Abstract
Objective
Drug-resistant epilepsy is a devastating disorder associated with diminished quality of life (QOL). Surgical resection leads to seizure freedom and improved QOL in many epilepsy patients, but not all individuals are candidates for resection. In these cases, neuromodulation-based therapies such as vagus nerve stimulation (VNS) are often used, but most VNS studies focus exclusively on reduction of seizure frequency. QOL changes and predictors with VNS remain poorly understood.
Method
Using the VNS Therapy Patient Outcome Registry, we examined 7 metrics related to QOL after VNS for epilepsy in over 5,000 patients (including over 3,000 with ≥ 12 months follow-up), as subjectively assessed by treating physicians. Trends and predictors of QOL changes were examined and related to post-operative seizure outcome and likelihood of VNS generator replacement.
Results
After VNS therapy, physicians reported patient improvement in alertness (58-63%, range over follow-up period), post-ictal state (55-62%), cluster seizures (48-56%), mood change (43-49%), verbal communication (38-45%), school/professional achievements (29-39%), and memory (29-38%). Predictors of net QOL improvement included shorter time to implant (odds ratio [OR], 1.3; 95% confidence interval [CI], 1.1-1.6), generalized seizure type (OR, 1.2; 95% CI, 1.0-1.4), female gender (OR, 1.2; 95% CI, 1.0-1.4), and Caucasian ethnicity (OR, 1.3; 95% CI, 1.0-1.5). No significant trends were observed over time. Patients with net QOL improvement were more likely to have favorable seizure outcomes (chi square [χ2] = 148.1, p < 0.001) and more likely to undergo VNS generator replacement (χ2 = 68.9, p < 0.001) than those with worsened/unchanged QOL.
Significance
VNS for drug-resistant epilepsy is associated with improvement on various QOL metrics subjectively rated by physicians. QOL improvement is associated with favorable seizure outcome and a higher likelihood of generator replacement, suggesting satisfaction with therapy. It is important to consider QOL metrics in neuromodulation for epilepsy, given the deleterious effects of seizures on patient QOL.
Keywords: epilepsy, quality of life, vagus nerve stimulation, VNS, surgery
1. Introduction
Epilepsy is a devastating neurological disorder affecting approximately 1% of the population, and seizures are resistant to anti-epileptic drugs (AEDs) in about one-third of patients [1, 2]. Drug-resistant epilepsy leads to significantly diminished quality of life (QOL), increased morbidity, and a 2-3 fold elevated risk of mortality [3, 4]. In many patients with focal seizures and a localizable epileptogenic zone (EZ), surgical resection or ablation may lead to a cessation of seizure activity and improved QOL. After resection, seizure freedom is achieved by approximately 60-80% of patients with mesial temporal lobe epilepsy and 40-60% of individuals with focal neocortical epilepsy [5-7]. Numerous studies have demonstrated improvement in various aspects of QOL after resective epilepsy surgery, as well as reduced morbidity and mortality [8-11]. However, not all individuals with drug-resistant seizures are candidates for surgical resection.
Candidacy for resective epilepsy surgery may be prevented by a primary generalized epilepsy syndrome, non-localizable or multifocal seizure onset, or an epileptogenic zone in an eloquent location [12]. In these individuals, neuromodulation-based surgical therapies are often considered, of which vagus nerve stimulation (VNS) is the most common [13]. These palliative neurostimulation therapies often result in reduced seizure frequency, although seizure freedom is significantly less common than with resection [14]. Unlike with resective therapy, very few studies of VNS or other forms of neuromodulation have specifically examined QOL outcomes with therapy, and the vast majority of investigations focus on percent decrease in seizure frequency and/or overall rate of response to stimulation [15-17]. Thus, even though improved QOL is a critical outcome goal with any therapy for epilepsy, changes in QOL with VNS are poorly understood.
Here we present the first large-scale study of QOL metrics with VNS therapy. Using registry-based data, we analyze physician survey responses for over 5,000 patients before and after implantation to gauge improvement or worsening in several QOL metrics and identify potential predictors of improvement. We also estimate net QOL change with VNS therapy, and relate QOL outcomes to both seizure outcomes and to the likelihood of generator (i.e., battery) replacement – an indirect estimate of patient satisfaction with therapy.
2. Methods
2.1 Patient outcome registry data collection
Data were obtained from the VNS Therapy Patient Outcome Registry maintained by the manufacturer of the device, Livanova (previously Cyberonics, Inc.), Houston, TX, USA. This database was established in 1999, after USA FDA approval of VNS therapy for epilepsy in 1997, in order to monitor patient outcomes systematically. Neurologists treating VNS patients were provided standard case report forms from the registry, and participation was fully voluntary. Although refusal to participate was not explicitly tracked, during active data collection, the registry included approximately 18% of all VNS devices implanted. Overall, data were prospectively collected by 1,285 prescribing physicians from 978 centers (911 within the USA and Canada and 67 international) at patients’ baseline before implantation and at various intervals during therapy. Previous studies have authenticated the integrity of the systems for collecting and processing data in the VNS registry using an independent auditing agency [18], and registry outcomes have been compared to and corroborated by outcomes reported across the independent literature [19]. The registry was IRB approved and individual patients provided consent.
At baseline, a patient history and implant form was submitted including information on patient demographics, epilepsy etiology and syndrome, historical seizure types and frequencies, and current AED use. At each follow-up visit, medications, VNS therapy settings, seizure frequency and type, generator replacements, device malfunctions, and QOL metrics were tracked. With respect to 7 metrics which may influence patient QOL, each individual was subjectively rated by the treating physician on a scale including worsened, no change, or improved compared to pre-implantation state. These metrics included: alertness, verbal communication, memory, social/professional achievements, mood changes, post-ictal state, and cluster seizures. In addition, as part of a “physician global assessment,” providers were asked to rate the patient’s overall condition as worsened, no change, or improved compared to pre-implantation state.
Data points collected in the registry at each follow-up visit included seizure frequency (overall and by seizure type), current AEDs, and QOL metrics. Seizure types classified as “generalized” included primary and secondarily generalized tonic-clonic, atonic (drop attacks), and absence seizures, while those classified as “partial” (focal) seizures included complex partial (focal with impairment of consciousness) and simple partial (focal without impairment of consciousness), including auras. Epilepsy etiologies classified as “lesional” included tumor, cyst, vascular malformation, mesial temporal sclerosis, tuber, and malformation of cortical development, while those classified as “non-lesional” included post-infectious, inflammatory, post-ischemic, Lennox-Gastaut or similar infantile syndrome, post-traumatic, cerebral palsy/perinatal event, or unknown/idiopathic. Between points of data submission by providers, it was assumed that therapy remained constant since the previous data point. For example, if data for a particular patient was submitted during a 4-month follow-up period and then again at an 12-month follow-up period, constant therapy was assumed between those data points.
2.2 Registry data analysis
The database was queried in February 2015, and all seizure and QOL outcomes reported with the 0-4, 4-12, 12-24, and 24-48 month time ranges after VNS device implantation were extracted and compared to patient pre-operative baseline. Duplicate visits for the same individual within the same time period were excluded from analysis. The patient was rated as worsened, no change, or improved on each individual QOL metric at each visit. In addition, to estimate net change across all seven QOL metrics (“net QOL change”), the total number of metrics rated as worsened (-1), no change (0), or improved (+1) was summed for each patient at each follow-up period. A positive total was estimated as improvement, a negative total was estimated as worsening, and a zero total was estimated as no change. Additionally, using the physician global assessment, providers recorded patient’s overall status at each visit as worsened, no change, or improved compare to pre-implantation state. Overall percent decrease (or increase) in seizure frequency compared to baseline was also calculated at each follow-up visit. Patients with ≥ 50% decrease in seizure frequency after VNS therapy compared to pre-operative baseline were designated seizure “responders” while those with < 50% reduction in seizure frequency were labeled seizure “non-responders,” and this overall responder rate was tracked over time.
2.3 Statistical analyses
Outcomes over time were evaluated for significant trends with linear regression analysis, including individual QOL metrics, estimated net QOL change, reported physician global assessment, change in seizure frequency, and seizure responder status. Univariate analysis with Pearson Chi square (χ2) with Yates correction was used to evaluate potential relationships between QOL metrics (improved vs. worsened/no change), seizure outcome (responder vs. non-responder), and VNS generator replacement status (replaced vs. not replaced). To examine potential predictors of net QOL improvement across all visits, multivariate analysis was performed using binary logistic regression with backward elimination of factors. Predictors were reported using odds ratio (OR) with a 95% confidence interval (CI). Bonferroni correction was applied for multiple comparisons for all analysis where appropriate, and the level of significance was set at 0.05 after correction. Statistical analysis was performed using JMP 10.0 and SAS version 9.2 (SAS Institute, Inc., Cary, NC).
3. Results
Query of the VNS Patient Outcome Registry revealed data from 12,319 unique physician visits by 5,554 patients with drug-resistant epilepsy. Of these, analysis included 4,666 unique visits during the 0-4 month follow-up period after VNS implantation, 3,277 visits at 4-12 months, 3,182 visits at 12-24 months, and unique 1,194 visits during the 24-48 month period. Altogether, 47% of patients in the registry were female, and the average age at implantation was 27 years (median 26, range 0-87 years). These and other patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Gender | Male | 2,696 (53%) |
| Female | 2,402 (47%) | |
| Race | Caucasian | 4,230 (83%) |
| Hispanic | 309 (6%) | |
| Other/Unknown | 264 (5%) | |
| African-American | 247 (5%) | |
| Asian | 48 (1%) | |
| Dominant seizure type | Partial/focal | 3,267 (64%) |
| Generalized | 1,831 (36%) | |
| Localization | Temporal | 1,348 (26%) |
| Extra-temporal | 1,689 (33%) | |
| Not-localized/Generalized | 2,061 (40%) | |
| Age of onset | mean ± SEM | 7.8 ± 10.0 |
| Age at implant | mean ± SEM | 27.1 ± 15.6 |
| Time to implant | < 10 years | 1,625 (32%) |
| ≥ 10 years | 3,473 (68%) |
3.1 Change in QOL metrics with VNS therapy
The percentage of epilepsy patients rated as improved, worsened, or no change with regard to 7 metrics related to QOL was determined during each follow-up period after VNS implantation (Fig. 1A). Improvement was most frequently reported with regard to alertness (58-63%, range over follow-up period), post-ictal state (55-62%), cluster seizures (48-56%), and mood change (43-49%), and was reported less frequently with regard to verbal communication (38-45%), school/professional achievements (29-39%), and memory (29-38%). A slight trend towards greater improvement over time was not significant for any factor (F < 11, p > 0.05 per metric, Bonferroni corrected). A net change in QOL metrics was also estimated and tracked over time, taking into account improvement, worsening, or no change across all 7 individual factors (Fig. 1B). Overall, net improvement was noted in 74-77% of patients, worsening in 4-6% of individuals, and no overall change in 18-22% of patients, with no significant trends observed over time (F = 0.1, p = 0.8, Bonferroni corrected). Finally, as part of the physician global assessment, providers rated 80-85% of patients as overall improved (range over follow-up period), 4-5% as worsened, and 11-16% as no change. These data suggest that overall, epilepsy patients are rated as having improvement in factors related to QOL after VNS implantation, according to subjective responses from providers.
Figure 1. Change in QOL metrics with VNS.
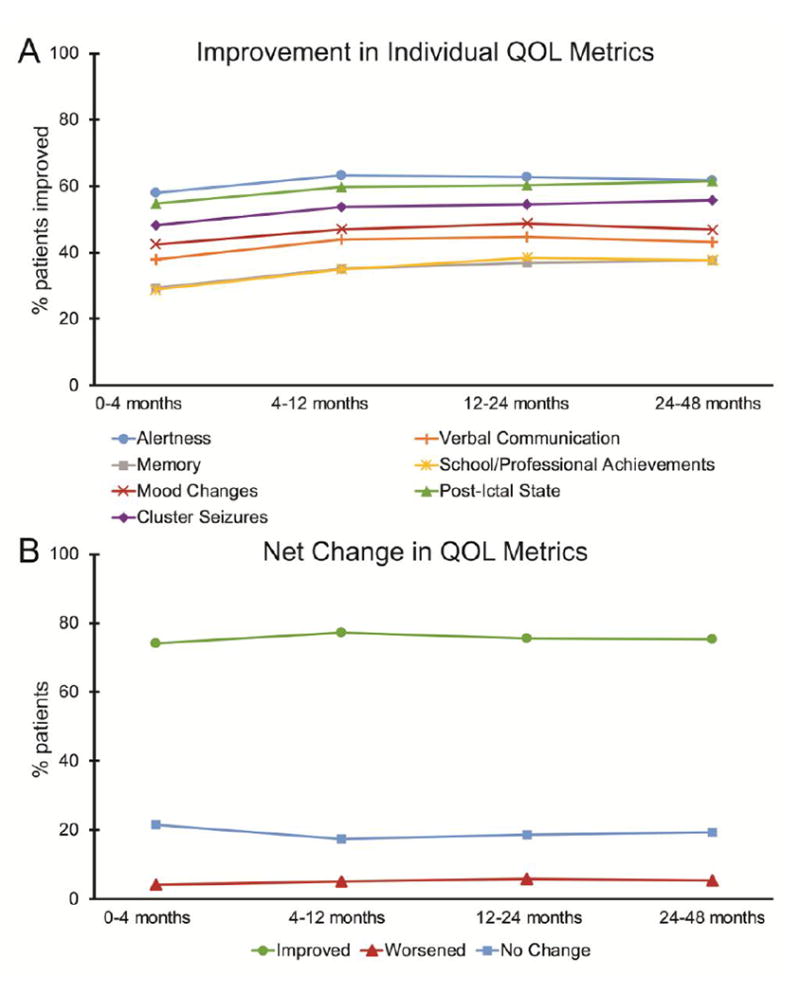
A) Improvement rates in various metrics related to QOL are shown. Patients were subjectively rated by the treating physician as improved, worsened, or no change with regard to each metric, compared to pre-operative baseline. B) Percentage of patients rated as net improved, worsened, or no change across all 7 QOL metrics. For A and B, no significant trends over time were observed (F < 11, p > 0.05 per metric, Bonferroni corrected). N = 4,666 (0-4 months), 3,277 (4-12 months), 3,182 (12-24 months), and 1,194 (24-48 months) patients. QOL, quality of life; VNS, vagus nerve stimulation.
Overall QOL and seizure outcomes at 12-24 months after implantation stratified by seizure type, localization, and time to implant are summarized in Table 2. Multivariate analysis was used to investigate potential predictors of net QOL improvement at 12-24 months, including age of epilepsy onset (< 12 vs. ≥ 12 years), gender, ethnicity (Caucasian vs. other ethnicities), time to VNS implant after diagnosis (< 10 vs. ≥10 years), epilepsy subtype (focal/partial vs. generalized), and epilepsy etiology (lesional vs. non-lesional). As summarized in Table 3, net QOL improvement was significantly predicted by female gender, Caucasian ethnicity, time to implant < 10 years, and generalized seizure subtype. Other factors examined were not predictive.
Table 2.
Summary of QOL and Seizure Outcomes
| Net QOL Improvement | Seizure Reduction ≥ 50% | Generator Replacement Rate | ||
|---|---|---|---|---|
| Dominant seizure type | Partial | 74.7% | 59.7% | 68.0% |
| Generalized | 76.4% | 60.7% | 70.9% | |
| Localization | Temporal | 76.6% | 59.0% | 68.8% |
| Extra-temporal | 74.0% | 59.9% | 65.6% | |
| Not- localized/Generalized | 75.6% | 60.8% | 72.0% | |
| Time to implant | < 10 years | 79.8% | 62.0% | 68.6% |
| ≥ 10 years | 73.4% | 59.2% | 69.2% |
Table 3.
Predictors of improvement in QOL metrics
| OR | 95% CI | |
|---|---|---|
|
|
||
| Female gender | 1.2 | 1.0-1.4 |
| Caucasian ethnicity | 1.3 | 1.0-1.5 |
| Time to implant < 10 years | 1.3 | 1.1-1.6 |
| Primarily generalized seizures | 1.2 | 1.0-1.4 |
Results of binary logistic regression analysis. CI, confidence interval; OR, odds ratio.
3.4 QOL metrics in seizure responders vs. non-responders
Next, the relationship between QOL outcome and seizure outcome was evaluated (Fig. 2). The median decrease in seizure frequency after VNS therapy increased significantly over time (F = 214.6, p < 0.01, Bonferroni corrected), from a 45% reduction at 0-4 months to a 63% reduction at 24-48 months (Fig. 2A). A progressive increase was also seen in the seizure responder rate over time (F = 43.0, p = 0.04, Bonferroni corrected), with 49% of patients responding to therapy at 0-4 months, to 61% of individuals responding at 24-48 months, with responder status defined as ≥ 50% decrease in seizure frequency (Fig. 2A). While net QOL improvement was estimated in 81-84% of seizure responders (Fig. 2B), net QOL improvement was significantly less common (64-70%) in seizure non-responders (Fig. 2C) (χ2 = 148.1, p < 0.001). This finding suggests a positive relationship between favorable seizure outcome and improved QOL metrics with VNS therapy.
Figure 2. QOL metrics in seizure responders vs. non-responders after VNS.
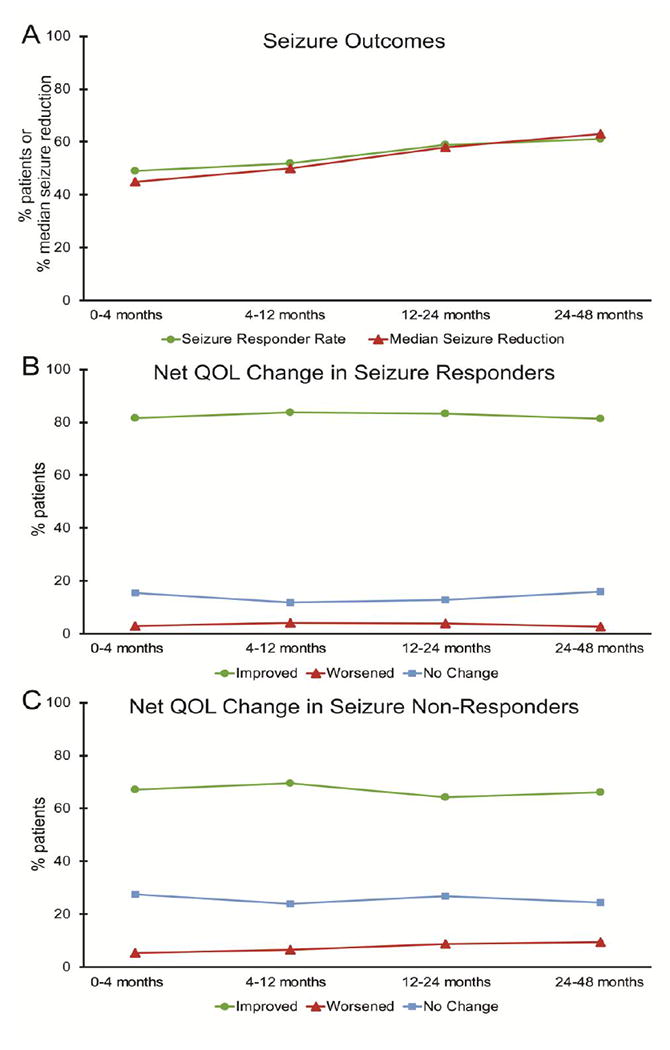
A) Over time with VNS, a significant increase is seen in both the median reduction in seizure frequency (F = 214.6, p < 0.01, Bonferroni corrected), and in the proportion of patients who respond to VNS, which is defined as ≥ 50% reduction in seizure frequency (F = 43.0, p = 0.04, Bonferroni corrected). B-C) Seizure responders (B) are significantly more likely to show improvement in QOL metrics than non-responders (C) (χ2 = 148.1, p < 0.001), though no significant trend over time is seen in either group (F < 5, p > 0.05, Bonferroni corrected). and N = 4,666 (0-4 months), 3,277 (4-12 months), 3,182 (12-24 months), and 1,194 (24-48 months) patients. QOL, quality of life; VNS, vagus nerve stimulation.
3.5 Relationship between QOL metrics and seizure outcome with generator replacement
Next, given that VNS generator replacement may help indicate satisfaction with therapy, we examined the potential relationship between net QOL improvement or seizure responder status to generator replacement in all patients with generator data available (Fig. 3). Of 3,085 patients, 2,203 (71%) underwent replacement of the VNS generator. Among patients with net improvement in QOL metrics, 75% underwent generator replacement, compared to only 59.4% of individuals with worsening/no change in QOL metrics (χ2 = 68.9, p < 0.001, Bonferroni corrected). Furthermore, while 75% of patients who were seizure responders received generator replacement, generators were exchanged in only 65% of seizure non-responders (χ2 = 40.0, p < 0.001, Bonferroni corrected). Overall, QOL improvement had a stronger association with generator replacement than seizure response (χ2 = 149.4, p < 0.001, Bonferroni corrected). These data suggest that provider-rated QOL metrics predict generator replacement, and may therefore help reflect satisfaction with therapy.
Figure 3. VNS generator replacement rates stratified by QOL metrics and seizure response.
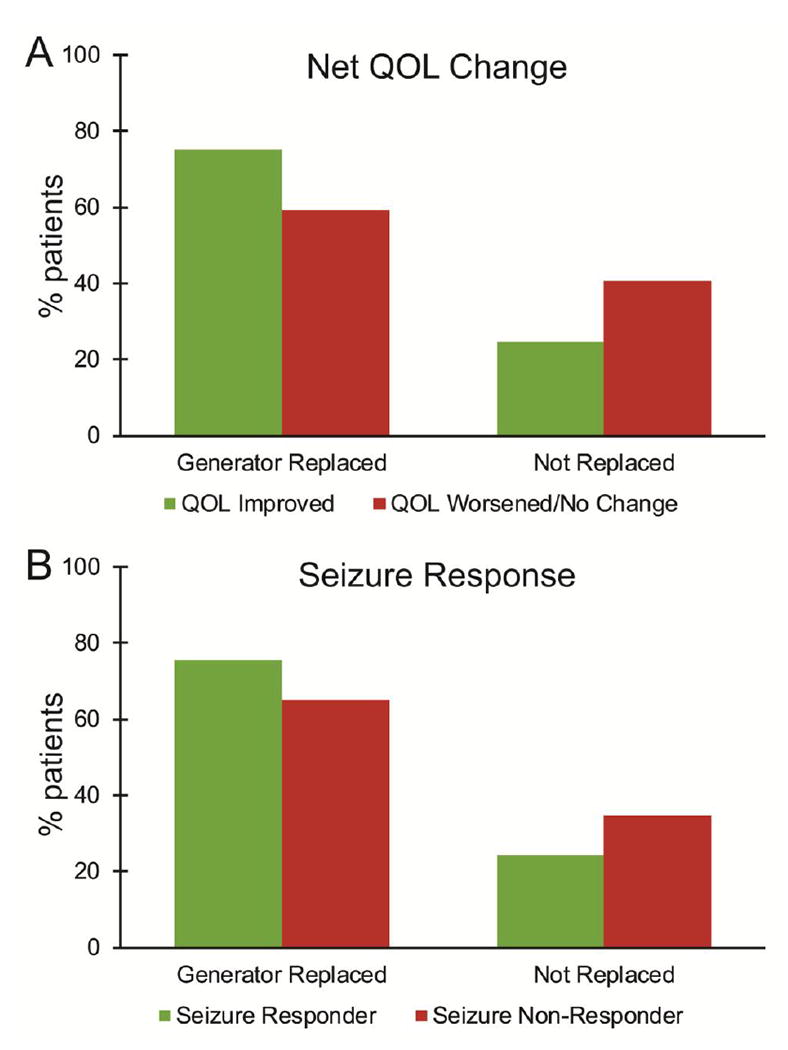
Overall, 71.4% of patients underwent VNS generator replacement, suggesting perceived benefit from continued therapy in these individuals. A) Patients with net improvement in QOL metrics were significantly more likely to undergo VNS generator replacement than those with worsened or unchanged QOL metrics (χ2 = 68.9, p < 0.001, Bonferroni corrected). B) Individuals who were seizure responders (≥ 50% reduction in seizure frequency) were also significantly more likely to undergo generator replacement than non-responders (χ2 = 40.0, p < 0.001, Bonferroni corrected). N = 3,085 patients with generator replacement data. QOL, quality of life; VNS, vagus nerve stimulation.
4. Discussion
Here we report the largest study of QOL metrics in VNS therapy, including data from over 5,000 patients, including more than 3,000 individuals with ≥ 12 months post-operative follow-up. Compared to their pre-operative assessments, VNS patients were rated as improved in several QOL-related metrics, including alertness, post-ictal state, cluster seizures, mood, and other variables. It is important to note that QOL metrics in this study were subjectively reported by physicians, and not based on standardized QOL testing. Therefore, QOL outcome metrics may be biased by a physician’s desire to see improvement in a patient’s status, which may lead to overestimation of favorable outcomes. This may have influenced the high rates of improvement reported in the “physician global assessment” (80-85%) which exceeded rates of improvement reported across individual QOL metrics (29-63%). Nevertheless, we did observe a significant relationship between provider QOL metrics and seizure outcomes in this study, which is consistent with previous studies linking seizure status to QOL in epilepsy [9-11]. Furthermore, patients with improvement in QOL metrics were significantly more likely to undergo generator replacement than those without positive QOL changes – a somewhat more objective indicator of satisfaction with therapy. Overall, it is useful to interpret out results in the context of previous smaller studies of QOL with VNS, including those with standardized QOL evaluations.
While the majority of previous VNS studies in epilepsy have focused on seizure frequency, the largest study addressing QOL was the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial [20]. This trial measured Quality of Life in Epilepsy Inventory-89 (QOLIE-89) scores in 112 adults receiving VNS vs. best medical therapy alone for drug resistant epilepsy. Overall, significantly improved QOLIE-89 scores were observed with VNS, but the trial was terminated prematurely due to recruitment difficulties. Klinkenberg and colleagues also examined QOLIE-89 scores before and 6 months after VNS in 41 adults with epilepsy, observing significant improvements in mood and overall QOL with stimulation [21]. In pediatric patients with epilepsy, a few small VNS studies have reported improved alertness or social interactions based on retrospective chart review [22, 23] or neuropsychological evaluation [24]. One investigation indirectly inferred increased quality-adjusted life years with VNS based on reduced hospitalizations recorded in U.S. Medicaid data [25]. Surveys in children with epilepsy based on subjective family member impressions of QOL have been less consistent, with some showing improvement[26] and others no change with VNS therapy [27, 28]. Overall, our observation of improved QOL metrics with VNS in drug-resistant epilepsy is consistent with the majority of prior reports.
It is important to note that the single most important predictor of QOL in epilepsy is complete seizure freedom [29, 30]. Complete freedom from seizures is significantly more likely with resective or ablative epilepsy surgery, reported in 50-80% of surgical cases [31], than with VNS, observed in approximately 8% of patients after > 24 months of therapy [19]. With VNS, approximately 50-60% of epilepsy patients achieve ≥ 50% reduction in seizure frequency after 12-24 months of treatment [16], and similar seizure outcomes have been reported with other neurostimulation therapies for epilepsy [13]. Thus, seizure freedom must be the ultimate goal in epilepsy treatment whenever possible, but VNS and other neuromodulation-based treatments represent additional treatment options for patients who are not candidates for or who refuse resection or ablation.
In comparing trends and predictors of QOL improvement vs. seizure response in VNS, we noted both differences and similarities. First, while there is a progressive improvement in seizure reduction with VNS during the first few years of therapy, we did not observe a similar trend in QOL metrics over time. Furthermore, while greater QOL improvement was observed in patients of female gender or Caucasian ethnicity in this study, these are not typical predictors of seizure response in VNS studies. However, a predominantly generalized seizure type predicted favorable QOL outcome in the present study, and has also been shown to predict a better seizure outcome in prior VNS studies [16, 19]. This is an important consideration, as patients with primary generalized epilepsy are unlikely to be candidates for surgical resection or ablation. Earlier implantation was also a positive predictor of QOL metrics in this study, and several previous investigations have suggested favorable seizure outcomes with early surgical intervention for drug resistant epilepsy, including studies of VNS [32, 33] and resection [2, 5]. Consistent with established recommendations, epilepsy patients who have failed ≥ 2 well-tolerated AED trials should be referred to a comprehensive epilepsy center for multidisciplinary evaluation and surgical consideration, due to the deleterious effects of persistent seizures on patient QOL and elevated risk of mortality [34, 35].
In addition to subjectivity and potential bias in our results discussed above, there are other limitations to this study that should be mentioned. Notably, registry data were collected from a database sponsored by the manufacturer of VNS therapy, and conflict of interest must be considered in the interpretation of our findings. Furthermore, as participation in the study was voluntary and not controlled, this may lead to bias related to which providers elected to participate. Next, data in the registry include only a subset of patients implanted during that time, and patient attrition in the registry leads to a smaller sample size at later follow-up periods, which may lead to further bias at later follow-up periods. However, the integrity of collecting and processing data in the registry has been previously authenticated by an independent auditing agency [18], and registry outcomes have been shown to resemble outcomes in the literature [19]. Further prospective investigation is warranted to assess changes and predictors of QOL in VNS therapy, ideally with a large-scale independent study including standardized QOL tests administered before and after treatment. Importantly, however, while registry-based studies provide data of significantly lower quality than randomized-controlled trials, the strength of this evaluation lies in the ability to pool a very large number of patients that would be difficult to achieve even in a multi-institutional trial.
Conclusions
In a large registry-based study of QOL in over 5,000 epilepsy patients treated with VNS, we observed improvement in various metrics subjectively reported by treating physicians, including alertness, mood, and post-ictal state. Improvement in QOL metrics was significantly related to more favorable seizure outcome, and was associated with higher rates of VNS generator replacement. A large, prospective study using standardized measurements is needed to further evaluate QOL outcomes with VNS and other neuromodulation-based therapies. Given the deleterious effects of recurrent seizures on QOL in epilepsy, improved QOL metrics represent an important treatment goal in this disorder.
Highlights.
- VNS for epilepsy is associated with improved QOL metrics, as subjectively rated by physicians
- Patients with greater QOL improvement are more likely to have favorable seizure outcomes
- Generator replacement is more common in patients with greater improvement in QOL metrics
Acknowledgments
This work was supported in part by NIH K99 NS097618 (DJE).
Footnotes
Disclosures of conflicts of interest: KH in an employee of Lilanova, the manufacturer of VNS therapy and sponsor of the VNS therapy Patient Outcome Registry. The other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37:389–404. doi: 10.1007/s10143-014-0527-9. discussion 404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jette N, Engel J., Jr Refractory epilepsy is a life-threatening disease: Lest we forget. Neurology. 2016 doi: 10.1212/WNL.0000000000002707. [DOI] [PubMed] [Google Scholar]
- 4.Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl 2):96–8. doi: 10.1111/j.1528-1167.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englot DJ, Raygor KP, Molinaro AM, Garcia PA, Knowlton RC, Auguste KI, Chang EF. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648–56. doi: 10.1227/NEU.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englot DJ, Han SJ, Rolston JD, Ivan ME, Kuperman RA, Chang EF, Gupta N, Sullivan JE, Auguste KI. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014;14:386–95. doi: 10.3171/2014.7.PEDS13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. 2016 doi: 10.1212/WNL.0000000000002700. [DOI] [PubMed] [Google Scholar]
- 9.Choi H, Sell RL, Lenert L, Muennig P, Goodman RR, Gilliam FG, Wong JB. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300:2497–505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 10.Wachi M, Tomikawa M, Fukuda M, Kameyama S, Kasahara K, Sasagawa M, Shirane S, Kanazawa O, Yoshino M, Aoki S, Sohma Y. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001;42(Suppl 6):4–8. [PubMed] [Google Scholar]
- 11.Westerveld M, Sass KJ, Chelune GJ, Hermann BP, Barr WB, Loring DW, Strauss E, Trenerry MR, Perrine K, Spencer DD. Temporal lobectomy in children: cognitive outcome. J Neurosurg. 2000;92:24–30. doi: 10.3171/jns.2000.92.1.0024. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Jr, Wiebe S. Who is a surgical candidate? Handb Clin Neurol. 2012;108:821–8. doi: 10.1016/B978-0-444-52899-5.00030-7. [DOI] [PubMed] [Google Scholar]
- 13.Rolston JD, Englot DJ, Wang DD, Shih T, Chang EF. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus. 2012;32:E14. doi: 10.3171/2012.1.FOCUS11335. [DOI] [PubMed] [Google Scholar]
- 14.Chang EF, Englot DJ, Vadera S. Minimally invasive surgical approaches for temporal lobe epilepsy. Epilepsy Behav. 2015;47:24–33. doi: 10.1016/j.yebeh.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurg Clin N Am. 2011;22:443–8. v. doi: 10.1016/j.nec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 17.Amar AP, DeGiorgio CM, Tarver WB, Apuzzo ML. Long-term multicenter experience with vagus nerve stimulation for intractable partial seizures: results of the XE5 trial. Stereotact Funct Neurosurg. 1999;73:104–8. doi: 10.1159/000029764. [DOI] [PubMed] [Google Scholar]
- 18.Amar AP, Apuzzo ML, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004;55:1086–93. doi: 10.1227/01.neu.0000141073.08427.76. [DOI] [PubMed] [Google Scholar]
- 19.Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and Predictors of Seizure Freedom With Vagus Nerve Stimulation for Intractable Epilepsy. Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, Zamponi N, Aguglia U, Wagner L, Minotti L, Stefan H, Boon P, Sadler M, Benna P, Raman P, Perucca E. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014;55:893–900. doi: 10.1111/epi.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinkenberg S, Majoie HJ, van der Heijden MM, Rijkers K, Leenen L, Aldenkamp AP. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clin Neurol Neurosurg. 2011 doi: 10.1016/j.clineuro.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, Griens R, Vallee L, Boon P, Rittey C, Jayewardene AK, Bunker M, Arzimanoglou A, Lagae L. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014;55:1576–84. doi: 10.1111/epi.12762. [DOI] [PubMed] [Google Scholar]
- 23.Mikati MA, Ataya NF, El-Ferezli JC, Baghdadi TS, Turkmani AH, Comair YG, Kansagra S, Najjar MW. Quality of life after vagal nerve stimulator insertion. Epileptic Disord. 2009;11:67–74. doi: 10.1684/epd.2009.0244. [DOI] [PubMed] [Google Scholar]
- 24.Hallbook T, Lundgren J, Stjernqvist K, Blennow G, Stromblad LG, Rosen I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure. 2005;14:504–13. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Helmers SL, Duh MS, Guerin A, Sarda SP, Samuelson TM, Bunker MT, Olin BD, Jackson SD, Faught E. Clinical outcomes, quality of life, and costs associated with implantation of vagus nerve stimulation therapy in pediatric patients with drug-resistant epilepsy. Eur J Paediatr Neurol. 2012;16:449–58. doi: 10.1016/j.ejpn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Ulate-Campos A, Cean-Cabrera L, Petanas-Argemi J, Garcia-Fructuoso G, Aparicio J, Lopez-Sala A, Palacio-Navarro A, Mas MJ, Muchart J, Rebollo M, Sanmarti FX. Vagus nerve stimulator implantation for epilepsy in a paediatric hospital: outcomes and effect on quality of life. Neurologia. 2015;30:465–71. doi: 10.1016/j.nrl.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 27.McGlone J, Valdivia I, Penner M, Williams J, Sadler RM, Clarke DB. Quality of life and memory after vagus nerve stimulator implantation for epilepsy. Can J Neurol Sci. 2008;35:287–96. doi: 10.1017/s0317167100008854. [DOI] [PubMed] [Google Scholar]
- 28.Sherman EM, Connolly MB, Slick DJ, Eyrl KL, Steinbok P, Farrell K. Quality of life and seizure outcome after vagus nerve stimulation in children with intractable epilepsy. J Child Neurol. 2008;23:991–8. doi: 10.1177/0883073808315417. [DOI] [PubMed] [Google Scholar]
- 29.Elliott I, Kadis DS, Lach L, Olds J, McCleary L, Whiting S, Snyder T, Smith ML. Quality of life in young adults who underwent resective surgery for epilepsy in childhood. Epilepsia. 2012;53:1577–86. doi: 10.1111/j.1528-1167.2012.03594.x. [DOI] [PubMed] [Google Scholar]
- 30.Macrodimitris S, Sherman EM, Williams TS, Bigras C, Wiebe S. Measuring patient satisfaction following epilepsy surgery. Epilepsia. 2011;52:1409–17. doi: 10.1111/j.1528-1167.2011.03160.x. [DOI] [PubMed] [Google Scholar]
- 31.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 32.Ghaemi K, Elsharkawy AE, Schulz R, Hoppe M, Polster T, Pannek H, Ebner A. Vagus nerve stimulation: outcome and predictors of seizure freedom in long-term follow-up. Seizure. 2010;19:264–8. doi: 10.1016/j.seizure.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Renfroe JB, Wheless JW. Earlier use of adjunctive vagus nerve stimulation therapy for refractory epilepsy. Neurology. 2002;59:S26–30. doi: 10.1212/wnl.59.6_suppl_4.s26. [DOI] [PubMed] [Google Scholar]
- 34.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 35.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, Guerrini R, Mathern GW International League against Epilepsy SfPES, Commissions of N, Paediatrics. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–9. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]


