levels were measured at Mayo Clinic Immunochemical Core Lab. In vivo plasmid transfection was performed using TurboFect in vivo Transfection Reagent (Thermo Scientific, #R0541). In brief, 50 μg of pcDNA3 or pcDNA3-MEG3 were diluted in 400 μl 5% glucose solution, mixed with 6 μl of in vivo transfection reagent and incubated for 20 min before injected to WT mice through tail vein. Serum and liver tissue were collected at day 4 after injection. Experiments were performed on males at the age of 8 weeks (n=5/group, repeated twice), unless stated otherwise. Protocols for animal use were approved by the Institutional Animal Care and Use Committee (IACUC) at University of Connecticut.
Plasmids, Reagents and Antibodies
Expression plasmids for SHP (37), CREB (38), PTBP1 (39) and MEG3 promoter luciferase reporter (MEG3-Luc) (40) were described previously. MEG3 expression vector was cloned in our laboratory and confirmed by sequencing. Cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid. (UDCA), taurocholic acid (TCA), and actinomycin D (ACD) were purchased from Sigma. Bile acid standards were purchased from Steraloids (Newport, RI). Deuterated isotopes used as analytical standards were from C/D/N Isotopes, Inc. (Pointe-Claire, Quebec, Canada). The following antibodies were used for RNA precipitation and Western blot (WB): antibodies against PTBP1 (ThermoFisher, 32-4800), β-actin (Sigma, A-1978), and CREB (Santa cruz, sc-69374).
Cell culture, Transient Transfection and Luciferase Assay
Human Huh7 (Health Science Research Resources Bank JCRB0403), mouse Hepa-1 (ATCC CRL-1830) and human 293T (ATCC CRL-3216) were maintained as described previously (37, 41). For ACD treatment, exponentially growing cells were treated with 500 ng/ml ACD for 0, 30 and 60 min. Standard methods were used for RNA and protein isolation, cDNA synthesis, qPCR, WB, transient transfection, and luciferase reporter assay (42, 43). Experiments were done in triplicate from three independent experiments.
Biotinylated RNA Pull-down Assay
Biotin-labelled RNA transcripts were synthesized by in vitro transcription with T7 RNA polymerase in the presence of biotin—UTP. PCR fragments were amplified with forward primers containing T7 RNA polymerase promoter sequences and reverse primers containing T3 RNA polymerase promoter sequences. Purified PCR products were used as DNA template for in vitro transcription. Whole cell lysates (300–500 μg per sample) were incubated with purified biotinylated RNA probes (~10 pmole) for 25 mins at 30°C. RNA-protein complexes were further isolated by streptavidin sepharose high performance beads (GE Healthcare). The recruited proteins were detected by mass spectrometry (LC-MS) or Western blot. Primers containing T7 or T3 promoter sequences used for synthesizing biotin-labelled MEG3, CDS, 3′-UTR of SHP mRNA and also negative control RNA (complementary to CDS of SHP mRNA) are listed in Tables S1–S2.
Native RNA immunoprecipitation (RIP)
For RNA immunoprecipitation (RIP), cells (5×106) were UV-cross-linked at 254 nm (2000 J/m2). After washing with cold PBS, cells were incubated in 200 μl of lysis solution (0.5% NP40, 0.5% C24H39O4Na, 200 U/ml RNase inhibitor (Promega), and protein inhibitor (Roche) for 35 mins with vigorous shaking. Followed by DNase I treatment for 30 min, whole cell lysate was incubated with anti-PTBP1 antibody or mouse IgG (Sigma) for 2 hr at 4°C with gently shaking. 40 μl protein A/G agarose beads were added to recruit RNA-protein complexes. RNAs associated with PTBP1 were recovered by Trizol-chloroform and analyzed by RT-PCR or qPCR.
Statistical analysis
All the experiments were performed in triplicate and repeated at least two times. The data presented as the mean values ± standard error of the mean (SEM). Statistical analysis was carried out using the Student t test for unpaired data to compare the values between the two groups. P<0.01 was considered statistically significant.
Results
RNA binding protein PTBP1 serves as a MEG3 binding partner
lncRNAs exert their functions via acting as scaffolds to recruit proteins, guides or decoys for RNA binding proteins (44). To identify MEG3 interacting proteins, we conducted native RNA pull-down assay combined with mass spectrometry (LC-MS). Biotin labeled sense and antisense (negative control) MEG3RNA probes synthesized by in vitro transcription (Fig. 1A) were incubated with whole cell lysate from HEK293T cells for 20 min at 30°C. Next, streptavidin beads were used to capture biotin-labeled RNAs and their associated proteins. The RNA-protein complexes were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by coomassie brilliant blue staining (Fig. 1B). Compared with antisense MEG3, a distinct band was captured by sense MEG3 and was subjected to LC-MS. The proteins identified by LC-MS (Tables S3) were classified for biological function using PANTHER (Protein Analysis Through Evolutionary Relationships). In Biological process classification, the proteins associated with metabolic process and cellular process were predominated, whereas the proteins associated with binding and catalytic activity were predominated in molecular function classification (Supporting Fig. 1). PTBP1 was further analyzed because of its multiple roles in gene expression regulation (45). Western blot with anti-PTBP1 antibody confirmed the existence of PTBP1 within MEG3 sense RNA probe pull-downed samples (Fig. 1C). In contrast, PCNA protein was not associated with MEG3 (a negative control).
Fig. 1. PTBP1 serves as a MEG3 binding protein.
(A) Biotin-labeled sense and antisense (negative control) MEG3RNAs were synthesized by in vitro transcription. (B) In vitro RNA pull-down assay. HEK293T cell lysates were incubated with biotin-labeled oligos. After pull-down, proteins were subjected to SDS-PAGE and stained by coomassie brilliant blue. The band indicated by arrow was subjected to mass spectrometry. (C) Western blot to determine the specific interaction of sense MEG3RNA with PTBP1 protein, but not with PCNA protein (negative control). (D) RNA immunoprecipitation (RIP) of PTBP1 interaction with MEG3RNA in vivo in HEK293T cells. Protein-RNA complexes immunoprecipited by anti-PTBP1 or IgG were determined by RT-PCR using specific primers for MEG3 or Hprt1 (negative control).
Since the results of RNA pull-down reflect RNA-protein interaction in vitro (46), we performed cross-linked RIP assay to detect endogenous association between PTBP1 and MEG3. Results in Fig. 1D showed that MEG3 was significantly enriched in samples immunoprecipitated with anti-PTB or anti-PARP1 (another potential MEG3 binding protein) antibodies, as compared with samples immunoprecipitated with lgG. Taken together, the results demonstrated that the PTBP1 protein bound to MEG3 RNA.
MEG3 enhances PTBP1 recruitment to SHP mRNA to facilitate its decay
PTBP1 belongs to a family of ribonuclear proteins (RNPs) known for its role in mRNA metabolism via binding to its target mRNAs. Therefore, we hypothesized that the association between MEG3 and PTBP1 may influence the effect of PTBP1 on its target mRNAs. Several online bioinformatics analysis tools (RBPmap, StarBase v2.0) were used to predict PTBP1 targets. SHP mRNA was predicted to contain potential PTBP1 binding sites within its 3′UTR and CDS (Fig. 2A).
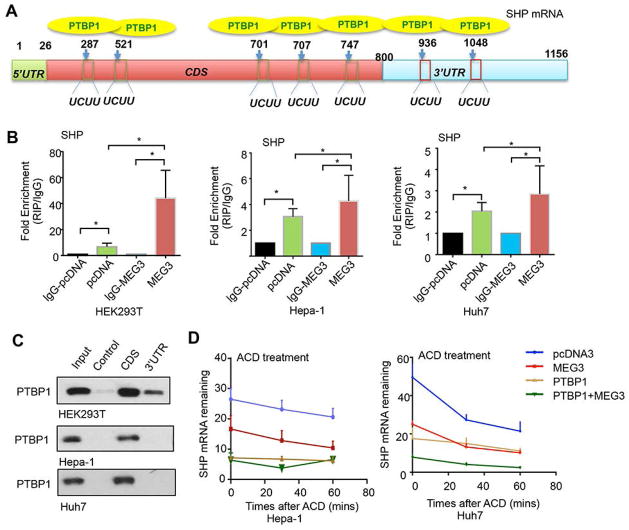
Fig. 2. MEG3 decreases SHP mRNA stability by enhancing PTBP1 binding.
(A) Schematic of predicted PTBP1 binding sites (UCUU) within the CDS and 3′UTR of SHP mRNA. (B) RNA IP to determine the interaction between SHP mRNA (full length) and PTBP1 protein using anti-PTBP1 or IgG (negative control) in HEK293T (left), Hepa-1 (middle), and Huh7 (right) cells in the presence (+) or absence (−) of MEG3RNA overexpression. (C) RNA pull-down followed by Western blot. Cell lysates of HEK293T, Hepa-1 and Huh7 were incubated with biotin-labeled CDS, 3′UTR of SHP mRNA, or a negative control transcript. After pull-down, the recruitment of PTBP1 to SHP CDS and 3′UTR was examined by Western blot with anti-PTBP1 antibody. (D) Hepa-1 (left) and Huh7 (right) cells transfected with pcDNA3 or MEG3RNA with or without PTBP1 were treated with actinomycin D (5 μg/ml). RNA was extracted at different time points (0, 30, 60 mins) and SHP mRNA was analyzed by qPCR and normalized to β-actin. Data are shown in mean ± SEM from triplicate assays. * p<0.01; **p<0.001; ***p<0.0001.
Next, RIP assay was carried out using anti-PTBP1 and mouse lgG antibodies, respectively, in cells that were transiently expressed with Flag-SHP in the presence or absence of MEG3 co-expression (Fig. 2B). The results illustrated that SHP mRNA was enriched by PTBP1 vs control lgG antibody In HEK293T cells (~10-fold) (left). Moreover, this enrichment was significantly increased by MEG3 overexpression (~40-fold). Similar results were observed in hepatocyte Hepa-1 (mouse) and Huh7 (human) cells (middle and right). The lesser enrichments of SHP mRNA by PTBP1 and MEG3 in Hepa-1 or Huh7 vs HEK293T were likely attributed by the cell’s lower transfection efficiency. Overall, the results suggested that the interaction of MEG3 and PTBP1 facilitated PTBP1 binding to SHP mRNA.
To determine the binding regions of PTBP1 in SHP mRNA, RNA probes containing CDS and 3′UTR of SHP mRNA were used for RNA pull-down, respectively. Interestingly, the CDS region of SHP mRNA recruited PTBP1 protein in all three cells, whereas SHP 3′UTR also showed binding to PTBP1 protein to a lesser extent in HEK293T cells (Fig. 2C).
Considering PTBP1 as a crucial regulator of mRNA fate, we further examined the effect of PTBP1 on SHP mRNA stability. Actinomycin D (ACD) was used to block de novo transcription in Hepa-1 or Huh7 cells transfected with MEG3 and PTBP1, alone or in combination. Endogenous SHP mRNA levels were determined at the indicated time points and normalized to GADPH mRNA; the latter has been proven to be stable during ACD treatment (6). Curves showed in Fig. 2D suggested that overexpression of PTBP1 or MEG3 facilitated SHP mRNA degradation, which were enhanced by their co-expression. Because the results obtained in Hepa-1 and Huh7 cells were compatible, we focused our studies in Huh7 cells in subsequent experiments.
MEG3 and PTBP1 activate Cyp7a1/Cyp8b1 by decreasing SHP mRNA
To determine the direct cell autonomous regulation of SHP mRNA by MEG3 and PTBP1, static levels of SHP mRNA were further examined in vitro in Huh7 cells. Forced expression of MEG3 (Fig. 3A) or PTBP1 (Fig. 3B) alone for 48 hr markedly decreased SHP mRNA expression. We repeated the experiment by overexpressing MEG3 and/or PTBP1 for 24 hr. MEG3 and PTBP1 not only inhibited SHP mRNA independently, but also exerted a synergistic effect when co-expressed (Fig. 3C). As expected, MEG3 and PTBP1, alone or in combination, activated SHP targets Cyp7a1 and Cyp8b1 (Fig. 3D). Interestingly, FXR mRNA was induced by MEG3 and PTBP1 as well, suggesting that PTBP1 may target FXR. Knockdown of endogenous PTBP1 using specific siRNA (Fig. 3E) blocked MEG3-mediated inhibition of SHP and activation of Cyp8b1 expression (Fig. 3F), indicating that PTBP1 acted downstream of MEG3 to directly control SHP expression. Overall, the results demonstrated that MEG3 inhibited SHP mRNA expression via PTBP1-mediated mechanism.
Fig. 3. MEG3 and PTBP1 induce Cyp7a1 and Cyp8b1 expression by downregulating SHP.
(A–B) qPCR of SHP mRNA in Huh7 cells overexpressed with pcDNA3 (−), MEG3RNA (A) or PTBP1 (B) for 48 h. (C–D) qPCR of SHP (C), Cyp7a1, Cyp8b1 and FXR mRNA (D) in Huh7 cells overexpressed with pcDNA3 (−), MEG3RNA or PTBP1, alone or in combination for 24 h. (E) Western blot of PTBP1 protein in Huh7 cells transfected with control (Con) or siRNA against PTBP1. (F) qPCR of SHP and Cyp8b1 mRNA in Huh7 cells overexpressed with MEG3 in the presence or absence of siPTBP1. Data are shown in mean ± SEM from triplicate assays. * p<0.01; **p<0.001; ***p<0.0001.
MEG3 induces cholestatic liver injury by downregulating SHP expression
The critical role of SHP in BA-induced liver injury (27, 36) promoted us to determine the in vivo functionality of MEG3 in BA metabolism. We established liver specific MEG3 overexpressed mouse model by hydrodynamic tail vein injection of control or MEG3 expression vector into C57BL/6J mouse liver.
The exogenously expressed MEG3RNA was confirmed by qPCR using liver RNA (Fig. 4A, left). As expected, SHP mRNA was abolished by MEG3 (middle, and Supporting Fig. 3), and this was accompanied by the upregulation of Cyp7a1 and Cyp8b1 (right) but not other BA regulators and transporters. The hepatic protein level of CYP7A1 was increased in MEG3 mice as well (upper right corner). The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), two classical markers of liver injury, were induced by MEG3 (Fig. 4B).
Fig. 4. Hepatic overexpression of MEG3 disrupts bile acid homeostasis and induces cholestatic liver injury.
(A) qPCR of the expression of hepatic MEG3RNA, Shp mRNA, and bile acid metabolic genes (left), and Western blot of CYP7A1 protein (right). Liver specific MEG3RNA overexpressed mice were generated by hydrodynamic tail vein injection of control or MEG3 expression vectors. (B) Serum levels of ALT and AST in MEG3RNA overexpressed mice (+) or control mice (−). (C) qPCR of liver (left six panels) or ileal (right panel) gene expression in MEG3RNA overexpressed mice (+) or control mice (−). (D) Measurement of serum BA, fecal BA excretion, BA pool size, and serum C4 levels. A–D: Data are shown in mean ± SEM (n=5 mice/group with triplicate assays). * p<0.01; **p<0.001; ***p<0.0001. (E) Serum BA composition was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in MEG3 vs control mice. Left: fold change below 10; Right: fold change above 50. (F) Representative images of H&E (left) and PAS (right) staining of liver sections from MEG3RNA overexpressed mice or control mice. Red circles: injury areas; black arrow: collagen deposition.
Overexpression of MEG3 also induced expression of hepatic genes in various pathways (Fig. 4C), including Srebp1c (lipid synthesis), F4/80 (inflammation), Gsk-3β (glycogen synthesis), Epcam (stem cell), and Ucp2 (energy metabolism), suggesting a broad disruption of liver metabolic homeostasis. Interestingly, F4/80, but not Gsk-3β, Epcam, and Ucp2, was upregulated in Shp−/− mouse livers (Supporting Fig. 4). The results suggest that the increased expression of Gsk-3β, Epcam, and Ucp2 in MEG3 mice is likely due to a direct effect of MEG3 activation but not an indirect effect of loss of Shp. The ileal expression of apical sodium-bile acid transporter (ASBT), a gene responsible for the initial uptake of bile acids, particularly conjugated bile acids, from the intestine as part of their enterohepatic circulation, was highly elevated in MEG3 mice (Fig. 4C, right).
Consistent with results observed in Shp−/− mice (26), serum BA and fecal BA excretion were markedly elevated in MEG3 mice (Fig. 4D). Interestingly, BA pool size remained at similar levels in control and MEG3 mice, despite upregulation of Cyp7a1 and Cyp8b1. We further measured serum C4 (7α-Hydroxy-4-cholesten-3-one) levels which are indicative of Cyp7a1 activity (47) and did not observe significant differences between control and MEG3 mice. This may explain the paradoxical increase of Cyp7a1 expression and the unchanged BA pool.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of serum BA composition revealed that the most highly elevated BAs in MEG3 mice were taurine or glycine conjugated BAs as compared to unconjugated BAs (Fig. 4E, right vs left). On the contrary, the secondary BAs including LCA and DCA were reduced in MEG3 mice (left). Histological analysis of liver sections revealed areas of injury (red circle) as well as increased glycogen storage (black arrow) in MEG3 mice (Fig. 4F). The retention of high levels of conjugated BAs but low levels of secondary BAs in blood in MEG3 mice suggest a deficiency in BA dehydroxylation and increased reabsorption, thus a disruption of the enterohepatic circulation.
SHP inhibits MEG3 expression via a CREB-dependent feedback regulation
Similar to other lncRNAs (27), MEG3 maintained a high level of expression in developmental liver and its expression was shut off in adult liver (Fig. 5A). To our surprise, RNA-seq revealed ~95 fold induction of MEG3RNA in Shp−/− vs WT liver (Fig. 5B, left). qPCR confirmed the drastic elevation of MEG3RNA in Shp−/− liver collected over a 12h/12h light/dark cycle (right). Interestingly, MEG3RNA displayed a rhythmic expression pattern that peaked at ZT14. The results suggested SHP as a potential transcriptional repressor of MEG3.
Fig. 5. MEG3 expression is transcriptionally inhibited by SHP.
(A) Integrated genome browser visualization of RNA-Seq read coverage for liver MEG3 in WT mice at E14, E18 and P60. (B) Left: Genome browser view of RNA-seq reads in MEG3 loci in Shp−/− and WT liver. Right: qPCR of hepatic MEG3RNA in Shp−/− (red) and WT (blue) liver. Samples were collected over a 24 hr light/dark cycle. (C) Left: qPCR of MEG3RNA in Huh7 cells transfected with transcription factors CREB, SREBP1c or pcDNA3, respectively. Middle: Luciferase reporter assay in HEK293T cells transfected with human MEG3 promoter reporter and expression plasmids for SHP and CREB. Top right: Western blot of liver CREB protein in Shp−/− or WT liver. (D) qPCR of MEG3RNA and PTBP1 mRNA in human liver specimens (normal n=6; steatosis n=8; fibrosis n=6; NASH cirrhosis n=8). Data are shown in mean ± SEM from triplicate assays. * p<0.01; **p<0.001; ***p<0.0001. (E) Western blot of PTBP1 protein in human liver specimens. Equal amounts of protein from individual specimen were pooled and loaded in duplicate and the band intensity was quantified. (F) Schematic of MEG3/PTBP1/SHP circuit to modulate BA homeostasis. MEG3 interacts with PTBP1 protein to form a RNA-protein complex. This complex can guide more PTBP1 binding to SHP mRNA to cause its degradation. Loss of SHP results in disruption of BA homeostasis and cholestatic liver injury. In contrast, SHP inhibits CREB-mediated activation of MEG3 expression. The feedback inhibition between MEG3RNA/PTBP1 and SHP mRNA forms a fine-tuned regulation of BA metabolism.
To explore the underlying molecular basis, we identified a CREB binding site in the MEG3 promoter (Supporting Fig. 5). Overexpression of CREB but not SREBP1c (neg. con) induced MEG3RNA in Huh7 cells (Fig. 5C, left). In addition, MEG3 promoter was activated by CREB, which was inhibited by SHP (middle). Furthermore, CREB protein was induced in Shp−/− liver as compared to WT liver (top right). Because loss of Shp in mice elevated serum BA levels (26, 27), we next determined the effects of BAs on MEG3 expression. Importantly, MEG3RNA and PTBP1 mRNA (Fig. 5D) and PTBP1 protein (Fig. 5E) were markedly increased in human fibrosis and NASH cirrhosis specimens, suggesting their relevant roles in human liver diseases. Taken together, the results demonstrated that the induction of MEG3 in Shp−/− mice was due to a direct loss of SHP inhibition.
Discussion
LncRNAs are emerging as crucial regulators of a variety of human diseases, particularly in cancers (48). In hepatocellular carcinoma (HCC), DANCR induces stemness of tumor cells through reducing CTNNB1 expression (49). H19 is induced by BA, which may contribute to Bcl2-induced cholestatic liver fibrosis (27). Lnc-HC exerts its crucial role in cholesterol metabolism by association with hnRNPA2B1 to modulate Cyp7a1 expression (50). Despite these advances, lncRNAs involved in BA metabolism remains to be identified. The present study unveils MEG3 as a novel regulator of BA homeostasis via PTBP1 mediated degradation of SHP mRNA (Fig. 5F).
LncRNAs often exert their functions through proteins they interact with. The first important step to better understand the function of MEG3 is to determine its associated protein in a regulatory network of certain biological processes. To achieve this goal, we identified PTBP1 as a MEG3 interacting protein. Of note, PTBP1 regulates degradation of its target mRNA mainly via binding to cis-acting element within 3′UTR and PTBP1 binding to CDS of mature mRNA has not been reported. Interestingly, PTBP1 interacted with CDS of SHP in HEK293T, Huh7 and Hepa-1 cells, but only interacted with 3′UTR of SHP in HEK293T, suggesting that the association of PTBP1 with 3′UTR of SHP mRNA may be cell type-dependent. Therefore, PTBP1 binding to its target mRNA is likely to be tightly controlled by cellular environments under different physiological conditions.
Recruiting RBPs appears to be a common mechanism among lncRNAs. lncLSTR and gadd7 recruit TDP-43 to control lipid metabolism (9). We found that MEG3 guided PTBP1 to SHP mRNA to facilitate its decay. MEG3 may utilize four potential mechanisms to enhance PTBP1 binding to SHP mRNA: 1) MEG3 could recognize SHP mRNA through a conserved RNA element; 2) MEG3 could serve as a protein scaffold to recruit not only PTBP1 but also other proteins to the MEG3-PTBP1 complex as sensors of SHP mRNA; 3) Binding to MEG3 may alter PTBP1 protein structure and expose its binding sites in SHP mRNA; and 4) Because another typical characteristic of PTBP1 in mRNA regulation is its nucleocytoplasmic shuttling, the association between MEG3 and PTBP1 may facilitate PTBP1 nuclear to cytoplasmic translocation, resulting in enhanced PTBP1 activity in the control of its target mRNA stability. Future studies would be necessary to elucidate how MEG3 guides RBPs to its target mRNAs.
Identifying MEG3 as a SHP target gene is another important finding of this study. Despite intensive efforts aimed at understanding the function of lncRNAs, little is known how lncRNAs are regulated transcriptionally. In this regard, we demonstrated SHP as a potent transcriptional repressor of MEG3. Interestingly, SHP also served as a transcriptional inhibitor of H19 (27), implicating SHP as a central component of the general transcriptional repressive machinery (17).
We observed an interesting circadian diurnal expression pattern for MEG3, which peaked at beginning of the dark phase when SHP expression was the lowest (34). We speculate that during fasting, the accumulation of glucagon would activate adenylate cyclase in the liver to increase the synthesis of MEG3 via CREB activation. This would allow increased BA synthesis to replace that lost during feeding. Moreover, it has been reported that PTBP1 is phosphorylated by protein kinas A (39). This might allow the MEG3:PTBP1 complex to be activated and/or move between the nucleus and cytoplasm during fasting. During the feeding cycle glucagon would be relatively low allowing Shp to be synthesized to repress BA synthesis.
Taken together, our studies identified the MEG3-PTBP1 axis in fine-tuning BA metabolism by controlling SHP mRNA stability. We results shed lights on the importance of lncRNAs as new regulators of liver function and diseases.
Supplementary Material
Acknowledgments
Grant Support: L.W. is supported by NIH R01DK104656, R01DK080440, R01ES025909, R21AA022482, R21AA024935, VA Merit Award 1I01BX002634, P30 DK34989 (Yale Liver Center) and the National Natural Scientific Foundation of China (Grant No. 81572443). OB is supported by grants from the Canadian Institute of Health Research (CIHR; grant#119331), the Canadian Foundation for Innovation (CFI; grant#25712), the Canadian Liver Foundation and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Myb-PTBP1 was kindly provided by Dr. Douglas L. Black (UCLA) and MEG3 promoter was kindly provided by Dr. Sunita K. Agarwal (NIDDK). We thank Dr. TuKiet T. Lam at Yale Keck Proteomics Center for the LC-MS analysis.
Abbreviations
- LncRNA
long noncoding RNA
- MEG3
maternally expressed gene 3
- PTBP1
polypyrimidine tract-binding protein 1
- SHP
small heterodimer partner
- BA
bile acids
- RIP
RNA immunoprecipitation
- CDS
coding region
- HCC
hepatocellular carcinoma
- CREB
cAMP response element-binding protein
- XIST
X-inactive specific transcript
- NEAT1
nuclear enriched abundant transcript 1
- HOTAIR
HOX transcript antisense RNA
- KCNQ1OT1
KCNQ1 overlapping transcript 1
- RBPs
RNA bind proteins
- HuR
Hu antigen R
- Gtl2
gene trap locus2
- PRC2
polycomb repressive complex 2
- FXR
farnesoid X receptor
- CYP7A1
cholesterol 7α-hydroxylase
- CYP8B1
sterol 12α-hydroxylase
- hnRNPs
heterogeneous nuclear ribonucleoproteins
- NSCs
neural stem cells
- ESCs
embryonic stem cells
- CA
Cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- LCA
lithocholic acid
- UDCA
ursodeoxycholic acid
- TCA
taurocholic acid
- ACD
actinomycin D
- LC-MS
liquid chromatography–mass spectrometry
- 3′UTR
3′ untranslated region
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- RNPs
ribonuclear proteins
- ALT
aminotransferase
- AST
aspartate aminotransferase
- NASH
nonalcoholic steatohepatitis
Footnotes
No conflicts of interests exist for all authors.
Author’s contributions: L.Z, Z.Y., J.T. and O.B. performed experiments and analyzed data. L.W. supervised the study. L.Z and L.W. wrote the manuscript.
References
- 1.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 3.Wutz A, Jaenisch R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell. 2000;5:695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 4.West JA, Davis CP, Sunwoo H, Simon MD, Sadreyev RI, Wang PI, Tolstorukov MY, et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell. 2014;55:791–802. doi: 10.1016/j.molcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y, Chen N, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Li D, Zhang W, Guo M, Zhan Q. Long non-coding RNA gadd7 interacts with TDP-43 and regulates Cdk6 mRNA decay. EMBO J. 2012;31:4415–4427. doi: 10.1038/emboj.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, White EJ, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gejman R, Batista DL, Zhong Y, Zhou Y, Zhang X, Swearingen B, Stratakis CA, et al. Selective loss of MEG3 expression and intergenic differentially methylated region hypermethylation in the MEG3/DLK1 locus in human clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2008;93:4119–4125. doi: 10.1210/jc.2007-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal T, Subhash S, Vaid R, Enroth S, Uday S, Reinius B, Mitra S, et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun. 2015;6:7743. doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Wu YB, Zhou J, Kang DM. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun. 2016;469:319–325. doi: 10.1016/j.bbrc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Wu YT, Huang C, Meng XM, Ma TT, Wu BM, Xu FY, et al. Inhibitory effects of long noncoding RNA MEG3 on hepatic stellate cells activation and liver fibrogenesis. Biochim Biophys Acta. 2014;1842:2204–2215. doi: 10.1016/j.bbadis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudraiah S, Zhang X, Wang L. Nuclear Receptors as Therapeutic Targets in Liver Disease: Are We There Yet? Annu Rev Pharmacol Toxicol. 2016;56:605–626. doi: 10.1146/annurev-pharmtox-010715-103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Xu N, Xu J, Kong B, Copple B, Guo GL, Wang L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology. 2014;60:919–930. doi: 10.1002/hep.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Zhu Y, Tawfik O, Kong B, Williams JA, Zhan L, Kassel KM, et al. Mechanisms of STAT3 activation in the liver of FXR knockout mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G829–837. doi: 10.1152/ajpgi.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Bonzo JA, Gonzalez FJ, Wang L. Diurnal regulation of the early growth response 1 (Egr-1) protein expression by hepatocyte nuclear factor 4alpha (HNF4alpha) and small heterodimer partner (SHP) cross-talk in liver fibrosis. J Biol Chem. 2011;286:29635–29643. doi: 10.1074/jbc.M111.253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smalling RL, Delker DA, Zhang Y, Nieto N, McGuiness MS, Liu S, Friedman SL, et al. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G364–374. doi: 10.1152/ajpgi.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Yang Z, Whitby R, Wang L. Regulation of miR-200c by nuclear receptors PPARalpha, LRH-1 and SHP. Biochem Biophys Res Commun. 2011;416:135–139. doi: 10.1016/j.bbrc.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu C, Barbier O, Smalling R, Tsuchiya H, Lee S, Delker D, et al. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci Rep. 2016;6:20559. doi: 10.1038/srep20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawicka K, Bushell M, Spriggs KA, Willis AE. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem Soc Trans. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 29.Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz CC, Salinas RD, et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16:439–447. doi: 10.1016/j.stem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin N, Chang KY, Li Z, Gates K, Rana ZA, Dang J, Zhang D, et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53:1005–1019. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SM, Zhang Y, Tsuchiya H, Smalling R, Jetten AM, Wang L. Small heterodimer partner/neuronal PAS domain protein 2 axis regulates the oscillation of liver lipid metabolism. Hepatology. 2015;61:497–505. doi: 10.1002/hep.27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Soto J, Park K, Viswanath G, Kuwada S, Abel ED, Wang L. Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth. Mol Cell Biol. 2010;30:1341–1356. doi: 10.1128/MCB.01076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchiya H, da Costa KA, Lee S, Renga B, Jaeschke H, Yang Z, Orena SJ, et al. Interactions Between Nuclear Receptor SHP and FOXA1 Maintain Oscillatory Homocysteine Homeostasis in Mice. Gastroenterology. 2015;148:1012–1023. e1014. doi: 10.1053/j.gastro.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabbi-Anneni I, Cooksey R, Gunda V, Liu S, Mueller A, Song G, McClain DA, et al. Overexpression of nuclear receptor SHP in adipose tissues affects diet-induced obesity and adaptive thermogenesis. Am J Physiol Endocrinol Metab. 2010;298:E961–970. doi: 10.1152/ajpendo.00655.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem. 2003;278:44475–44481. doi: 10.1074/jbc.M305258200. [DOI] [PubMed] [Google Scholar]
- 37.Zhou T, Zhang Y, Macchiarulo A, Yang Z, Cellanetti M, Coto E, Xu P, et al. Novel polymorphisms of nuclear receptor SHP associated with functional and structural changes. J Biol Chem. 2010;285:24871–24881. doi: 10.1074/jbc.M110.133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Tsuchiya H, Zhang Y, Hartnett ME, Wang L. MicroRNA-433 inhibits liver cancer cell migration by repressing the protein expression and function of cAMP response element-binding protein. J Biol Chem. 2013;288:28893–28899. doi: 10.1074/jbc.M113.502682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie J, Lee JA, Kress TL, Mowry KL, Black DL. Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc Natl Acad Sci U S A. 2003;100:8776–8781. doi: 10.1073/pnas.1432696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modali SD, Parekh VI, Kebebew E, Agarwal SK. Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol. 2015;29:224–237. doi: 10.1210/me.2014-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang L. Characterization of the mitochondrial localization of the nuclear receptor SHP and regulation of its subcellular distribution by interaction with Bcl2 and HNF4alpha. PLoS One. 2013;8:e68491. doi: 10.1371/journal.pone.0068491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Andrews GK, Wang L. Zinc-induced Dnmt1 expression involves antagonism between MTF-1 and nuclear receptor SHP. Nucleic Acids Res. 2012;40:4850–4860. doi: 10.1093/nar/gks159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang L. Nuclear receptor SHP inhibition of Dnmt1 expression via ERRgamma. FEBS Lett. 2011;585:1269–1275. doi: 10.1016/j.febslet.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner M, Galloway A, Vigorito E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat Immunol. 2014;15:484–491. doi: 10.1038/ni.2887. [DOI] [PubMed] [Google Scholar]
- 45.Stern MZ, Gupta SK, Salmon-Divon M, Haham T, Barda O, Levi S, Wachtel C, et al. Multiple roles for polypyrimidine tract binding (PTB) proteins in trypanosome RNA metabolism. RNA. 2009;15:648–665. doi: 10.1261/rna.1230209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marin-Bejar O, Huarte M. RNA pulldown protocol for in vitro detection and identification of RNA-associated proteins. Methods Mol Biol. 2015;1206:87–95. doi: 10.1007/978-1-4939-1369-5_8. [DOI] [PubMed] [Google Scholar]
- 47.Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K, Yan I, Haga H, Patel T. Long noncoding RNA in liver diseases. Hepatology. 2014;60:744–753. doi: 10.1002/hep.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan SX, Wang J, Yang F, Tao QF, Zhang J, Wang LL, Yang Y, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63:499–511. doi: 10.1002/hep.27893. [DOI] [PubMed] [Google Scholar]
- 50.Lan X, Yan J, Ren J, Zhong B, Li J, Li Y, Liu L, et al. A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology. 2016;64:58–72. doi: 10.1002/hep.28391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.