Abstract
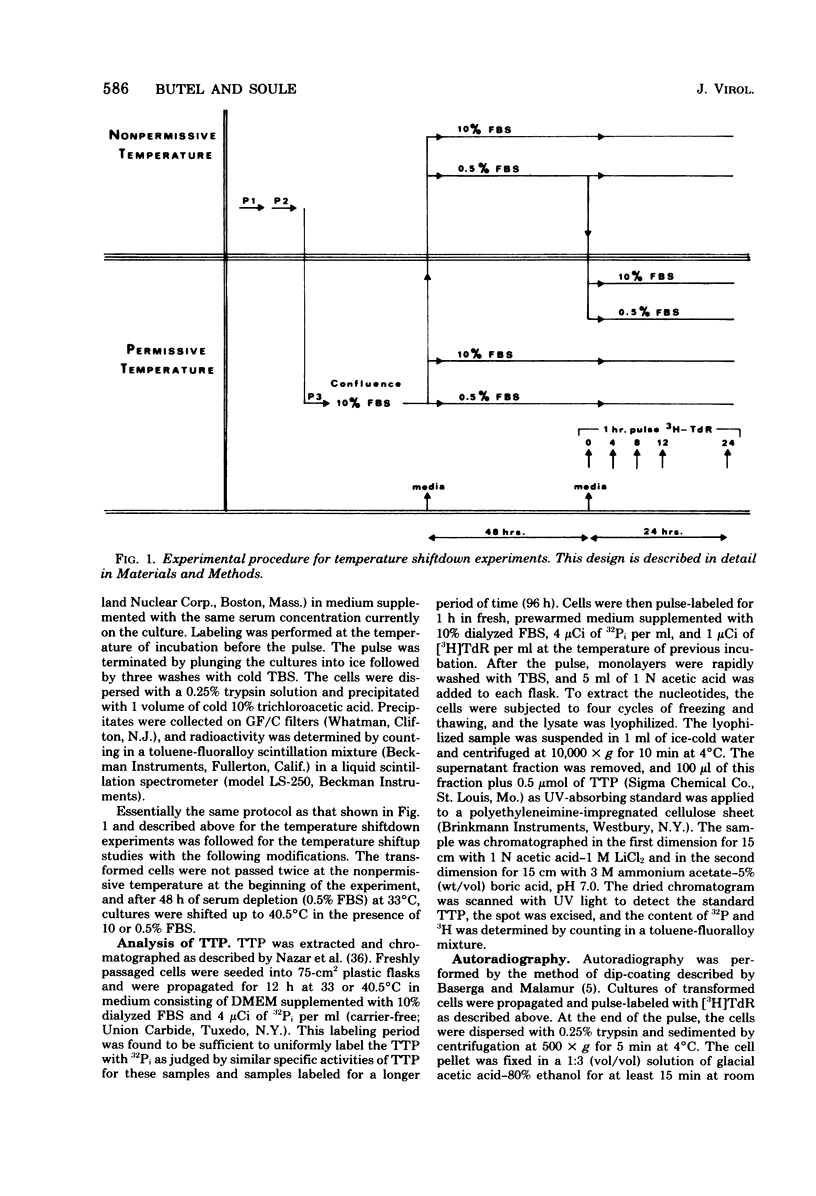
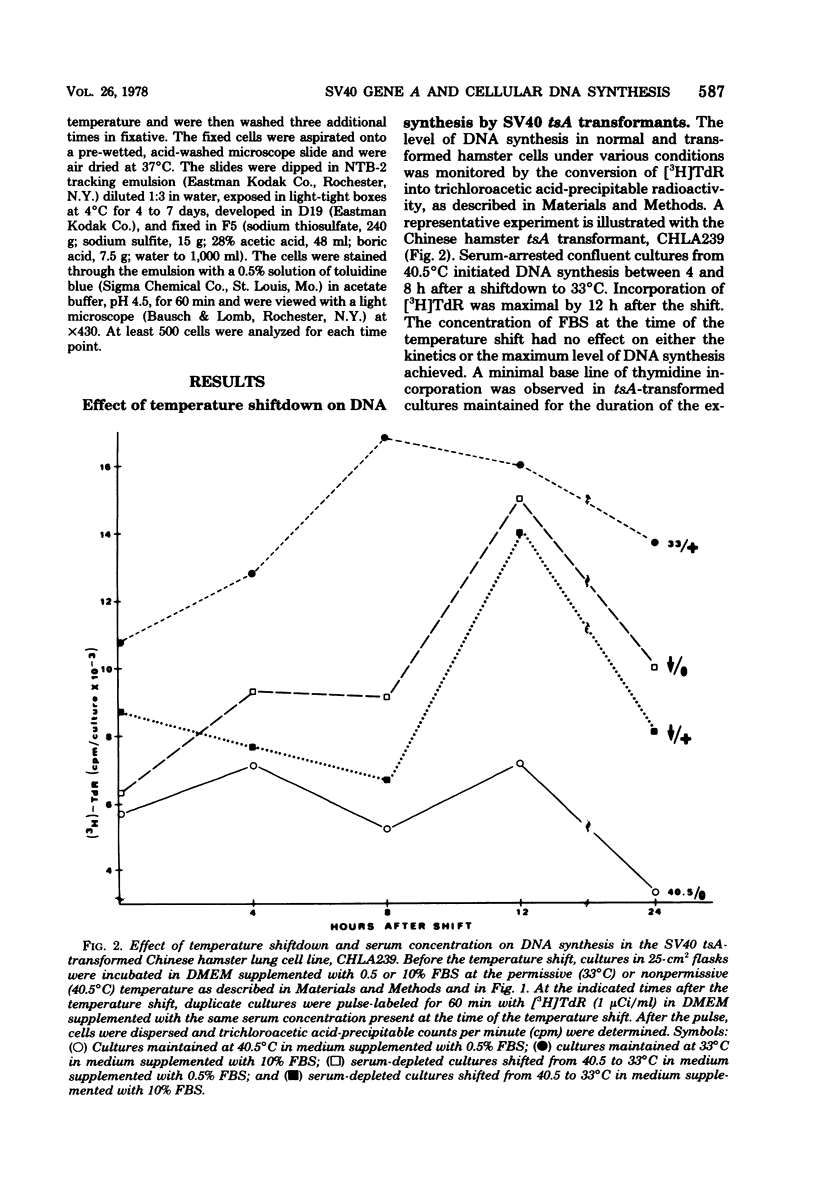
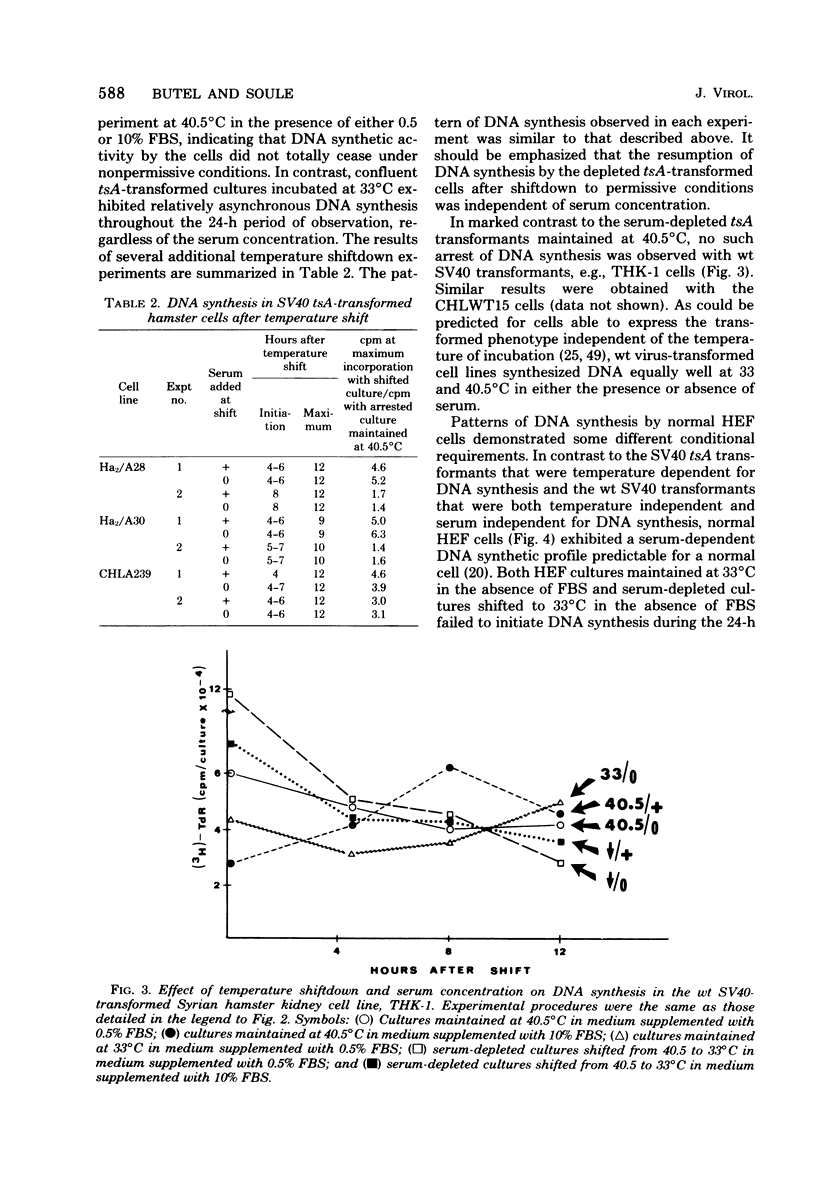
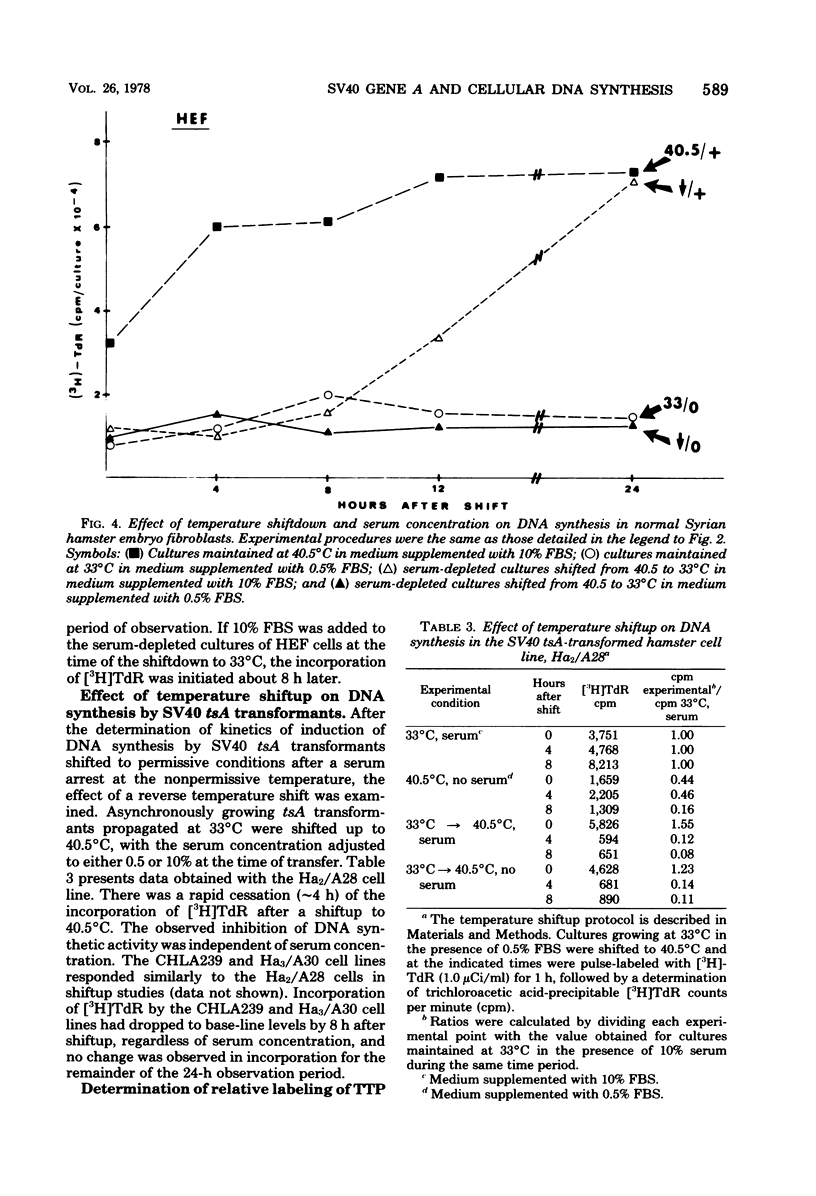
Cells transformed by tsA mutants of simian virus 40 (SV40) are temperature sensitive for the maintenance of the transformed phenotype. The kinetics of induction of DNA synthesis were determined for hamster cell transformants shifted to the permissive temperature after a 48-h serum arrest at the nonpermissive temperature. DNAsynthesis was initiated in the tsA transformants by 8 h after shiftdown was maximal by 12 h. The presence or absence of fetal bovine serum at the time of temperature shift had no effect on the kinetics of initiation of DNA synthesis. Analysis of TTP in tsA transformants revealed similar levels of incorporation of [3H]thymidine into TTP at both permissive and nonpermissive temperatures. Autoradiography revealed that by 12 h after a shift to the permissive temperature, approximately 50% of the cells exhibited labeled nuclei after a 60-min pulse with [3H]thymidine, indicating that a majority of the cells were actively synthesizing DNA. By 8 to 12 h after a shiftup of confluent tsA transformants to the nonpermissive temperature, the number of labeled nuclei was reduced to approximately 16%, regardless of serum concentration. These data indicate that the SV40 gene A product, either directly or indirectly, regulates cellular DNA synthesis in transformed cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Chang C., Mora P. T., Martin R. G. Expression and thermal stability of simian virus 40 tumor-specific transplantation antigen and tumor antigen in wild type- and tsA mutant-transformed cells. J Virol. 1977 Feb;21(2):459–467. doi: 10.1128/jvi.21.2.459-467.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Martin R. G., Chang C., Mora P. T., Livingston D. M. Nuclear preparations of SV40-transformed cells contain tumor-specific transplantation antigen activity. Virology. 1977 Jan;76(1):420–425. doi: 10.1016/0042-6822(77)90314-2. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P. AN ANALYSIS OF SV40-INDUCED TRANSFORMATION OF HAMSTER KIDNEY TISSUE IN VITRO. I. GENERAL CHARACTERISTICS. Proc Natl Acad Sci U S A. 1963 Oct;50:606–613. doi: 10.1073/pnas.50.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. G., Wyke J. A., Macpherson I. A. Transformation by a temperature sensitive mutant of Rous sarcoma virus in the absence of serum. J Gen Virol. 1975 May;27(2):127–134. doi: 10.1099/0022-1317-27-2-127. [DOI] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Brugge J. S., Noonan C. A. Transformation of primate and rodent cells by temperature-sensitive mutants of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):25–36. doi: 10.1101/sqb.1974.039.01.006. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Tevethia S. S., Melnick J. L. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res. 1972;15:1–55. doi: 10.1016/s0065-230x(08)60371-1. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Sauer G., Sokol F. The effect of actinomycin D on the transcription and replication of simian virus 40 deoxyribonucleic acid. Virology. 1969 Feb;37(2):214–226. doi: 10.1016/0042-6822(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Products of complementation between temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):127–136. doi: 10.1128/jvi.15.1.127-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Simian virus to (SV40) tumor-specific proteins in nucleus and plasma membrane of HeLa cells infected by adenovirus 2-SV40 hybrid virus Ad2+ND2. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2505–2509. doi: 10.1073/pnas.73.7.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation between temperature-sensitive (ts) and host range nontransforming (hr-t) mutants of polyoma virus. Virology. 1977 Apr;77(2):589–597. doi: 10.1016/0042-6822(77)90484-6. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Stein S. Resting state in normal and simian virus 40 transformed Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1655–1659. doi: 10.1073/pnas.73.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar R. N., Lawford H. G., Wong J. T. An improved procedure for extraction and analysis of cellular nucleotides. Anal Biochem. 1970 Jun;35(2):305–313. doi: 10.1016/0003-2697(70)90189-2. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. Transformation by polyoma virus and simian virus 40. Adv Cancer Res. 1972;16:141–180. [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Stimulation of glucose transport in cultures of density-inhibited chick embryo cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3154–3157. doi: 10.1073/pnas.68.12.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Fluck M. M., Benjamin T. L. Host range selection of transformation-defective hr-t mutants of polyoma virus. Virology. 1977 Apr;77(2):598–609. doi: 10.1016/0042-6822(77)90485-8. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weissman S. M. The early region of SV40 DNA may have more than one gene. Cell. 1977 Aug;11(4):837–843. doi: 10.1016/0092-8674(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Basilico C. SV40-transformed cells with temperature-dependent serum requirements. Cell. 1975 Mar;4(3):255–262. doi: 10.1016/0092-8674(75)90173-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Kuchino T. Temperature-sensitive mutants of simian virus 40 selected by transforming ability. J Virol. 1975 Jun;15(6):1297–1301. doi: 10.1128/jvi.15.6.1297-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


