Abstract
We report 9 consecutive percutaneous image-guided cryoablation procedures of head and neck tumors in 7 patients (4 males, 3 females; mean age 68 years, range 50-78). Entire tumor ablation for local control or regional ablation for pain relief or functional status preservation was achieved in 8 of 9 procedures. One patient experienced intraprocedural bradycardia while another developed a neopharyngeal abscess. There were no deaths, permanent neurological or functional deficits, vascular complications, or adverse cosmetic sequelae.
Introduction
Salvage head and neck surgery results in complications ranging from 13% to 40%, with 2.7% mortality, and with a reported 16% major complication rate in a relatively typical sample (1). Due to these complication rates, there has been much recent active research into alternative, less invasive, new potential treatment options for recurrent and metastatic head and neck cancer including superselective intra-arterial chemotherapy infusion (2–4) as well as re-irradiation with protons (5,6), carbon ions (6), charged particle beams (7), and stereotactic body radiotherapy (8). Superselective intra-arterial chemotherapy has not demonstrated superiority to standard intravenous chemotherapy (4) while long-term follow-up will be needed for the radiation studies to evaluate associated late toxicities, which lead to speech and swallowing impairment in 43% of patients receiving traditional radiation therapy (9).
Percutaneous image-guided thermal ablation also offers a potentially less morbid minimally-invasive treatment option. While CT-guided and MRI-guided percutaneous aspiration of head and neck tumors is well documented (10,11), the literature of head and neck ablation is sparse, limited primarily to case reports, and limited primarily to CT- and US-guided radiofrequency ablation (for example 12–15) with a few reported successful CT-guided cryoablation and MRI-guided laser-induced thermotherapy procedures (16,17). None of these techniques visualize the precise margins of ablation. We have adopted a technique of percutaneous cryoablation utilizing CT, PET, or MRI-guidance to treat head and neck tumors with precise cryoprobe positioning and near real-time monitoring of the margins of the cryoablation ice ball to ensure adequate coverage of the target while avoiding injury to adjacent structures.
Materials and Methods
We retrospectively reviewed 9 consecutive percutaneous image-guided cryoablation procedures of head and neck tumors performed at our institution by a single interventional neuroradiologist between May 2013 and July 2015. These procedures were performed on 7 patients (4 males, 3 females) with a mean age of 68 years (range, 50-78). Patient demographics and tumor information are presented in Table 1. All patients were evaluated by a surgeon and each patient was either a poor surgical candidate or declined surgery. Standard procedural informed consent was obtained from each patient prior to the procedure, with thorough explanation of the procedure and explanation of the potential risks, benefits, and alternatives. This retrospective review was approved by our institutional review board and performed in compliance with HIPAA. Informed consent for participation in the retrospective review was waived.
TABLE 1.
Demographics of Head and Neck Ablations in Chronological Order
| Patient # | Age | Gender | Location | Size (mm) | Pathology | Procedure Goal |
|---|---|---|---|---|---|---|
| 1 | 76 | M | Right neck level 3 lymph node | 19 | Metastasis – Squamous Cell Carcinoma | Complete ablation |
| 1 | 78 | M | Right neck level 2 and 3 lymph nodes | 20, 17 | Metastasis – Squamous Cell Carcinoma | Complete ablation |
| 2 | 73 | F | Right neck retropharyngeal lymph node | 18 | Metastasis – Squamous Cell Carcinoma | Partial ablation for local control |
| 3 | 75 | M | Neopharynx | 37 | Recurrence – Squamous Cell Carcinoma | Partial ablation to prevent airway occlusion |
| 4 | 59 | M | Left neck, extensive and ill-defined with focal area encasing and narrowing the carotid and vertebral arteries | 26 | Metastasis – Squamous Cell Carcinoma | Partial ablation to minimize mass effect |
| 5 | 50 | M | Left parapharyngeal | 19 | Metastasis – Adenoid Cystic Carcinoma | Complete ablation |
| 2 | 74 | F | Right neck retropharyngeal lymph node | 14 | Metastasis – Squamous Cell Carcinoma | Partial ablation for local control |
| 6 | 65 | F | Right foramen ovale | 27 | Metastasis – Adenoid Cystic Carcinoma | Partial ablation for pain relief |
| 7 | 61 | F | Right trigeminal nerve/foramen ovale/pterygoid muscles | 37 | Metastasis – Squamous Cell Carcinoma | Partial ablation for pain relief |
Imaging guidance modality, imaging techniques, and the reasons for choosing the modality were recorded for each patient. Similarly, cryoablation technical parameters were reviewed and recorded for each patient.
The initial technical objective was either cryoablation of an entire tumor for local control or cryoablation of a pre-determined partial tumor region for pain relief or preservation of functional status. As this was not a prospective study, there were no formal measures taken to determine level of pain relief or level of functional status. Pain relief was reported subjectively by patients in follow-up clinical encounters. Functional status deficits were reported subjectively by patients and were evaluated with physical neurological exam in follow-up clinical encounters. For purposes of this review, a procedure was considered technically successful if the ablation zone included the targeted tumor volume on intraprocedural imaging.
All follow-up imaging examinations and clinic notes were reviewed to establish outcomes to the time of writing.
Results
All cryoablation procedures were performed in a dedicated image-guided operating room or interventional suite with either a 3-Tesla MAGNETOM Verio MRI System (Siemens Medical Solutions, Malvern, PA), Biograph mCT 64 PET-CT System (Siemens Medical Solutions, Malvern, PA), or SOMATOM Sensation 64 Fluoroscopy CT System (Siemens Medical Solutions, Malvern, PA). Rationale for choice of guidance modality is outlined in Table 2 for each patient.
TABLE 2.
Methods of Head and Neck Ablations in Chronological Order
| Patient # | Guidance Modality | Reasons for Choice of Guidance Modality | Cryoprobe Type/Position | Ablation Times in minutes and Cryoprobe Flow Rates | Adjacent Major Structures Within 1mm |
|---|---|---|---|---|---|
| 1 | CT | PET-CT not yet utilized as guidance modality at our institution | IceSphere/middle aspect of mass IceSphere/inferior aspect of mass IceSeed/superior aspect of mass |
15 freeze / 10 thaw / 10 freeze − 20% / 0% / 20% − 20% / 0% / 20% − 50% / 0% / 100% |
carotid artery, jugular vein, vagus nerve |
| 1 | PET-CT | Confirm small target, ensure ice ball covers metabolically active region | IceSphere x2 | 15 freeze / 10 thaw / 10 freeze − 100% / 0% / 100% |
carotid artery, jugular vein, vagus nerve |
| 2 | MRI | Deep location with many surrounding structures requiring precision placement | IceSeed x2 | 15 freeze / 10 thaw / 15 freeze - 100% / 0% / 100% |
carotid artery, jugular vein, vagus nerve |
| 3 | PET-CT | Confirm ill-defined target, ensure ice ball covers metabolically active region | IceSphere x4 IceSphere/left aspect not in ice ball |
15 freeze / 10 thaw / 15 freeze -100% / 0% / 100% -100% / 0% / 100% |
airway stoma, neopharyngeal mucosa |
| 4 | PET-CT | Confirm ill-defined target, ensure ice ball covers metabolically active region | IceSeed x2/hypermetabolic region adjacent to vasculature | 11 freeze / 4 freeze / 10 thaw/ 11 freeze / 4 freeze − 100% / 40% / 0% / 100% / 40% |
vertebral artery, carotid artery, jugular vein, vagus nerve |
| 5 | PET-CT | Confirm small target, ensure ice ball covers metabolically active region | IceSphere/center ofmass | 15 freeze / 10 thaw / 15 freeze − 100% / 0% / 100% |
carotid artery, facial artery, esophagus |
| 2 | PET-CT | Deep location with many surrounding structures requiring precision placement | IceSeed/center of hyper metabolic region | Not performed | carotid artery, jugular vein, vagus nerve |
| 6 | MRI | Deep location with many surrounding structures requiring precision placement | IceRod/center ofmass | 15 freeze / 10 thaw / 15 freeze − 100% / 0% / 100% |
right temporal lobe, cavernous carotid artery |
| 7 | MRI | Deep location with many surrounding structures requiring precision placement | IceSeed/center of mass, submandibular approach | 15 freeze / 10 thaw / 6 freeze / 4 freeze / 5 freeze − 60% / 0% / 60% / 80% / 100% |
right temporal lobe, cavernous carotid artery |
All CT examinations were performed in the axial plane. Sagittal, coronal, or oblique reconstructions were generated at the scanner whenever necessary to optimize cryoprobe or ice ball visualization. All MRI examinations were performed with a turbo spin-echo (“TSE”) T2-weighted sequence (TR: 5,820; TE: 103; slice thickness: 2 mm; slice spacing 2 mm). MRI plane of imaging was chosen to optimize visualization of the tumor, cryoprobe, and ice ball relative to the adjacent vascular and neural structures and mucosa. Intraprocedural monitoring MRI and/or CT imaging was performed at approximately 3 minute intervals.
Cryoablation was performed with the following equipment: 17-gauge IceSphere, IceSeed, and IceRod cryoprobes (Galil Medical, Minneapolis, MN) with the SeedNet cryoablation system (Galil Medical, Minneapolis, MN). Specific cryoprobe types, position, and power settings used for each patient are outlined in Table 2. An anesthesiologist administered general endotracheal anesthesia for all cases. Prophylactic antibiotics were not administered. Skin at the entry site was typically kept warm with warm saline soaked gauze or sponges. The guidance modalities and major structures located within 1 mm are outlined in Table 2 for each procedure.
Complications, technical success, and outcomes are succinctly outlined in Table 3. Patient 1 experienced minimal hoarseness and dysphagia that resolved over several weeks following cryoablation of a 19 mm level III lymph node metastasis that was immediately adjacent to the vagus nerve, likely due to vagal nerve stunning. Patient 2 experienced transient bradycardia from 80 bpm to 40 bpm with cryoprobe placement into a right retropharyngeal lymph node metastasis with subsequent immediate bradycardia to 40 bpm and severe hypotension upon commencement of cryoablation. The procedure was discontinued; heart rate and blood pressure immediately returned to normal. Patient 3 developed an abscess in the region of ablation and required surgical debridement approximately 1 week following cryoablation of a 37 mm neopharyngeal recurrent tumor, which was causing increased mass effect on an airway stoma and which involved neopharyngeal mucosa, with no residual tumor found.
TABLE 3.
Results of Head and Neck Ablations in Chronological Order
| Patient # | Complications | Technical Success* | Follow-up Imaging Length | Follow-up Imaging Modality | Follow-up Clinical Length | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Minimal hoarseness and dysphagia; resolved over several weeks | Yes | 18 months | CT | 18 months | Imaging: No FDG-avid recurrence at the ablation site; increasing size of right level 2 and 3 lymph nodes Clinical: No symptoms |
| 1 | None | Yes | 4 months | CT | 5 months | Imaging: Involution of both lesions Clinical: Death reportedly from “natural causes” |
| 2 | None | Yes | 3 months | PET-CT | 3 months | Imaging: Enlarging residual/recurrent disease Clinical: No symptoms, plan for second ablation |
| 3 | Post-procedure abscess requiring surgical debridement | Yes | - | - | 1 week | Imaging: None available. Clinical: No residual viable tumor in the ablation bed per outside hospital surgical report |
| 4 | None | Yes | 3 months | CT | 5 months | Imaging: Necrosis in ablation bed with patent vessels; increased bulk of residual tumor peripheral to the ablation zone Clinical: Deceased while on hospice |
| 5 | None | Yes | 1 month | CT (report only) | 3 months | Imaging: New nasopharyngeal lesion, no mention of ablation areas. Clinical: Completed additional radiation therapy, no symptoms |
| 2 | Bradycardia with severe hypotension; resolved with discontinuation of the procedure | No | - | - | 6 months | Imaging: No further imaging performed Clinical: Home hospice; No neck pain and no neck or neurological symptoms |
| 6 | None | Yes | 3 months | MRI | 3 months | Imaging: Residual 1.1 cm lobulation along lateral edge of ablation zone Clinical: Complete reported subjective pain relief, plan for ablation of residual tumor |
| 7 | None | Yes | 1 month | CT | 2 months | Imaging: Regions of necrosis in the ablation zone but increased leftward extent of non-ablation portion of mass Clinical: Partial reported subjective pain relief with slow subsequent return to baseline pain over approximately 2 months |
Technical success determined by whether the ablation zone included the targeted tumor volume on intraprocedural imaging. Outcomes do not all correlate with technical success of the procedure.
Technical success was achieved in 8 of 9 procedures. The only procedure in which technical success was not achieved was in the discontinued second ablation of Patient 2 due to bradycardia and hypotension.
Patient 5 was lost to follow-up. Good imaging and/or clinical outcomes were achieved in 5 of the 7 other technically successful procedures. Patient 2 had increasing disease following the first ablation. Patient 7 experienced only partial pain relief with slow subsequent return to baseline following partial cryoablation of a 37 mm lobulated mass focused over the foramen ovale, likely due to tumor infiltrating superior to the foramen ovale into Meckel's cave, beyond the zone of ablation.
Discussion
Percutaneous image-guided cryoablation offers a potentially less morbid minimally-invasive treatment option compared to salvage head and neck surgery, which has complication rates of 13% to 40% (1), potentially fewer long-term side effects compared to radiation therapy, which results in a 43% rate of impaired speech and swallowing (9), and potentially fewer severe complications as reported with heat-based ablative techniques, including carotid blowout, stroke, and death (18,19) due to superior visualization of the region of ablation using the ice ball as an ablation zone proxy and due to the inherent protection of vascular structures and spinal cord by warm flow of blood and cerebrospinal fluid. For these reasons, at our institution we are currently using cryoablation as the exclusive thermal ablation modality to treat head, neck, and spine tumors, which are typically adjacent or close to critical central nervous system and vascular structures.
There were two complications in this study cohort. First, a patient with a right retropharyngeal mass encasing the carotid sheath developed bradycardia and hypotension resulting in discontinuation of the procedure. Multiple other tumors adjacent to the vagus nerve were ablated without similar complication, likely due to the protective heat sink effects of carotid and jugular flow. In this symptomatic patient, the jugular vein was thrombosed. Second, a patient with a recurrent tumor of the neopharynx developed an abscess approximately 1 week post-ablation and required surgical debridement. The neomucosa involved by the recurrent tumor was likely devascularized during the ablation and the patient was asymptomatic in the first week postoperatively since the the neopharynx was not innervated. Post-ablation infection in the head and neck has previously been reported (20). In the future we plan to administer antibiotics covering oral flora to patients with mucosal involvement. Hydrodissection of adjacent but uninvolved mucosa or mucosal protection with a saline-filled balloon may also be helpful in future studies. We postulate that use of cryoablation, particularly with intraprocedural MRI monitoring, should make severe complications such as carotid blowout and stroke less likely since structures such as the carotid artery and CSF-filled thecal sac have warm flow that provides protection from freezing and because the ice ball margins can be precisely visualized in near real time.
Choice of imaging modality is based largely on three factors: visibility, depth, and presence of complex critical adjacent anatomy along the planned cryoprobe trajectory. In our experience, PET-CT is a valuable tool to identify small or ill-defined target tumors that are not visible in postoperative regions on MRI but that are distinctly hypermetabolic on PET-CT (Figure 1). When the lesion is visible by MRI and the patient has no contraindication to MRI, MRI is preferred since it more clearly shows the ice ball as a black zone as opposed to a subtle gray zone on PET-CT. MRI is also superior for visualizing adjacent nerves and vessel flow-voids without need for contrast (Figures 2 and 3).
Figure 1.
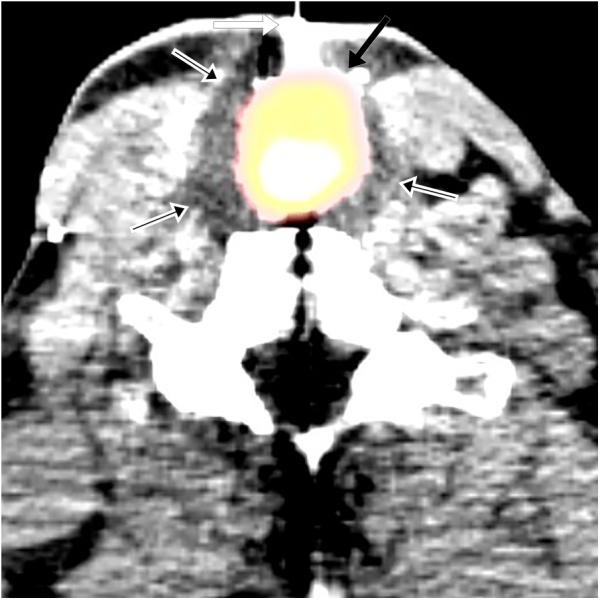
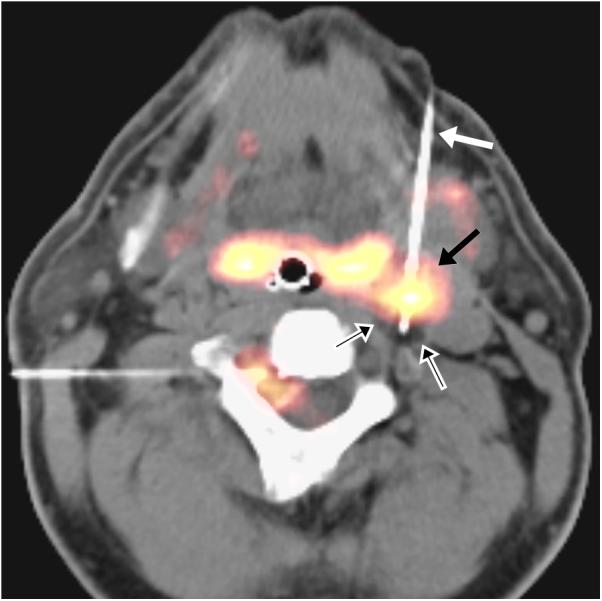
(a) 75 year-old male with recurrent squamous cell carcinoma of the neopharynx. Intraprocedural axial fused PET-CT image demonstrates cryoprobe (white arrow) within FDG-avid lesion (black arrow) with ice ball encompassing FDG-avid lesion (black arrows with white outlines) and the entire neopharynx. (b) 50 year-old male with adenoid cystic carcinoma metastases. Intraprocedural axial fused PET-CT image demonstrates cryoprobe (white arrow) within the hypermetabolic lesion (black arrow) with faintly visible ice ball (black arrows with white outlines) encompassing the lesion. A second cryoprobe is demonstrated in a right vertebral foramen metastasis, which was simultaneously ablated.
Figure 2.
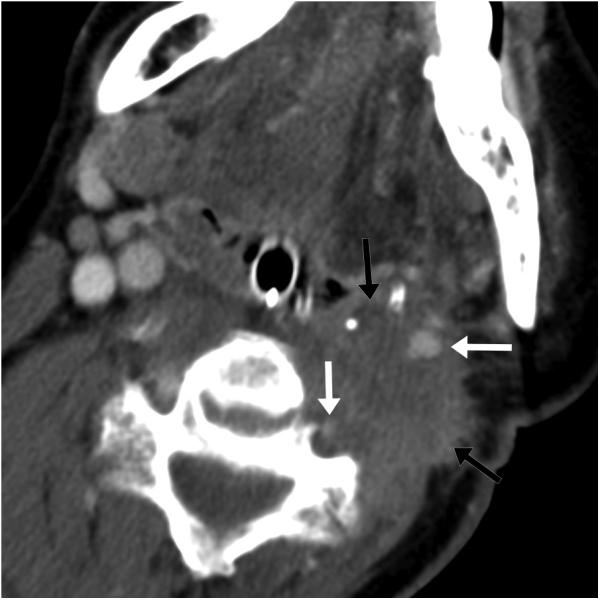
59 year-old male with squamous cell carcinoma metastases. (a) Preprocedure contrast-enhanced axial CT image demonstrates an ill-defined lesion (black arrows) involving the carotid and vertebral arteries (white arrows). (b) Intraprocedural axial TSE T2-weighted MR image demonstrates ice ball (black arrows with white outlines) adjacent to carotid and vertebral arteries (white arrows) with deformed ice ball contour due to flow-related heat sink effect and with preserved flow voids and mild surrounding edema; warm saline soaked gauze overlies the skin.
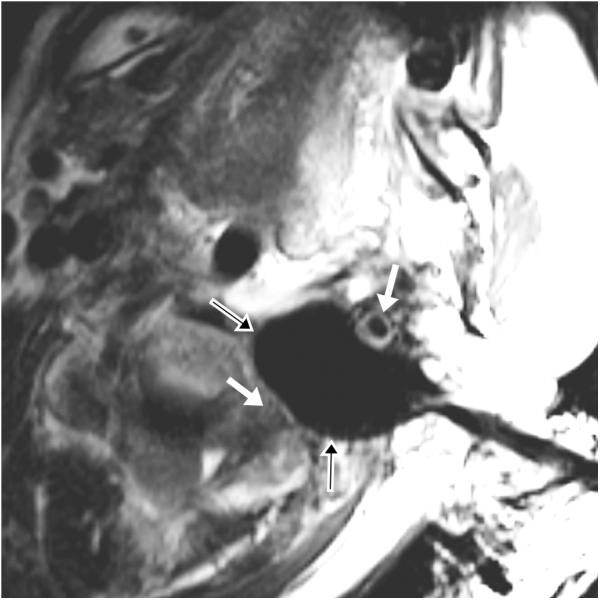
Figure 3.
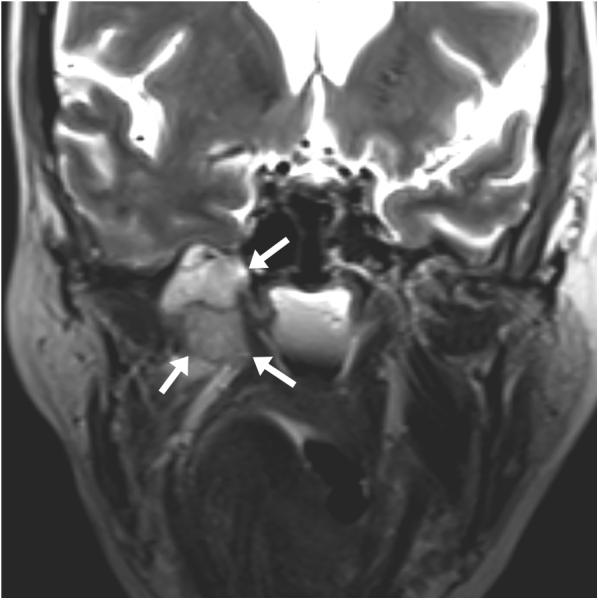
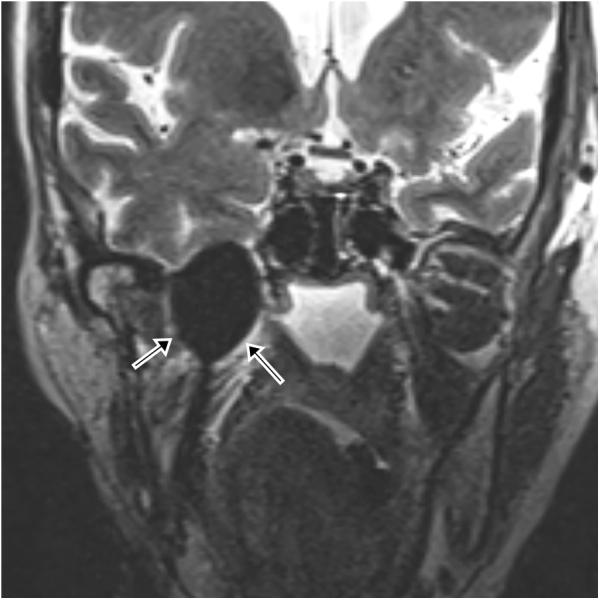
65 year-old female with metastatic adenoid cystic carcinoma metastasis causing severe right-sided facial pain. (a) Preprocedure and (b) intraprocedural coronal TSE T2-weighted MR images demonstrate a mass (white arrows) and a cryoprobe with ice ball (black arrows with white outlines) covering the entire mass and extending to the right temporal lobe. The ice ball was also adjacent to the right cavernous carotid artery several millimeters posterior to this plane.
The primary limitations of this study are the small number of treated patients and short-term follow-up. Larger studies and more operator experience will be required to establish complication rates and safety profile.
In summary, this report describes the technical success, complications, and outcomes of 9 consecutive percutaneous image-guided cryoablation procedures of head and neck tumors. Guidance and monitoring modalities were chosen on a case-by-case basis based upon tumor visibility and location. The technical objective was either cryoablation of entire tumor for local control or cryoablation of a pre-determined partial tumor region for goal of pain relief or preservation of functional status. Technical success was achieved in 8 of 9 procedures. There were no deaths, permanent neurological or functional deficits, vascular complications, or adverse cosmetic sequelae. The complications that we encountered may be largely avoidable with increased experience. Further work is needed to continue improving the safety and efficacy and to continue expanding the use of cryoablation for non-operative head and neck tumor patients.
Acknowledgments
Grant Support: The Advanced Multimodality Image Guided Operating Suite was supported in part through NIH grant P41 EB015898.
The study was approved by our institutional review board and performed in compliance with HIPAA. The need for patient informed consent was waived.
Footnotes
Disclosures: None
Not previously presented at or submitted to any academic meeting.
References
- 1.Temam S, Koka V, Mamelle G, Julieron M, Carmantrant R, Marandas P, et al. Treatment of the N0 neck during salvage surgery after radiotherapy of head and neck squamous cell carcinoma. Head Neck. 2005 Aug;27(8):653–8. doi: 10.1002/hed.20234. [DOI] [PubMed] [Google Scholar]
- 2.Homma A, Onimaru R, Matsuura K, Robbins KT, Fujii M. Intra-arterial chemoradiotherapy for head and neck cancer. Jpn J Clin Oncol. 2016 Jan;46(1):4–12. doi: 10.1093/jjco/hyv151. [DOI] [PubMed] [Google Scholar]
- 3.Fuwa N, Ito Y, Matsumoto A, Kamata M, Kodaira T, Furutani K, et al. A combination therapy of continuous superselective intraarterial carboplatin infusion and radiation therapy for locally advanced head and neck carcinoma. Phase I study. Cancer. 2000 Nov 15;89(10):2099–105. [PubMed] [Google Scholar]
- 4.Robbins KT, Homma A. Intra-arterial chemotherapy for head and neck cancer: experiences from three continents. Surg Oncol Clin N Am. 2008 Oct;17(4):919–933. xi. doi: 10.1016/j.soc.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys. 2016 May 1;95(1):386–95. doi: 10.1016/j.ijrobp.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto K, Demizu Y, Hashimoto N, Mima M, Terashima K, Fujii O, et al. Particle radiotherapy using protons or carbon ions for unresectable locally advanced head and neck cancers with skull base invasion. Jpn J Clin Oncol. 2014 May;44(5):428–34. doi: 10.1093/jjco/hyu010. [DOI] [PubMed] [Google Scholar]
- 7.Jensen AD, Nikoghosyan A, Ellerbrock M, Ecker S, Debus J, Münter MW. Reirradiation with scanned charged particle beams in recurrent tumours of the head and neck: acute toxicity and feasibility. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011 Dec;101(3):383–7. doi: 10.1016/j.radonc.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Strom T, Wishka C, Caudell JJ. Stereotactic Body Radiotherapy for Recurrent Unresectable Head and Neck Cancers. Cancer Control J Moffitt Cancer Cent. 2016 Jan;23(1):6–11. doi: 10.1177/107327481602300103. [DOI] [PubMed] [Google Scholar]
- 9.Denaro N, Russi EG, Adamo V, Merlano MC. State-of-the-art and emerging treatment options in the management of head and neck cancer: news from 2013. Oncology. 2014;86(4):212–29. doi: 10.1159/000357712. [DOI] [PubMed] [Google Scholar]
- 10.Sherman PM, Yousem DM, Loevner LA. CT-guided aspirations in the head and neck: Assessment of the first 216 cases. Am J Neuroradiol. 2004 Oct;25(9):1603–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Lü Y, Liu M, Li C, Wu L, Fritz J. MRI-guided biopsy and aspiration in the head and neck: evaluation of 77 patients. Eur Radiol. 2012 Feb;22(2):404–10. doi: 10.1007/s00330-011-2270-8. [DOI] [PubMed] [Google Scholar]
- 12.Bui QT, Dupuy DE. Percutaneous CT-guided radiofrequency ablation of an adenoid cystic carcinoma of the head and neck. AJR Am J Roentgenol. 2002 Nov;179(5):1333–5. doi: 10.2214/ajr.179.5.1791333. [DOI] [PubMed] [Google Scholar]
- 13.Schirmang TC, Davis LM, Nigri PT, Dupuy DE. Solitary fibrous tumor of the buccal space: Treatment with percutaneous cryoablation. Am J Neuroradiol. 2007 Oct;28(9):1728–30. doi: 10.3174/ajnr.A0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenette JP, Monchik JM, Dupuy DE. Image-guided Ablation of Postsurgical Locoregional Recurrence of Biopsy-proven Well-differentiated Thyroid Carcinoma. J Vasc Interv Radiol. 2013;24(5):672–9. doi: 10.1016/j.jvir.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Belfiore MP, Sciandra M, Romano F, Tartaglione T, De Lucia G, Della Volpe T, et al. Preliminary Results in Unresectable Head and Neck Cancer Treated by Radiofrequency and Microwave Ablation: Feasibility, Efficacy, and Safety. J Vasc Interv Radiol JVIR. 2015 Aug;26(8):1189–96. doi: 10.1016/j.jvir.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Dar SA, Love Z, Prologo JD, Hsu DP. CT-guided cryoablation for palliation of secondary trigeminal neuralgia from head and neck malignancy. J Neurointerventional Surg. 2013 May;5(3):258–63. doi: 10.1136/neurintsurg-2012-010265. [DOI] [PubMed] [Google Scholar]
- 17.Vogl TJ, Mack MG, Müller P, Phillip C, Böttcher H, Roggan A, et al. Recurrent nasopharyngeal tumors: preliminary clinical results with interventional MR imaging--controlled laser-induced thermotherapy. Radiology. 1995 Sep;196(3):725–33. doi: 10.1148/radiology.196.3.7644636. [DOI] [PubMed] [Google Scholar]
- 18.Brook AL, Gold MM, Miller TS, Gold T, Owen RP, Sanchez LS, et al. CT-guided radiofrequency ablation in the palliative treatment of recurrent advanced head and neck malignancies. J Vasc Interv Radiol JVIR. 2008 May;19(5):725–35. doi: 10.1016/j.jvir.2007.12.439. [DOI] [PubMed] [Google Scholar]
- 19.Owen RP, Khan SA, Negassa A, Beitler JJ, Bello JA, Brook A, et al. Radiofrequency Ablation of Advanced Head and Neck Cancer. Arch Otolaryngol-Head Neck Surg. 2011 May;137(5):493–8. doi: 10.1001/archoto.2011.62. [DOI] [PubMed] [Google Scholar]
- 20.Widmann G, Pototschnig C, Bale R. Three-dimensionally Navigated Image-guided Radiofrequency Ablation in the Head and Neck. J Vasc Interv Radiol. 2010 Jan;21(1):165–6. doi: 10.1016/j.jvir.2009.10.002. [DOI] [PubMed] [Google Scholar]


