Abstract
Substance-use disorders are a global public health problem that arises from behavioral misallocation between drug use and more adaptive behaviors maintained by nondrug alternatives (e.g., food or money). Preclinical drug self-administration procedures that incorporate a concurrently available nondrug reinforcer (e.g., food) provide translationally relevant and distinct dependent measures of behavioral allocation (i.e., to assess the relative reinforcing efficacy of the drug) and behavioral rate (i.e., to assess motor competence). In particular, preclinical drug versus food ‘choice’ procedures have produced increasingly concordant results with both human laboratory drug self-administration studies and double-blind placebo-controlled clinical trials. Accordingly, here we provide a heuristic framework of substance-use disorders based on a behavioral-centric perspective and recent insights from these preclinical choice procedures.
Keywords: choice, addiction, preclinical model, drug self-administration, substance-use disorder
Trends
Substance-use disorders (i.e., drug addiction) are increasingly being conceptualized as disorders of behavioral misallocation between drug and nondrug reinforcers.
Preclinical drug versus food choice procedures are increasingly being utilized to elucidate environmental, pharmacological, and biological mechanisms associated with this behavioral misallocation.
Preclinical drug versus food choice procedures provide distinct dependent measures that dissociate drug reinforcement (i.e., allocation of behavior) from motor competence (i.e., rate of behavior).
Alternative nondrug reinforcer availability and temporal delivery impact behavioral allocation.
Preclinical drug versus food choice procedures are being developed for abused drugs other than cocaine.
Biological variables are an emerging as important determinants of behavioral allocation between drug and nondrug reinforcers.
Drug Addiction
Drug addiction is an insidious and global public health problem. Estimates from the most recent World Drug Report indicate that 246 million adults aged 15–64 have used at least one illicit drug within the past year and, since 2008, this number of individuals has increased year on year [1]. Although both scientists and clinicians agree that drug addiction is a significant public health issue, there are disagreements regarding the operational definition of drug addiction [2,3]. For example, both the National Institute on Drug Abusei and the American Society for Addiction Medicineii define addiction as ‘a primary, chronic disease of brain reward, motivation, memory and related circuitry’. In the most recent version of the Diagnostic and Statistical Manual of Mental Disorders, the term ‘substance-use disorder’ is used and can be diagnostically categorized from mild to severe [4]. Furthermore, six of the 11 diagnostic criteria used for substance-use disorders are based on the allocation of behavior towards the procurement and use of the substance compared with other behaviors maintained by nondrug and presumably more adaptive alternative reinforcers (e.g., food, money, or social commendation). Thus, the diagnosis of substance-use disorders takes a behavioral-centric perspective and implies that such disorders arise from behavioral misallocation between the abused substance and these alternative nondrug reinforcers (Figure 1A, Key Figure). This behavioral-centric perspective is also supported by evidence from the scientific community [5,6] and the clinical deployment of both pharmacological and nonpharmacological treatment strategies for substance-use disorders [7,8]. Furthermore, based on these diagnostic criteria, treatment goals for substance-use disorders include not only decreasing drug use, but also increasing behaviors maintained by adaptive nondrug reinforcers [9–12] (Figure 1B). Accordingly, in this review, we highlight recent insights from preclinical drug versus food ‘choice’ procedures on the environmental, pharmacological, and biological determinants and treatment of substance-use disorders.
Figure 1.
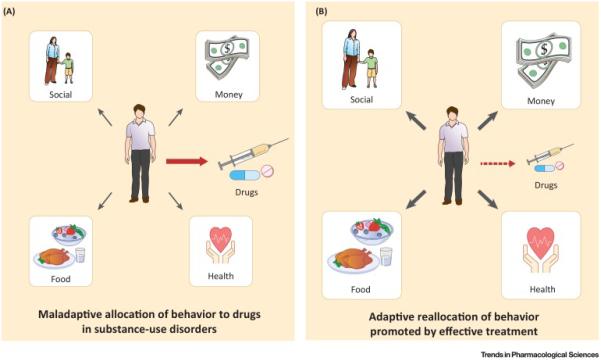
Conceptual Framework for Drug Addiction as a Disorder of Behavioral Misallocation between Concurrently Available Abused Substances and Nondrug Reinforcers. In a natural environment, a subject allocates its behavior based on numerous environmental, pharmacological, and biological factors. For most individuals, nondrug reinforcers (e.g., food, money, or social commendation) are effective to minimize or eliminate behavior directed towards the procurement and use of abused substances. However, for some individuals (A), behavior becomes predominantly misallocated (red arrow) to an abused substance at the expense of these nondrug reinforcers. This misallocation of behavior may be due to environmental, pharmacological, and biological factors that remain to be fully elucidated. Based on this conceptual framework, substance-use disorder treatments should not only decrease behavior maintained by abused drugs (faded red arrow), but also increase behavior (thicker black arrows) maintained by socially adaptive, nondrug reinforcers (B).
Preclinical Drug Choice
The development of preclinical drug self-administration procedures has been critical for improving our understanding of substance-use disorders. All drug self-administration procedures are founded on the principles of operant conditioning related to the three-term contingency [13] as follows (Equation 1):
| [1] |
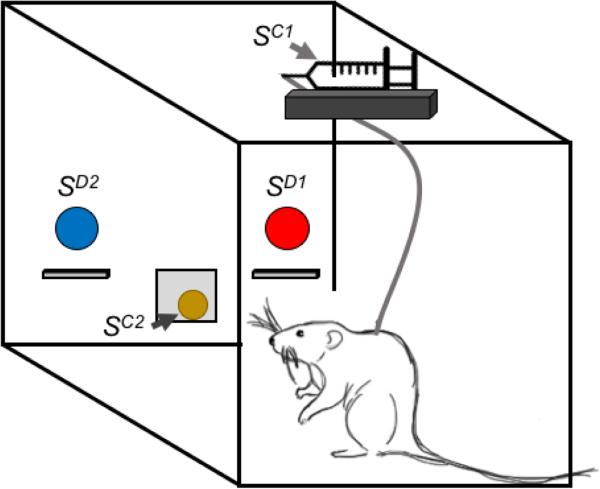
where SD denotes the discriminative stimulus (see Glossary), R denotes the response by the organism, and SC denotes the consequent stimulus. For example, in a simple type of drug self-administration procedure, the illumination of a stimulus light (the SD) might indicate that pressing a response lever (the R) will result in the delivery of an intravenous drug injection (the SC), and the primary dependent measure would be the rate of lever pressing or the number of injections delivered. In the specific case of concurrent ‘choice’ schedules of reinforcement, there are at least two SD, R, and SC to be concurrently arranged, as shown in Figure 2. In this example, a blue stimulus light (SD1) is illuminated over the left lever, and a response (R1) on the left lever will result in intravenous heroin delivery (SC1) delivery. Concurrently, there is also a red stimulus light (SD2) over the right lever, and a response (R2) on the right lever will result in food (SC2) delivery. Thus, the research organism is afforded the opportunity to allocate its behavior or ‘choose’ between these two concurrently available response options, and the primary dependent variables include both the proportion of behavior allocated toward drug choice and the overall rate of behavior maintained by both reinforcers. Inferences can then be made regarding the relative reinforcing efficacy of these two consequent stimuli that may have utility in understanding the neuropharmacological and neurobehavioral processes involved in substance-use disorders.
Figure 2.
Illustration of a Concurrent Schedule of Heroin and Food Pellet Availability in a Prototypical Intravenous Rat Drug Self-Administration Environment. In a typical intravenous rat drug self-administration procedure, the red light (SD1) over the right lever would be illuminated and responding (R1) on the right lever would produce intravenous heroin injections (SC1). Responding on the left lever would have no programmed consequences. In a heroin versus food choice procedure, the red light (SD1) over the right lever would also be illuminated and responding (R1) on the right lever would produce intravenous heroin injections (SC1). In addition, the blue light (SD2) over the left lever would also be concurrently illuminated and responding (R2) on the left lever would produce a food pellet (SC2). The rat is free to allocate its behavior between these two concurrently available reinforcers, depending upon the programmed experimental parameters.
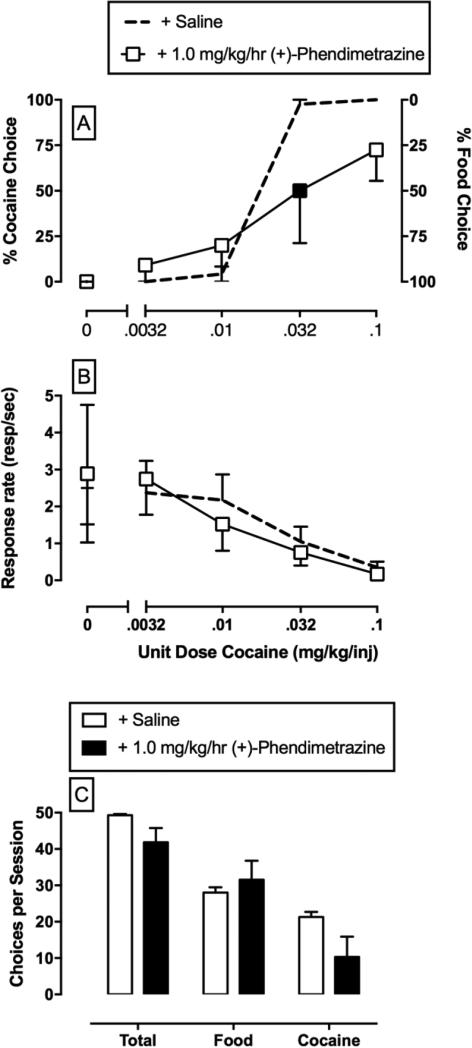
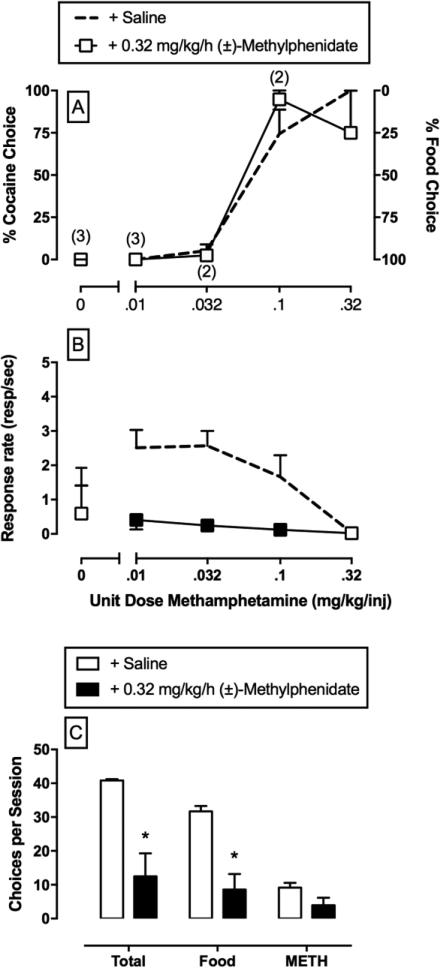
The deployment of preclinical drug versus food choice procedures to interrogate the environmental, pharmacological, and biological determinants of substance-use disorders may be advantageous for the following two reasons. First, choice procedures provide dependent measures of behavioral allocation (i.e., percent drug choice) and behavioral rate (i.e., overall rates of operant responding) that facilitate dissociation of experimental manipulations on the reinforcing efficacy of a drug from potential reinforcement-independent rate-altering effects [10]. For example, an experimental manipulation that decreases the relative reinforcing efficacy of a drug would be expected to not only decrease drug choice, but also increase choice of the alternative reinforcer without altering overall rates of behavior. An example of this selective effect (Figure 3) is continuous 14-day treatment with the prodrug phendimetrazine on cocaine versus food choice [14]. By contrast, an experimental manipulation that produces reinforcement-independent rate-altering effects (e.g., motor impairment) would be expected to decrease rates of behavior and may or may not alter drug choice. An example of this effect is shown (Figure 4) during continuous 7-day treatment with the monoamine uptake inhibitor methylphenidate on methamphetamine versus food choice [15]. Second, as noted above, clinical treatment goals of substance-use disorders include not only decreasing drug-maintained behavior, but also increasing nondrug-maintained behavior [9,11,12,16]. Preclinical choice procedures provide an assessment of behavioral allocation between drug and nondrug reinforcers during pharmacological treatments being evaluated as candidate pharmacotherapies.
Figure 3.
Effects of Continuous Treatment with the Prodrug Phendimetrazine on Choice between (−)-Cocaine and Food in Rhesus Monkeys (N = 4) [14]. (A,B) Saline and phendimetrazine treatment effects on cocaine choice dose–effect functions. Top and middle abscissae: unit cocaine dose in milligrams per kilogram per injection (log scale). Top Left ordinate: percent cocaine choice. Top Right ordinate: percent food choice. Middle ordinate: rates of responding in responses per second. (C) Summary data for response requirement completions ‘choices’ for the total session (total choices), food choices, and cocaine choices summed across all cocaine doses. All points and bars represent mean ± S.E.M. obtained during days 12–14 of each treatment period. Filled symbols indicate statistically different (P <0.05) from continuous saline treatment conditions (+ saline) within a cocaine dose.
Figure 4.
Effects of Continuous Treatment with the Monoamine Uptake Inhibitor (+)-Methylphenidate on Choice between (+)-Methamphetamine and Food in Rhesus Monkeys (N = 4) [15]. (A,B) Saline and methylphenidate treatment effects on methamphetamine choice dose–effect functions. Top and middle abscissae: unit methamphetamine dose in milligrams per kilogram per injection (log scale). Top Left ordinate: percent methamphetamine choice. Top Right ordinate: percent food choice. Middle ordinate: rates of responding in responses per second. (C) Summary data for response requirement completions ‘choices’ for the total session (total choices), food choices, and methamphetamine choices summed across all methamphetamine doses. All points and bars represent mean ± S.E.M. obtained during days 5–7 of each treatment period. Filled symbols and asterisks indicate statistically different (P <0.05) from continuous saline treatment conditions (+ saline) within a methamphetamine dose. Numbers in parentheses denote the number of subjects contributing to that data point if less than the total number of subjects tested. This indicates that a subject failed to complete at least one response requirement during that component of the choice session.
The potential of preclinical drug versus nondrug choice procedures was demonstrated decades before the earliest preclinical publication of intravenous drug self-administration [17]. For example, Spragg [18] assessed choice between intramuscular morphine and bananas in morphine-dependent chimpanzees and discovered that behavioral allocation between these two reinforcers was influenced by the state of morphine withdrawal, such that morphine withdrawal severity was positively correlated with the probability of the chimpanzees choosing morphine over bananas. Similarly, behavioral allocation between oral morphine and water in rats was influenced by the state of morphine withdrawal, such that morphine withdrawal increased the morphine versus water choice [19]. These early choice studies provided compelling evidence that the efficacy of a drug to function as a reinforcer when assessed under ‘choice’ conditions was influenced by the state of the subject (in these cases, by the state of morphine withdrawal). More recent studies have expanded on these early findings, and below we highlight insights from preclinical drug versus food choice studies on environmental, pharmacological, and biological determinants of drug choice.
Environmental Determinants
Concurrent Availability of an Alternative Reinforcer
To date, preclinical drug versus nondrug choice procedures have been established for the abused drugs cocaine [20–22], methamphetamine [23,24], 3,4-methylenedioxymethamphetamine [25], heroin [26,27], remifentanil [28], secobarbital and chlordiazepoxide [29], and nicotine [30] in either nonhuman primates or rats. With the exception of one heroin versus electrical brain stimulation choice study [31], all other preclinical drug versus nondrug choice procedures have used some food variant as the alternative nondrug reinforcer. This body of literature has suggested three main findings. First, preclinical drug versus nondrug choice has been established under a broad range of experimental conditions across different laboratories and different drug classes. These preclinical choice procedures provide an explicit, albeit simplified, model of the clinical context in which behavioral allocation occurs between drug use and other activities maintained by nondrug reinforcers. Second, these preclinical results are consistent with human laboratory studies demonstrating dose-dependent increases in the choice of the abused drugs cocaine [32,33], methamphetamine [34,35], opiates [36], Δ-9-tetrahydrocannabinol [37], and tobacco cigarettes [38] relative to choice of a nondrug alternative (usually money). Lastly, these results show that simply introducing an alternative nondrug reinforcer attenuates the potency of abused drugs to function as reinforcers compared with their potency in other drug self-administration procedures that do not include a concurrently available alternative reinforcer. Furthermore, in the case of the noncaloric sweetener saccharin, the availability of a nondrug reinforcer may attenuate the relative reinforcing efficacy of drugs in a subset of rats [24,30,39]. As described in more detail below, this potency shift may be particularly relevant in the evaluation of candidate pharmacotherapies for substance-use disorders in preclinical drug self-administration procedures.
Relative Magnitude and Cost of the Reinforcer
Both preclinical and human laboratory drug choice studies have examined the role of both reinforcer magnitude and cost on drug versus nondrug choice and examples of these manipulations that either decrease or increase drug versus food choice are listed in Table 1. In general, increasing the magnitude of the alternative reinforcer decreases behavioral allocation towards the drug and increases behavioral allocation towards the alternative reinforcer in both preclinical [22,40,41] and human laboratory [33,42] studies. Drug versus nondrug choice is also sensitive to changes in cost (e.g., response requirement) in both preclinical [41,43,44] and human laboratory [45] studies. However, drug versus nondrug choice sensitivity to cost manipulations may depend upon the self-administered drug. For example, recent preclinical and human laboratory methamphetamine versus nondrug choice studies suggest that methamphetamine choice is less sensitive to changes in the response requirement compared with previous cocaine choice studies [23,35]. Response requirement manipulations on drug versus nondrug choice for other abused drugs have not been published. Overall, these results demonstrate that the magnitude and relative cost of both nondrug and drug reinforcers may be important determinants of behavioral allocation that may contribute to the initiation, progression, and treatment of substance-use disorders.
Table 1.
Examples of Environmental, Pharmacological, and Biological Variables that Have Been Shown to Increase or Decrease Preclinical Drug versus Food Choice
| Variables | Refs |
|---|---|
| Environmental Manipulations that Decrease Drug versus Food Choice | |
| Increasing the price of the drug reinforcer | [43,44] |
| Increasing the magnitude of the alternative food reinforcer | [43,65] |
| Increasing the delay to drug reinforcer delivery | [28] |
| Environmental Manipulations that Increase Drug versus Food Choice | |
| Increasing the price of the alternative food reinforcer | [41,65] |
| Increasing the magnitude of the drug reinforcer | [40,65] |
| Increasing the delay to food reinforcer delivery | [28,51] |
| Pharmacological Manipulations that Decrease Drug versus Food Choice | |
| Chronic d-amphetamine treatment effects on cocaine versus food choice | [14,65] |
| Chronic xanomeline treatment effects on cocaine versus food choice | [109] |
| Pharmacological Manipulations that Increase Drug versus Food Choice | |
| Chronic buspirone treatment effects on cocaine versus food choice | [93] |
| Chronic U50,488 treatment effects on cocaine versus food choice | [82] |
| Biological Manipulations that Increase Drug versus Food Choice | |
| Opioid withdrawal on heroin versus food choice | [27,84] |
| Sex differences | [102,105] |
Temporal Delivery of the Reinforcer
The allocation of behavior between two concurrently available reinforcers depends upon not only the relative magnitude and cost of the reinforcers, as referenced above, but also the temporal nature of reinforcer delivery. Emerging clinical and preclinical evidence has indicated that an individual's sensitivity to the temporal nature of reinforcer delivery may be a potential risk factor for substance use and substance-use disorders (reviewed in [6,46]). The conceptual framework that relates the effectiveness of a reinforcer as a function of the delay to its presentation is called ‘delay discounting’ [47]. A subject will typically choose larger reinforcers over smaller reinforcers delivered immediately and choose reinforcers that are delivered without delay over reinforcers that are delivered following a delay [48,49]. For example, recent preclinical choice studies convincingly demonstrated that the efficacy of an alternative nondrug reinforcer to attenuate the relative reinforcing efficacy of a drug varies as a function of delay, such that longer delays to nondrug reinforcer delivery are associated with greater drug choice [28,50,51]. Furthermore, these preclinical results are also consistent with human laboratory data where cocaine users could choose between hypothetical amounts of cocaine and money either immediately or after some delay [52]. Overall, this body of literature highlights the temporal nature between behavior and reinforcer delivery as an important determinant of nondrug reinforcer efficacy to attenuate drug choice. Moreover, the degree to which pharmacological treatments might alter the delay sensitivity of nondrug reinforcers to attenuate drug choice remains to be empirically determined (see Outstanding Questions).
Pharmacological Determinants
One area of scientific discovery where preclinical choice procedures have emerged as having translational utility is in the development and evaluation of candidate pharmacotherapies for substance-use disorders. Examples of recent pharmacological variables that have either decreased or increased drug versus food choice are listed in Table 1. Preclinical drug versus food choice procedures evaluating subchronic pharmacological treatments have produced concordant results with both human laboratory drug self-administration studies and double-blind, placebo-controlled clinical trials for cocaine [16], opioids [53], and methamphetamine [54]. Three examples where this translational concordance between preclinical drug versus food-choice procedures, human laboratory drug versus money choice procedures, and double-blind, placebo-controlled clinical trials has been recently revealed are described below.
First, of the current US Food and Drug Administration (FDA)-approved pharmacotherapies for substance-use disorders, almost all have pharmacological ‘agonist-like’ properties similar to the abused substance. For example, the mu-opioid agonist methadone and the nicotinic acetylcholine receptor partial agonist varenicline are FDA approved for the treatment of opioid and tobacco-use disorder, respectively [55,56]. However, for other substance-use disorders, such as cocaine, methamphetamine, or marijuana, there are currently no FDA-approved pharmacotherapies. The pharmacological attributes and potential efficacy of an ‘agonist-like’ pharmacotherapy for cocaine-use disorder has received the most scientific attention (recently reviewed in [57,58]). In particular, maintenance on the monoamine transporter substrate d-amphetamine attenuated metrics of cocaine use in double-blind, placebo-controlled clinical trials [59–61]. Consistent with these clinical trials, d-amphetamine treatment attenuated cocaine versus money choice in human laboratory studies [62,63] and cocaine versus food choice in preclinical studies utilizing both nonhuman primates [14,41,64] and rats [65]. Furthermore, a recent analysis of d-amphetamine treatment effects on cocaine versus food choice suggested that d-amphetamine increased the relative price of cocaine compared with the alternative nondrug reinforcer [66]. However, the effectiveness of an ‘agonist-like’ pharmacotherapy approach does not completely translate to other substance-use disorders, such as methamphetamine [54]; and the effectiveness of ‘agonist-like’ pharmacotherapy approaches on preclinical nicotine versus nondrug choice is unknown. Overall, this body of literature suggests that candidate pharmacotherapies that share pharmacological attributes with some, but not all, abused drugs hold the most promise in both decreasing behaviors maintained by drugs and increasing behaviors maintained by alternative reinforcers.
Second, emerging evidence has implicated the dynorphin/kappa opioid receptor (KOR) as one potential neurobiological modulator of drug reinforcement and, as a result, KORs may have therapeutic potential as a substance-use disorder pharmacotherapy target (reviewed in [67,68]). Briefly, previous studies in humans [69,70], nonhuman primates [71], and rodents [72,73] reported prodynorphin mRNA upregulation in the striatum following cocaine exposure. Given that both dynorphin or exogenous KOR agonists decrease basal nucleus accumbens dopamine levels and block cocaine-induced increases in nucleus accumbens dopamine levels [74–76], this literature has been interpreted to suggest that chronic cocaine exposure increases dynorphin signaling that contributes to the hypodopaminergic state in substance-use disorders [77]. Therefore, preclinical drug self-administration studies have evaluated KOR antagonists as candidate pharmacotherapies for cocaine-use disorder with the neurobiological goal of blocking the increased dynorphin signaling and subsequently normalizing the dopaminergic tone.
Consistent with the neurobiological and neurochemical evidence described above, pharmacological blockade of KOR with acute administration of the long-acting KOR antagonist nor-binaltorphimine (nor-BNI) or mixtures of buprenorphine and naltrexone (intended to produce a KOR antagonist effect) attenuated cocaine [78,79], methamphetamine [80], and heroin [81] self-administration in rats given ‘extended or long drug access’ conditions. However, when acute nor-BNI treatment was evaluated under drug self-administration conditions that included an alternative, nondrug reinforcer, it failed to attenuate either cocaine versus food choice [82,83] or methamphetamine versus food choice (Banks, unpublished observations, XXXX) in nonhuman primates. Furthermore, acute treatment with another long-acting KOR antagonist, 5′-guanidonaltrindole, also failed to block withdrawal-associated increases in heroin versus food choice in nonhuman primates [84]. Whether these differential KOR antagonist treatment effects represent procedural differences related to the schedule of reinforcement and presence, or absence, of an alternative nondrug reinforcer or species differences between rats and nonhuman primates remains an outstanding question. Until recently, there were no KOR antagonist clinical treatment results available to provide critical feedback regarding the translatability of these preclinical KOR antagonist results. Recently, chronic buprenorphine+naloxone plus naltrexone (to produce a KOR antagonist effect) treatment was evaluated in a double-blind, placebo-controlled clinical trial for the treatment of cocaine-use disorder, and no significant differences were reported compared with placebo treatment conditions [85]. Overall, the results of this single clinical trial support the translational concordance between pharmacological treatment results in preclinical drug versus food choice procedures and pharmacological treatment results in the clinical setting. Furthermore, these results do not support the therapeutic potential of KOR antagonists for the treatment of cocaine-use disorder.
Lastly, emerging evidence has also implicated a role of dopamine D3 receptors as a potential neurobiological modulator of drug reinforcement and, thus, a potential pharmacotherapeutic target for substance-use disorders (reviewed in [86,87]). Briefly, both postmortem and brain-imaging studies in humans reported higher dopamine D3 receptors in cocaine users compared with controls [88,89]. Therefore, preclinical drug self-administration studies have evaluated dopamine D3 antagonists as candidate pharmacotherapies for cocaine-use disorder with the neurobiological goal of normalizing dopaminergic tone. Consistent with the neurobiological evidence cited above, continuous 7-day treatment with the dopamine D3 antagonist buspirone [90,91] selectively attenuated cocaine- versus food-maintained responding under a multiple schedule of reinforcement in nonhuman primates, although see [92] for different buspirone treatment results in nonhuman primates. When repeated buspirone treatment was evaluated under a cocaine versus food choice procedure in nonhuman primates, buspirone failed to attenuate cocaine choice [93,94]. Similar treatment results have also been reported for methamphetamine versus food choice [93,95]. However, more nuanced effects of buspirone on cocaine versus food choice was unmasked when group-housed nonhuman primates were categorized based on their social hierarchy, highlighting one potential utility of nonhuman primate studies utilizing preclinical choice procedures [94]. Specifically, buspirone decreased cocaine versus food choice in dominant monkeys and either had no effect or increased cocaine versus food choice in subordinate monkeys. Overall, and consistent with the preclinical KOR antagonist treatment results described above, buspirone treatment effects appear to depend upon the schedule of reinforcement. Furthermore, dopamine D3 treatment effects may also depend upon social hierarchy and corresponding dopamine D2-like receptor levels.
Recently, buspirone treatment was evaluated in both a double-blind, placebo-controlled clinical trial [96] and human laboratory drug self-administration study [97] to provide critical feedback on the translatability of preclinical results. Under both clinical conditions, buspirone treatment failed to significantly decrease cocaine use. Overall, these clinical results support the translational concordance between pharmacological treatment results in preclinical drug versus food choice procedures. Furthermore, these results do not support the broad therapeutic potential of dopamine D3 antagonists for the treatment of cocaine or methamphetamine-use disorder.
There are at least two potential reasons for the differential pharmacological treatment results between traditional drug self-administration procedures and drug versus food choice procedures in the preclinical studies. First, although traditional drug self-administration procedures can detect pharmacological treatment effects that decrease rates of drug self-administration, these procedures lack the capability of detecting whether the treatment promoted a reallocation of behavior. It is this reallocation of behavior that is hypothesized to be critical for the treatment of substance-use disorders [9,10,12]. Second, drugs are generally more potent to maintain responding under both fixed-ratio [90] and progressive-ratio [98] schedules of reinforcement compared with concurrent schedules of cocaine and food availability [14,65].
Pharmacological treatment selectivity to decrease drug self-administration or drug choice has been shown to be inversely related to the unit drug dose, such that greater selectivity is reported at smaller drug doses [44,99]. However, a desirable attribute of a candidate medication might be behavioral selectivity to decrease large-dose drug self-administration, both because drug abuse typically involves the use of large doses, and because behavior maintained by smaller unit drug doses can often be reduced by nonpharmacological strategies (e.g., contingency management) [33,42].
Biological Determinants
Emerging preclinical evidence has implicated sex as a biological determinant of both substance-use initiation and progression to substance-use disorders (recently reviewed in [100,101]). The role of sex as a determinant of preclinical drug versus nondrug choice has been recently investigated in both rats [65,102–104] and monkeys [105], and these results suggest two main findings. First, there was no effect of estrous cycle on intravenous cocaine versus food choice in rats or an effect of menstrual cycle on oral cocaine versus saccharin choice in monkeys. Second, both female rats and monkeys preferred cocaine over the alternative nondrug reinforcer to a greater degree than did males, although see [65] for no sex difference in rat cocaine choice ED50 values. The results from these choice studies are generally consistent with sex differences in the potency of cocaine to function as a reinforcer under simple schedules of reinforcement [106,107]. The degree to which sex differences exist in the potency of other drugs to function as reinforcers under different schedules of reinforcement remains an outstanding question. In addition, a recent clinical trial [96] noted that buspirone treatment worsened cocaine use-dependent measures in women, but not in men. This single clinical trial suggests that biological variables also impact pharmacological treatment sensitivity for substance-use disorders. Overall, this limited literature suggests the potential for sex differences in cocaine versus food choice, but little effect of cycling ovarian hormones across the estrous/menstrual cycle in females.
Furthermore, the degree to which biological variables impact the environmental or pharmacological determinants of behavioral allocation between drug and nondrug reinforcers remains relatively unexplored. For example, a decrease in drug versus nondrug choice could occur either because the reinforcing efficacy of the drug has been reduced relative to the nondrug alternative or the reinforcing efficacy of the nondrug alternative has been enhanced relative to the drug alternative. A recent study in rats reported that individual differences in baseline behavioral economic ‘demand’ curves for cocaine and an alternative nondrug (food pellets or saccharin) reinforcer alone significantly correlated with subsequent baseline cocaine versus nondrug choice [108]. Specifically, rats that displayed a more inelastic demand curve for cocaine alone and a more elastic demand curve for food alone had a higher probability of choosing cocaine over food under a concurrent schedule. Moreover, this study highlights the potential utility of rodent species to elucidate the biological determinants of drug versus nondrug choice.
Concluding Remarks
Substance-use disorders occur in the context of numerous competing alternative nondrug reinforcers. The diagnosis of substance-use disorders is founded in part on the maladaptive allocation of behavior toward drug use at the expense of other more adaptive behaviors maintained by nondrug alternative reinforcers. This diagnosis implies that the treatment of substance-use disorders seeks not only to decrease behaviors maintained by the abused substance, but also to increase behaviors maintained by socially adaptive nondrug alternatives. Preclinical drug versus food choice procedures provide an experimental tool for preclinical research to elucidate environmental, pharmacological, and biological determinants of this behavioral allocation. Furthermore, preclinical drug versus food choice procedures evaluating the efficacy of both nonpharmacological and pharmacological treatment strategies have produced translationally relevant and concordant results with both human laboratory studies and clinical trials. Although most preclinical drug versus nondrug choice studies have used nonhuman primates, rat drug versus nondrug choice studies are accelerating and provide unique opportunities to elucidate mechanisms of drug versus nondrug choice.
In conclusion, recent evidence from these preclinical choice studies has provided an empirical foundation to explore new scientific territories for improving our understanding of the environmental, pharmacological, and biological determinants of substance abuse and substance-use disorders (see Outstanding Questions). The hope is that these new insights will translate into both safer and more effective treatment strategies for substance-use disorders.
Outstanding Questions.
Can nondrug reinforcers, other than food or saccharin, be utilized in preclinical drug versus nondrug choice procedures?
Do FDA-approved pharmacotherapies for tobacco-use disorders attenuate preclinical nicotine versus nondrug choice?
Do both candidate and clinically used pharmacological treatments for substance-use disorders alter the delay sensitivity to nondrug reinforcer delivery in a delay-discounting procedure?
How do biological variables, such as sex, impact behavioral allocation between drug and nondrug reinforcers? What effects do these biological variables have on sensitivity to alternative nondrug reinforcers and their associated costs and magnitudes? What role do biological variables have on pharmacological treatment sensitivity in preclinical drug versus nondrug choice procedures?
How do biological variables, such as species or strain of the research subject, impact behavioral allocation between drug and nondrug reinforcers?
TRENDS BOX.
Substance use disorders (i.e., drug addiction) are increasingly being conceptualized as disorders of behavioral misallocation between drug and nondrug reinforcers.
Preclinical drug vs. food choice procedures are increasingly being utilized to elucidate environmental, pharmacological, and biological mechanisms associated with this behavioral misallocation.
Preclinical drug vs. food choice procedures provide distinct dependent measures that dissociates drug reinforcement (i.e., allocation of behavior) from motor competence (i.e., rate of behavior).
Alternative nondrug reinforcer availability and temporal delivery impact behavioral allocation.
Preclinical drug vs. food choice procedures are being developed for abused drugs other than cocaine.
Biological variables are an emerging as important determinants of behavioral allocation between drug and nondrug reinforcers.
Acknowledgments
The authors’ research related to this topic has been supported by the National Institute on Drug Abuse of the National Institutes of Health under award numbers R01DA031718 (M.L.B.), R01DA033364 (M.L.B. and S.S.N.), and R01DA026946 (S.S.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the artistic assistance of Megan Jo Moerke.
Glossary
- Concurrent schedule of reinforcement
one category of reinforcement schedules where two different schedules of reinforcement are presented to the organism simultaneously or concurrently. Responding on one operant manipulandum to obtain one of the concurrently available reinforcers implies a ‘choice’ and subsequent forfeiture of the other reinforcer available during that particular experimental trial or session.
- Consequent stimulus (SC)
one category of stimuli in operant terminology; defined as a stimulus whose presentation follows a response.
- Delay discounting
a theoretical framework that relates the effectiveness of a consequent stimulus to function as a reinforcer with the delay to its presentation, such that the reinforcing effectiveness of a consequent stimulus decreases as a function of the delay to its presentation. Delay-discounting procedures utilize concurrent schedules of reinforcement.
- Discriminative stimulus (SD)
another category of stimuli in operant terminology. These stimuli are usually visual or auditory based, but may represent any environmental stimulus, including drug-induced stimuli. These stimuli are correlated with the availability of a consequent stimulus and, thus, are presented before a response.
- Elastic demand
consumption of a commodity (e.g., abused drug or food) is sensitive to price changes. As the price of a commodity increases, commodity consumption decreases.
- Inelastic demand
consumption of a commodity (e.g., abused drug or food) is relatively insensitive to price changes. As the price of a commodity increases, commodity consumption does not decrease across a broader range of prices.
- Reinforcing stimulus (SR)
one category of consequent stimuli; defined as a stimulus in which its presentation following a response increases the probability that the response will recur.
- Schedule of reinforcement
the experimental parameters programmed by the investigator to establish the relation between the response and the presentation of a reinforcing stimulus. For example, a fixed-ratio ten schedule of intravenous heroin reinforcement indicates that ten consecutive responses on the operant manipulandum are required for delivery of an intravenous heroin injection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNODC . World Drug Report 2015. United Nations Office on Drugs and Crime; 2015. [Google Scholar]
- 2.Volkow ND, Koob G. Brain disease model of addiction: why is it so controversial? Lancet Psychiatry. 2015;2:677–679. doi: 10.1016/S2215-0366(15)00236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall W, et al. Brain disease model of addiction: misplaced priorities? Lancet Psychiatry. 2015;2:867. doi: 10.1016/S2215-0366(15)00417-4. [DOI] [PubMed] [Google Scholar]
- 4.APA . Diagnositc and Statistical Manual Of Mental Disorders. 5th edn American Psychiatric Association; 2013. [Google Scholar]
- 5.Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4:31. doi: 10.3389/fpsyt.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb RJ, et al. Determinants of choice, and vulnerability and recovery in addiction. Behav. Processes. 2016;127:35–42. doi: 10.1016/j.beproc.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu. Rev. Clin. Psychol. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- 8.Czoty PW, et al. Evaluation of the ‘pipeline’ for development of medications for cocaine use disorder: a review of translational preclinical, human laboratory, and clinical trial research. Pharmacol. Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 10.Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv. Pharmacol. Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comer SD, et al. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner B. Science and Human Behavior. Simon and Schuster; 1953. [Google Scholar]
- 14.Banks ML, et al. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwienteck KL, Banks ML. Effects of continuous 7-day d-amphetamine, methylphenidate, and cocaine treatment on choice between methamphetamine and food in male rhesus monkeys. Drug Alcohol Depend. 2015;155:16–23. doi: 10.1016/j.drugalcdep.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks ML, et al. Use of preclinical drug versus food choice procedures to evaluate candidate medications for cocaine addiction. Curr. Treat Options Psychiatry. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- 18.Spragg SDS. Morphine addiction in chimpanzees. Comp. Psychol. Monogr. 1940;15:1–132. [Google Scholar]
- 19.Nichols JR, Davis WM. Drug addiction. II. Variation of addiction. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1959;48:259–262. doi: 10.1002/jps.3030480502. [DOI] [PubMed] [Google Scholar]
- 20.Woolverton WL, Balster RL. The effects of lithium on choice between cocaine and food in the rhesus monkey. Commun. Psychopharmacol. 1979;3:309–318. [PubMed] [Google Scholar]
- 21.Lenoir M, et al. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AR, et al. development of a translational model to screen medications for cocaine use disorder i: choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2016;165:103–110. doi: 10.1016/j.drugalcdep.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology. 2015;40:2198–2206. doi: 10.1038/npp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caprioli D, et al. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addiction Biol. 2015;20:913–926. doi: 10.1111/adb.12220. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks ML, et al. Effects of ambient temperature on the relative reinforcing strength of mdma using a choice procedure in monkeys. Psychopharmacology. 2008;196:63–70. doi: 10.1007/s00213-007-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths RR, et al. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol. Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- 27.Lenoir M, et al. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire DR, et al. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology. 2013;229:323–330. doi: 10.1007/s00213-013-3121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Findley JD, et al. Addiction to secobarbital and chlordiazepoxide in the rhesus monkey by means of a self-infusion preference procedure. Psychopharmacologia. 1972;26:93–114. doi: 10.1007/BF00422097. [DOI] [PubMed] [Google Scholar]
- 30.Huynh C, et al. Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addict. Biol. 2015 doi: 10.1111/adb.12306. Published online September 16, 2015 http://dx.doi.org/10.1111.adb.12306. [DOI] [PubMed]
- 31.Gerber GJ, et al. Concurrent heroin self-administration and intracranial self-stimulation in rats. Pharmacol. Biochem. Behav. 1985;23:837–842. doi: 10.1016/0091-3057(85)90079-6. [DOI] [PubMed] [Google Scholar]
- 32.Hart CL, et al. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav. Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Lile JA, et al. Development of a translational model to screen medications for cocaine use disorder ii: choice between intravenous cocaine and money in humans. Drug Alcohol Depend. 2016;165:111–119. doi: 10.1016/j.drugalcdep.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart CL, et al. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- 35.Bennett JA, et al. Alternative reinforcer response cost impacts methamphetamine choice in humans. Pharmacol. Biochem. Behav. 2013;103:481–486. doi: 10.1016/j.pbb.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comer SD, et al. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav. Pharmacol. 2013;24:504–516. doi: 10.1097/FBP.0b013e328363d1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart CL, et al. Reinforcing effects of oral δ9-thc in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- 38.Cassidy RN, et al. Increasing the value of an alternative monetary reinforcer reduces cigarette choice in adolescents. Nicotine Tob Res. 2015;17:1449–1455. doi: 10.1093/ntr/ntv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantin L, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- 41.Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- 42.Donny EC, et al. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- 43.Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 1992;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- 44.Banks ML, et al. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013;38:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoops WW, et al. Alternative reinforcer response cost impacts cocaine choice in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:189–193. doi: 10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bickel WK, et al. The behavioral- and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachlin H, et al. Subjective probability and delay. J. Exp. Anal. Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catania AC. Concurrent performances: a baseline for the study of reinforcement magnitude1. J. Exp. Anal. Behav. 1963;6:299–300. doi: 10.1901/jeab.1963.6-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung S-H, Herrnstein RJ. Choice and delay of reinforcement1. J. Exp. Anal. Behav. 1967;10:67–74. doi: 10.1901/jeab.1967.10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolverton WL, Anderso KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 51.Huskinson SL, et al. Delay discounting of food by rhesus monkeys: cocaine and food choice in isomorphic and allomorphic situations. Exp. Clin. Psychopharmacol. 2015;23:184–193. doi: 10.1037/pha0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wesley MJ, et al. Choosing money over drugs: the neural underpinnings of difficult choice in chronic cocaine users. J. Addict. 2014;2014:189853. doi: 10.1155/2014/189853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negus SS, Banks ML. In: Medications development for opioid abuse. Addiction. Pierce RC, Kenny PJ, editors. Cold Springs Harbor Press; 2013. pp. XXX–YYY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banks ML. Utility of preclincial drug versus Food choice procedures to evaluate candidate medications for methampehtamine addiction. Ann. N.Y. Acad. Sci. 2016 doi: 10.1111/nyas.13276. doi: 10.1111/nyas.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell J. Pharmacological maintenance treatments of opiate addiction. Br. J. Clin. Pharmacol. 2014;77:253–263. doi: 10.1111/bcp.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cahill K, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Syst. Rev. 2012:CD006103. doi: 10.1002/14651858.CD006103.pub6. [DOI] [PubMed] [Google Scholar]
- 57.Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:815–825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pérez-Mañá C, et al. Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta-analysis of randomized controlled trials. J. Subst. Abuse Treat. 2011;40:109–122. doi: 10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Grabowski J, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Mariani JJ, et al. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol. Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuijten M, et al. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:2226–2234. doi: 10.1016/S0140-6736(16)00205-1. [DOI] [PubMed] [Google Scholar]
- 62.Rush CR, et al. Cocaine choice in humans during d-amphetamine maintenance. J. Clin. Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- 63.Greenwald MK, et al. Sustained release d-amphetamine reduces cocaine but not ‘speedball'-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banks ML, et al. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int. J. Neuropsychopharmacol. 2015;18:pyv009. doi: 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomsen M, et al. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J. Exp. Anal. Behav. 2013;99:211–233. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutsell BA, et al. A generalized matching law analysis of cocaine versus Food choice in rhesus monkeys: effects of candidate ‘agonist-based’ medications on sensitivity to reinforcement. Drug Alcohol Depend. 2015;146:52–60. doi: 10.1016/j.drugalcdep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Ann. Rev. Pharmacol. Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- 69.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 70.Frankel PS, et al. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fagergren P, et al. Temporal upregulation of prodynorphin mrna in the primate striatum after cocaine self-administration. Eur. J. Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 72.Daunais JB, et al. Short-term cocaine self administration alters striatal gene expression. Brain Res. Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- 73.Spangler R, et al. Regulation of kappa opioid receptor mRNA in the rat brain by ‘binge’ pattern cocaine administration and correlation with preprodynorphin mRNA. Mol. Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- 74.Maisonneuve IM, et al. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci. Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 75.Yokoo H, et al. Effect of opioid peptides on dopamine release from nucleus accumbens after repeated treatment with methamphetamine. Eur. J. Pharmacol. 1994;256:335–338. doi: 10.1016/0014-2999(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 76.Carlezon WA, et al. Depressive-like effects of the κ-opioid receptor agonist salvinorin a on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 77.Trifilieff P, Martinez D. Kappa-opioid receptor signaling in the striatum as a potential modulator of dopamine transmission in cocaine dependence. Front Psychiatry. 2013;4:44. doi: 10.3389/fpsyt.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wee S, et al. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wee S, et al. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci. Transl. Med. 2012;4:146ra10. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitfield TW, et al. Κ opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J. Neurosci. 2015;35:4296–4305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schlosburg JE, et al. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J. Neurosci. 2013;33:19384–19392. doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Negus SS. Effects of the kappa opioid agonist u50,488 and the kappa opioid antagonist norbinaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- 83.Hutsell BA, et al. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict. Biol. 2016;21:360–373. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ling W, et al. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the cocaine use reduction with buprenorphine (curb) study. Addiction. 2016;111:1416–11427. doi: 10.1111/add.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman AH, et al. Medication discovery for addiction: translating the dopamine d3 receptor hypothesis. Biochem. Pharmacol. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Foll B, et al. Chapter 11 – dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. In: Marco Diana GDC, Pierfranco S, editors. Prog Brain Res. Elsevier; 2014. pp. 255–275. [DOI] [PubMed] [Google Scholar]
- 88.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J. Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Payer DE, et al. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [lsqb]11c[rsqb]-(+)-PHNO. Neuropsychopharmacology. 2014;39:321–328. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mello NK, et al. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2013;38:455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergman J, et al. Modification of cocaine self-administration by buspirone (buspar®): potential involvement of d3 and d4 dopamine receptors. Int. J. Neuropsychopharmacol. 2013;16:445–458. doi: 10.1017/S1461145712000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gold LH, Balster RL. Effects of buspirone and gepirone on iv cocaine self-administration in rhesus monkeys. Psychopharmacology. 1992;108:289–294. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- 93.John WS, et al. Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology. 2015;232:1279–1289. doi: 10.1007/s00213-014-3760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Czoty PW, Nader MA. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology. 2015;40:1072–1083. doi: 10.1038/npp.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.John WS, et al. Differential effects of the dopamine D3 receptor antagonist PG01037 on cocaine and methamphetamine self-administration in rhesus monkeys. Neuropharmacology. 2015;92:34–43. doi: 10.1016/j.neuropharm.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winhusen TM, et al. Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J. Clin. Psychiatry. 2014;75:757–764. doi: 10.4088/JCP.13m08862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolin BL, et al. Buspirone reduces sexual risk-taking intent but not cocaine self-administration. Exp. Clin. Psychopharmacol. 2016;24:162–173. doi: 10.1037/pha0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- 99.Stafford D, et al. Response requirements and unit dose modify the effects of GBR. 12909 on cocaine-maintained behavior. Exp. Clin. Psychopharmacol. 2000;8:539–548. doi: 10.1037//1064-1297.8.4.539. [DOI] [PubMed] [Google Scholar]
- 100.Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carroll ME, Lynch WJ. How to study sex differences in addiction using animal models. Addict. Biol. 2016;21:1007–1029. doi: 10.1111/adb.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerstetter KA, et al. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perry AN, et al. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One. 2013;8:e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perry AN, et al. The roles of dopamine and [alpha]1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology. 2015;40:2696–2704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carroll ME, et al. Sex and menstrual cycle effects on chronic oral cocaine self-administration in rhesus monkeys: effects of a nondrug alternative reward. Psychopharmacology. 2016;233:2973–2984. doi: 10.1007/s00213-016-4343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morgan D, et al. Social dominance in monkeys: dopamine d2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 107.Nader MA, et al. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol. Psychiatry. 2012;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kearns DN, et al. Essential values of cocaine and non-drug alternatives predict the choice between them. Addict. Biol. 2016 doi: 10.1111/adb.12450. doi: 10.111/adb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomsen M, et al. Acute and chronic effects of the M1/M4-preferring muscarinic agonist xanomeline on cocaine versus Food choice in rats. Psychopharmacology. 2014;231:469–479. doi: 10.1007/s00213-013-3256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]