Abstract
Objective
We previously identified PAPSS2 as a transcriptional target of TGF-β in chondrocytes. PAPSS2 is required for proper sulfation of proteoglycans in cartilage. Defective sulfation in the matrix results in alterations in mechanical properties of the cartilage that would be expected to result in degeneration. The objective of this study was to identify factors that regulate PAPSS2 expression and compare to a known TGF-β responsive gene, PRG4. In this study, TGF-β-mediated regulation of SOX9 was characterized, and the involvement of SOX9 in regulation of PAPSS2 mRNA was investigated.
Design
Primary bovine articular chondrocytes grown in micromass culture and ATDC5 cells were used as the model system. Adenoviruses were used to express SOX9 and SMAD3. siRNA was used to knock-down Sox9 and Smad3. Western blot and real-time quantitative RT-PCR were used to measure changes in protein and mRNA levels in response to treatment.
Results
Over-expression of SOX9 was sufficient to up-regulate PAPSS2 mRNA. TGF-β treatment of SOX9-expressing cells resulted in enhanced up-regulation of PAPSS2 mRNA, suggesting that SOX9 cooperates with TGF-β signaling. Furthermore, Sox9 was required for full TGF-β-mediated induction of Papss2. In contrast, PRG4 was regulated by SMAD3 but not SOX9. SOX9 protein levels were increased after treatment with TGF-β although SOX9 mRNA was not. SOX9 protein was post-translationally stabilized after treatment with TGF-β.
Conclusions
TGF-β stabilizes SOX9 protein, and SOX9 is sufficient and necessary for TGF-β-mediated regulation of PAPSS2 mRNA, providing a novel mechanism for TGF-β-mediated gene regulation in chondrocytes.
Keywords: TGF-β, SMAD3, SOX9, PAPSS2, PRG4, cartilage
Introduction
Little is known about the molecular mechanisms that maintain healthy cartilage. Elucidating these mechanisms may help identify druggable targets that could be used to prevent, slow, or reverse the erosion of articular cartilage that occurs during osteoarthritis (OA). Transforming growth factor β (TGF-β) is a peptide that has been shown to be critical for maintenance of cartilage [1, 2]. TGF-β has many biological effects on multiple tissues, making it difficult to use TGF-β ligand directly as a therapy. Furthermore, changes in TGF-β signaling as cartilage ages complicate the use of TGF-β ligand in OA [3, 4]. An alternative treatment may involve the use of cartilage-specific downstream effectors of TGF-β’s chondroprotective pathway. However, the molecular mechanisms that regulate such downstream effectors are largely unknown.
To identify molecular targets of TGF-β in cartilage, we performed an Affymetrix-based microarray using primary bovine articular chondrocytes grown in micromass cultures and treated with TGF-β1 [5]. Known TGF-β-regulated genes, including proteoglycan 4/lubricin (PRG4), were identified in the microarray [6]. PRG4 acts as a lubricant in joints [7] and is down-regulated in animal models of OA [5, 8]. Over-expression of PRG4 is protective against the development of OA in mice [9]. 3’-phosphoadenosine 5’-phosphosulfate synthase 2 (PAPSS2), a previously unknown target of TGF-β, was also identified in this screen [5]. Furthermore, we showed that PAPSS2 mRNA and protein levels were significantly reduced in cartilage from mice with defective TGF-β signaling when compared to controls [5]. PAPSS2 is required for proper sulfation of chondroitin sulfate proteoglycans in cartilage matrix [10]. Sulfation is especially important in articular cartilage where the large amount of sulfation on the glycosaminoglycans linked to the core proteins of proteoglycans provide the necessary biomechanical properties for cartilage to function. PAPSS2’s main function is to generate 3’-phosphoadenosine 5’-phosphosulfate (PAPS), the sulfate donor for sulfotransferase reactions. Generation of PAPS is the rate-limiting step for these reactions [11, 12]. PAPSS2 is highly expressed in cartilage [13, 14]. The importance of this enzyme in maintaining the cartilage phenotype is clear from the largely cartilage-specific phenotypes found in the Pakistani type of spondyloepimetaphyseal dysplasia, associated with mutations in the PAPSS2 gene [14, 15]. Patients and mice with mutations in PAPSS2 demonstrate short stature and early-onset osteoarthritis [14–19]. Almost nothing is known about how PAPSS2 levels are regulated in cartilage.
A well-known mediator of TGF-β signaling is SMAD3. SMADs are directly phosphorylated by the TGF-β type I receptor, translocate to the nucleus and act as transcription factors. SMAD3 is required for the maintenance of articular cartilage [20] and regulates a number of downstream gene targets of TGF-β signaling, including parathyroid hormone-related peptide (PTHrP) [21], type II collagen (COL2A1) [22], and PRG4 [23]. SOX9 is another important transcription factor expressed in mature articular cartilage. High expression levels of SOX9 are associated with enhanced levels of aggrecan (ACAN) and COL2A1 as well as maintenance of articular cartilage [24, 25]. Both SOX9 and TGF-β have been shown to increase the amount of chondroitin sulfate proteoglycans produced by chondrocytes in vitro [26–28]. Due to the common functions of TGF-β and SOX9 in cartilage, it is possible that they can act in a common chondroprotective signaling pathway.
The goal of the present study was to determine the factors involved in TGF-β-mediated regulation of PAPSS2 mRNA. SOX9 was shown to be sufficient to up-regulate PAPSS2 and required for full TGF-β-mediated regulation of Papss2. In contrast, PRG4 was not regulated by SOX9. We also showed that TGF-β regulates the stability of SOX9 protein, providing a potentially novel mechanism for TGF-β-mediated regulation of gene expression in cartilage.
Materials and Methods
Isolation of primary bovine chondrocytes
Cartilage was harvested from bovine metacarpophalangeal joints, minced, washed, and placed in PBS containing 2 mg/mL collagenase D (Roche) and 1% penicillin-streptomycin (Thermo Fisher Scientific) at 37°C overnight [29]. The digested tissue was filtered to isolate cells. The donor animals ranged in age from 9 month to 18 months. If the cartilage was damaged or yellow, it was not used.
Cell culture
Chondrocytes were suspended in culture medium consisting of Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific), 10% heat-inactivated fetal bovine serum (FBS, Thermo Fisher Scientific), 50 µg/mL sodium-L-ascorbate (Sigma-Aldrich), 1% penicillin-streptomycin (Thermo Fisher Scientific), and 1% L-glutamine (Thermo Fisher Scientific), referred to as high-serum culture medium. Cells were cultured in micromasses in 35-mm culture dishes (Thermo Fisher Scientific). Specifically, six separate 20-µL drops, each containing 2 × 105 cells, were placed in each 35-mm culture dish, forming six micromasses per dish. Cells were left at 37°C overnight to attach to culture dishes, and culture dishes were flooded with culture medium consisting of DMEM, 0.5% heat-inactivated FBS, 50 µg/mL sodium-L-ascorbate, 1% penicillin-streptomycin, and 1% L-glutamine, referred to as low-serum medium. Since the full thickness of the cartilage was used, cultures consisted of a mixture of chondrocytes from all zones. The number of biological replicates, separate experiments with cells isolated from a different animal, is indicated in each figure legend (n=).
Adenoviruses
To over-express SMAD3 and SOX9 protein, adenoviruses were used: an adenovirus encoding for a wild-type, FLAG-tagged SMAD3 protein with an enhanced green fluorescent protein (eGFP) reporter (Ad-SMAD3), an adenovirus encoding for a wild-type, FLAG-tagged SOX9 protein with an eGFP reporter (Ad-SOX9), and an adenovirus encoding for only eGFP (Ad-eGFP) to control for the effects of adenoviral infection. eGFP expression was controlled by an IRES. Ad-SMAD3 was generated from pRK5F Smad3, a gift from Dr. Rik Derynck (Addgene plasmid # 12625) [30]. Ad-SOX9 was generated from SOX9-pcDNA-5 UT, provided by Dr. Veronique Lefebvre [31]. The adenoviruses were suspended in high-serum culture medium. Transduction of primary bovine chondrocytes was carried out by combining the cells and adenoviruses at an appropriate MOI in suspension at a concentration of 2 × 105 cells per 20 µL and by placing the suspensions on a nutating mixer at 37°C for 1 hour. Cells were plated in micromasses as described above and left to attach overnight, then the culture dishes were flooded with low-serum culture medium. Infection efficiency was determined by counting the number of green cells over the number of total cells from images of 4 separate experiments.
TGF-β1
After flooding culture dishes, micromass cultures that were transduced with virus were incubated overnight so that eGFP fluorescence reached full intensity, suggesting high expression of the proteins encoded by the adenoviruses. TGF-β1 (R&D Systems; 5 ng/mL) was then added to appropriate cultures, and the vehicle for TGF-β1, 4 mM HCl containing 0.1% bovine serum albumin, was added to control cultures. After treatment, mRNA and protein were collected at 24 hours or the indicated time points. In experiments where virus was not used, cells were cultured according to the same timeline used for the adenovirus-transduced cultures (i.e., chondrocytes were placed in micromasses, cells were incubated overnight, dishes were flooded with culture medium, cells were incubated overnight, and TGF-β1 was added to cells).
Luciferase assays
To verify that Ad-SOX9 made a functional protein, luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega). Briefly, 400 ng of an expression plasmid encoding for luciferase and a SOX9 binding element from the type II collagen gene (Col2a1-luc) [31], 4 ng of an expression plasmid encoding for renilla luciferase, and 3 µL of lipofectamine (Thermo Fisher Scientific) were diluted in Opti-MEM medium (Thermo Fisher Scientific) to a total volume of 100 µL. Mixtures were placed into each well of a 12-well plate containing pre-established monolayers of 293T cells seeded at a density of 4 × 105 cells per well. The cells were incubated for 24 hours, treated with adenoviruses at 75 MOI, and incubated for an additional 24 hours. Cell lysates were obtained, and relative luciferase units (RLUs) were measured. Microsoft Excel (version 14.4.0) was used to perform an Anderson-Darling normality test and an f-test for variance on the luciferase data, which showed that the data was normally distributed with unequal variance. Since the data was normally distributed, a Student’s t-test (two-tailed, unpaired, unequal variance) was used to determine whether there was a statistically significant difference between RLUs of Ad-eGFP-infected cultures and RLUs of Ad-SOX9-infected cultures.
Knock-down experiments
ATDC5 cells were cultured as previously described [32]. Mouse SOX9 siRNA (sc-36534, Santa Cruz), mouse SMAD 2/3 siRNA (sc-37239, Santa Cruz), and control siRNA (sc-37007, Santa Cruz) were transfected into ATDC5 cells using Lipofectamine RNAiMAX (13778-150, Thermo Fisher) for 48 hours, according to the manufacturer’s protocol. Cells were subsequently treated with TGF-β1 or vehicle control and mRNA or protein was collected.
Quantitative real-time RT-PCR (qPCR)
Cultures were harvested using TRIzol reagent (Thermo Fisher Scientific), and mRNA was extracted from the TRIzol reagent using a Direct-zol RNA MiniPrep kit (Zymo Research). A QuantiFast SYBR Green RT-PCR kit (QIAGEN) and a LightCycler 480 Instrument II (Roche) were used to determine crossing point (Cp) values, and the Cp values were converted to relative mRNA units using the Relative Expression Software Tool (REST) 2009 (QIAGEN) [33]. REST analysis includes correction for PCR efficiency and provides statistical testing for nonparametric data using a pair-wise fixed reallocation randomization test to determine significance. Gene expression levels were determined with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference/normalization gene in bovine cells and peptidylprolyl isomerase A (Ppia) in mouse ATDC5 cells [34]. Primer sequences are listed in Table 1.
Table 1.
Primer sequences for qPCR
| Gene | Efficiency % | Forward primer | Reverse primer |
|---|---|---|---|
| bGAPDH | 110 | GGG TCA TCA TCT CTG CAC CT | GGT CAT AAG TCC CTC CAC GA |
| bPAPSS2 | 114 | TGC CAT CTT CCC ATC TCC CAT GTT | ACA GGT CTC TCT TGG TCT CAG GAT |
| bPRG4 | 101 | TGC CCT GAC TTC AAG AAG GAA TGC | CCA TAA TCG GAA CAG CAC TTG CCA |
| bPTHrP | 134 | CGG TTA TTA TTT CGG AGG AGG C | CCT CTC GCT CTG GGG ACT TAT |
| bSOX9 | 117 | CCG GCT CCG ACA CCG AGA ACA | CCA GCG TCC AGT CGT AGC CCT |
| mPpia | 99 | CGC GTC TCC TTC GAG CTG TTT G | TGT AAA GTC ACC CTG GCA CAT |
| mPapss2 | 98 | CCC GTG ATG GAG TCA ACA TGA G | GTG CTT TGC AGT GGG TGT TCC |
| mAcan | 96 | AGG TTG CTA TGG TGA CAA GG | TGG AAG GTG AAT TTC TCT GGG |
| mSox9 | 102 | AGG AAG TCG GTG AAG AAC GG | TGG AAG GTG AAT TTC TCT GGG |
Western blot
Chondrocytes in micromass culture were lysed with radioimmunoprecipitation assay (RIPA) buffer containing phosphatase and protease inhibitors (Roche #04906837001 and #05892970001). Thirty µg of protein lysate per sample was separated by reducing electrophoresis on 4–15% polyacrylamide gels (Bio-Rad Laboratories). Protein was transferred from gels to polyvinylidene fluoride membranes (Bio-Rad Laboratories) using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories). Membranes for assessment of total protein were blocked with 3% Blotto non-fat dry milk (Santa Cruz Biotechnology) and incubated with either anti-FLAG primary antibody (1:500, Sigma-Aldrich #F1804), anti-SOX9 primary antibody (1:1000, Santa Cruz Biotechnology sc-20095), or anti-SMAD2/3 primary antibody (1:2000, Cell Signaling Technology #8685S). For phosphorylated SMAD3, membrames were blocked with 3% bovine serum albumin for 1 hour and incubated overnight in anti-phosphorylated-SMAD3 primary antibody (1:1000, Cell Signaling Technology #9520S). To assess whether equivalent amounts of protein were loaded in all wells, either an anti-GAPDH primary antibody (1:1000, Santa Cruz Biotechnology sc-25778) or anti-α-Tubulin primary antibody (1:2500, Rockland Immunochemicals #200-301-880) were used. Membranes were washed with Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with HRP-conjugated anti-rabbit (1:2000, Cell Signaling Technology) or anti-mouse (1:2500, Rockland Immunochemicals, #610-143-003) secondary antibody. Images and any quantitation of Western blots was acquired on a ChemiDoc MP system (Bio-Rad Laboratories).
Results
TGF-β regulates PRG4 and PAPSS2 mRNA in bovine chondrocytes over time
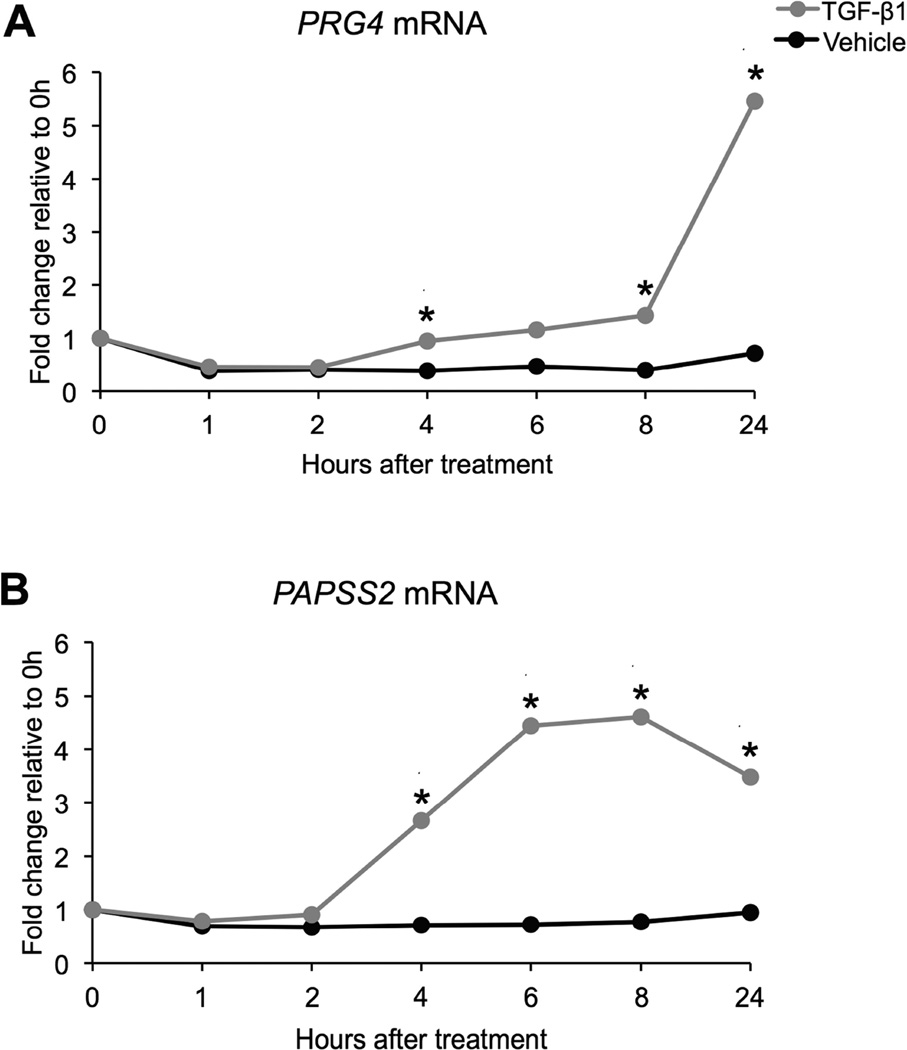
Previous work showed that PRG4 and PAPSS2 mRNA levels were up-regulated in bovine articular chondrocytes grown in micromass culture after 8 hours of TGF-β1 treatment when compared to mRNA levels of vehicle-treated controls [5, 6]. However, establishing a complete time course for PRG4 and PAPSS2 mRNA levels may help identify specific time points that should be further investigated when elucidating how PRG4 and PAPSS2 mRNA levels are regulated. Therefore, time course experiments were performed by treating primary bovine chondrocytes in micromass culture with either 5 ng TGF-β1/mL or a vehicle control for 0, 1, 2, 4, 6, 8, and 24 hours. mRNA was isolated from cultures, and relative mRNA levels were determined using qPCR (Figure 1). PRG4 mRNA levels started increasing by 4 hours of TGF-β1 treatment and increased dramatically between 8 and 24 hours of treatment (Figure 1A, Table S1A). PAPSS2 mRNA levels also started increasing by 4 hours of TGF-β1 treatment; however, maximum levels were observed between 6 and 8 hours of treatment (Figure 1B, Table S1B).
Figure 1. TGF-β1 regulates PRG4 mRNA and PAPSS2 mRNA in bovine chondrocyte micromass cultures over time.
PRG4 mRNA levels increased after 4, 8, and 24 hours of TGF-β1 treatment, when compared to mRNA levels of vehicle-treated controls at the same time points (REST, * p < 0.05, n = 5) (A). PAPSS2 mRNA levels increased after 4, 6, 8, and 24 hours of TGF-β1 treatment, when compared to mRNA levels of vehicle-treated controls at the same time points (REST, * p < 0.05, n = 5) (B).
SOX9 is sufficient to regulate PAPSS2 mRNA but not PRG4 mRNA in bovine chondrocytes
Next, to determine how TGF-β regulates PAPSS2 mRNA, we analyzed conserved regions of the PAPSS2 gene from human, mouse, and cow for potentially relevant transcription factor binding sites using Dialign TX [35] and the Genomatix software suite to identify potential protein binding sites [36]. Several SMAD3 binding sites were identified in potential regulatory regions of the PAPSS2 gene. In addition, paired SOX9 DNA binding elements were identified in the first intron of the mouse Papss2 gene. Paired SOX9 elements are thought to be indicative of cartilage-specific regulation [37, 38]. A recent ChIP-Seq analysis confirmed Sox9 binds elements in the first intron of the Papss2 gene [39]. We hypothesized that TGF-β may regulate PAPSS2 mRNA through SMAD3 and/or SOX9.
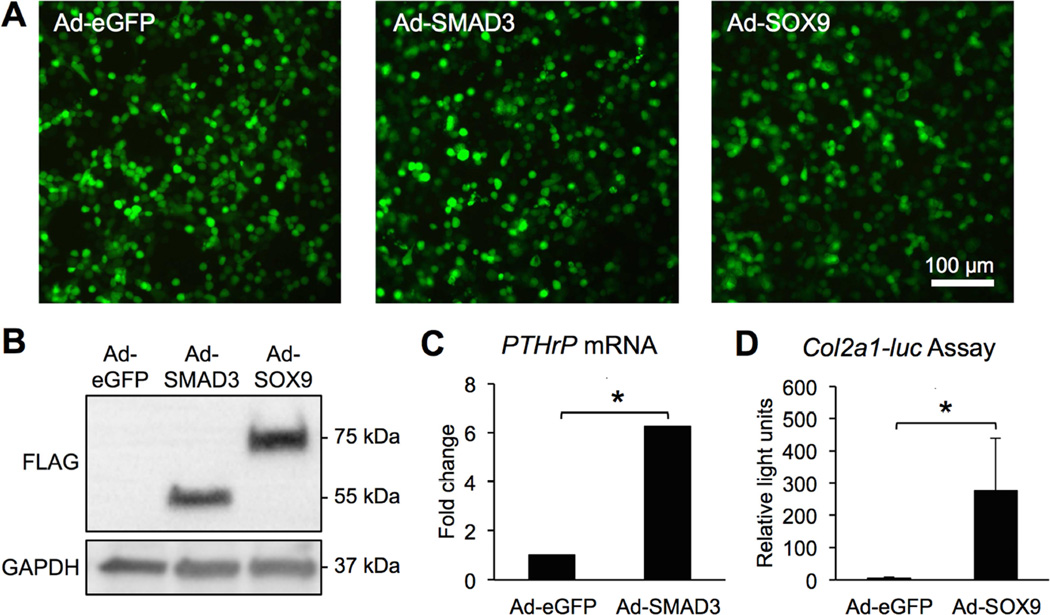
To directly test whether SMAD3 and/or SOX9 were sufficient to regulate PAPSS2 mRNA, adenoviral vectors encoding SMAD3 and SOX9 (Ad-SMAD3 and Ad-SOX9) were used to over-express SMAD3 and SOX9 in bovine primary articular chondrocyte cultures. First, the activity of the proteins encoded in the Ad-SMAD3 and Ad-SOX9 vectors was validated (Figure 2). eGFP reporters were used to calculate infection efficiency. Infection efficiency was comparable for all of the viruses used (Ad-GFP 69.4% ± 6.51; Ad-SMAD3 67.5% ± 14.7; Ad-SOX9 75.7% ± 4.33; p=0.326) (Figure 2A). Western blots using an anti-FLAG antibody revealed expression of FLAG-tagged proteins with appropriate molecular weights for FLAG-tagged SOX9 and SMAD3 in cells infected with the indicated adenoviruses (Figure 2B). Protein levels were measured as band intensities normalized to GAPDH on Western blots. SOX9 and SMAD3 protein levels were comparable (SMAD3 7.5 ± 1.2; SOX9 5.0 ± 2.3; p=0.088). As expected based on previous studies [21], Ad-SMAD3 induced up-regulation of PTHrP mRNA, indicating that the expressed SMAD3 protein was functional (Figure 2C, Table S2). In addition, Ad-SOX9 induced luciferase activity in 293T cells transfected with a Col2a1-luciferase expression plasmid [31], indicating that the expressed SOX9 protein was functional (Figure 2D).
Figure 2. SMAD3 and SOX9 adenoviral vectors infect cells, induce expression of FLAG-tagged proteins, and up-regulate SMAD3 and SOX9 function respectively.
Bovine chondrocytes that were infected with either Ad-eGFP, Ad-SMAD3, or Ad-SOX9 at an MOI of 75 fluoresced green and showed efficient viral transduction (n = 4) (A). Western blots of extracts from bovine chondrocytes showed expression of FLAG-tagged proteins at approximately 52 kDa and 75 kDa, corresponding to SMAD3 and SOX9 molecular weights respectively (n = 4) (B). Bovine chondrocytes transduced with Ad-SMAD3 exhibited increased PTHrP mRNA levels; Ad-SMAD3 mRNA is relative to Ad-eGFP mRNA (REST, * p < 0.001, n = 5) (C). 293T cells transfected with a Col2a1-luciferase plasmid exhibited increased luciferase activity (Student’s t-test, * p = 0.04, n = 4) (D).
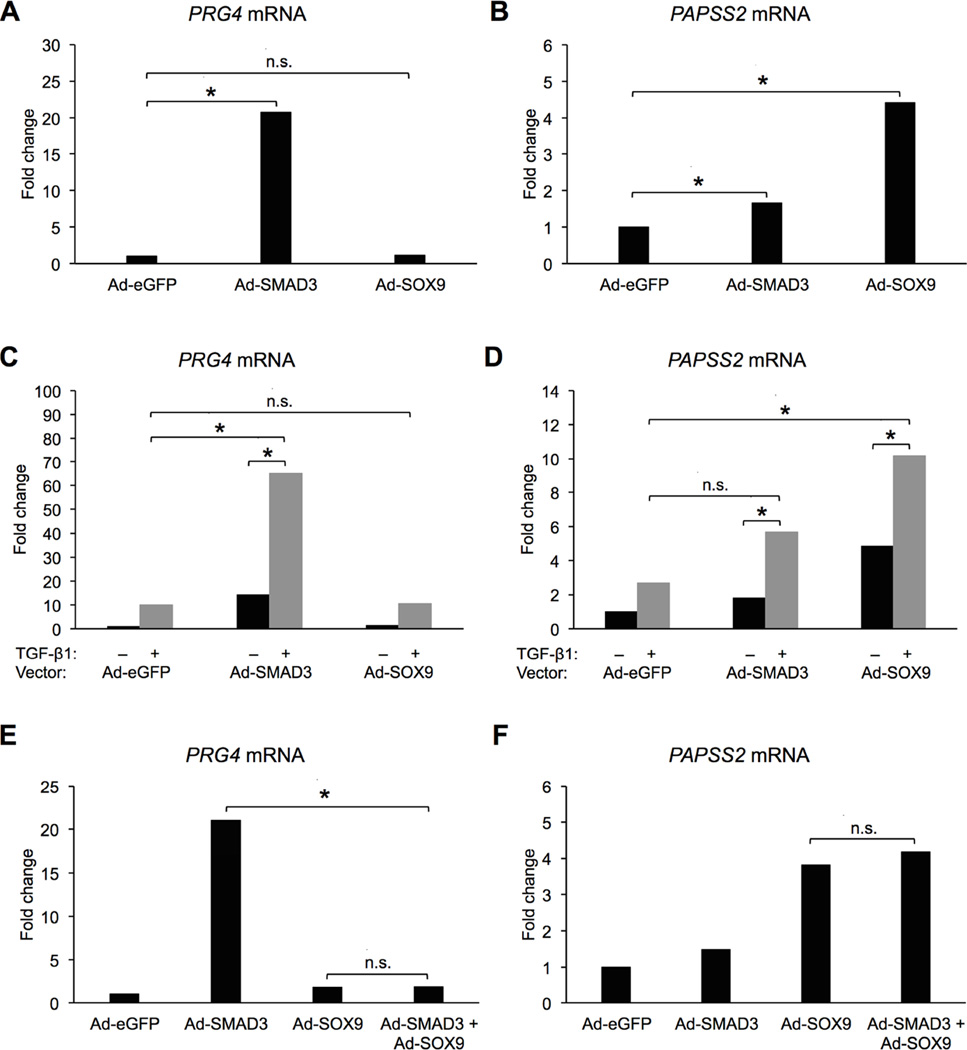
Next, primary articular chondrocytes were transduced with either Ad-eGFP, Ad-SMAD3, or Ad-SOX9 at an MOI of 75, and PRG4 and PAPSS2 mRNA levels were assessed by qPCR (Figure 3). As expected, based on previous studies, SMAD3 was sufficient to significantly up-regulate PRG4 mRNA [23]; however, PRG4 was not regulated by over-expression of SOX9 (Figure 3A, Table S3A). In contrast, SOX9 and SMAD3 were sufficient to up-regulate PAPSS2 mRNA (Figure 3B, Table S3B). The results indicate that regulation of PRG4 and PAPSS2 are different; SOX9 is sufficient to regulate PAPSS2 but not PRG4.
Figure 3. SOX9 regulates PAPSS2 mRNA but not PRG4 mRNA in bovine chondrocytes.
In A and B, cells were infected with the indicated viruses at 75 MOI, and relative mRNA levels for PRG4 and PAPSS2 were determined. (REST, * = p < 0.05, n.s. = not significant, n = 8). In C and D, cells were infected with the indicated viruses at 75 MOI and treated with either vehicle (−) or TGF-β1 (+), and relative mRNA levels for PRG4 and PAPSS2 were determined. (REST, * = p < 0.05, n.s. = not significant, n = 4). In E and F, cells were infected with either 150 MOI of AdeGFP (labeled Ad-eGFP), 75 MOI of Ad-eGFP + 75 MOI of Ad-SMAD3 (labeled Ad-SMAD3), 75 MOI of Ad-eGFP + 75 MOI of Ad-SOX9 (labeled Ad-SOX9), or 75 MOI of Ad-SMAD3 + 75 MOI of Ad-SOX9 (labeled Ad-SMAD3 + Ad-SOX9). (REST, * = p < 0.05, n.s. = not significant, n = 4). In all cases, mRNA levels are relative to mRNA levels of cultures treated with only Ad-eGFP (control).
Next, we tested whether the expressed transcription factors could cooperate with TGF-β to regulate gene expression. Cells were infected with Ad-eGFP, Ad-SMAD3, or Ad-SOX9 then treated with either vehicle or TGF-β1 (Figure 3C, D). Treatment of Ad-eGFP-infected cells with TGF-β resulted in an increase in PRG4 and PAPSS2 mRNA levels, as expected. Ad-SMAD3 was sufficient to stimulate PRG4 mRNA levels; however, in the presence of TGF-β the levels of PRG4 mRNA were significantly higher than either Ad-SMAD3 or TGF-β alone (Figure 3C, Table S3C). This cooperation to regulate PRG4 was likely due to increased phosphorylation and activation of the over-expressed SMAD3 through activation of the receptor kinases. In contrast, PRG4 mRNA was regulated to the same extent in cells infected with Ad-SOX9 and treated with TGF-β as it was in cells treated with TGF-β alone (Figure 3C, Table S3C) indicating SOX9 does not cooperate with TGF-β to regulate PRG4. However, SOX9 did cooperate with TGF-β to regulate PAPSS2 expression. PAPSS2 mRNA was significantly increased in cells infected with Ad-SOX9 and treated with TGF-β when compared to cells treated with either Ad-SOX9 or TGF-β alone (Figure 3D, Table S3D). PAPSS2 mRNA levels were also higher in cells that expressed the exogenous SMAD3 and were treated with TGF-β when compared to cells infected with SMAD3 (Figure 3D, Table S3D).
Since TGF-β and SOX9 cooperated to regulate PAPSS2 expression, we next tested the hypothesis that SMAD3 is the component of TGF-β signaling that cooperates with SOX9 to regulate PAPSS2. Cells were infected with both Ad-SOX9 and Ad-SMAD3, and mRNA levels were determined by qPCR (Figure 3E, F). As expected, Ad-SOX9 increased PAPSS2 expression; however, addition of SMAD3 did not further regulate PAPSS2 mRNA levels over what was observed with Ad-SOX9 alone (Figure 3F, Table S3F). Even when TGF-β was added to the cultures, the addition of SMAD3 did not increase PAPSS2 expression over that of SOX9 and TGF-β together (data not shown). PRG4 expression was up-regulated in the presence of SMAD3 as expected; however, when SOX9 was added together with SMAD3, PRG4 mRNA levels were unexpectedly reduced, suggesting that SOX9 inhibits SMAD3-mediated regulation of PRG4 through an unknown mechanism (Figure 3E, Table S3E). Together, the results suggest a model where TGF-β signaling regulates PAPSS2 expression through cooperation with the SOX9 transcription factor. In contrast, PRG4 mRNA is likely regulated through canonical activation of SMAD3.
Sox9 is required for full up-regulation of Papss2 mRNA by TGF-β in ATDC5 cells
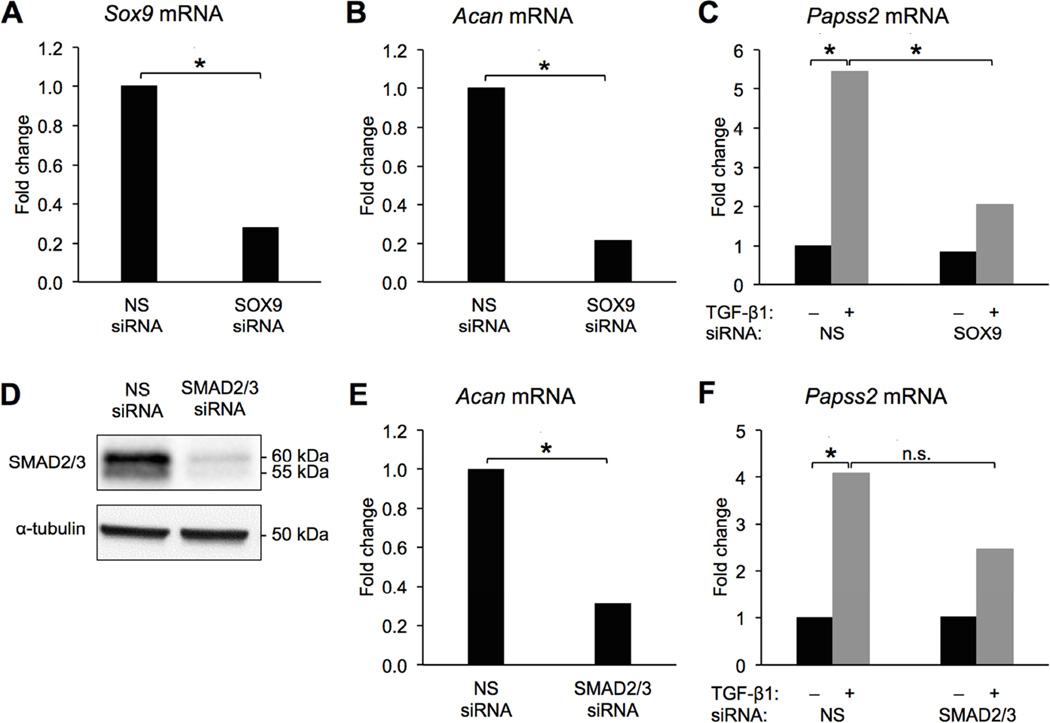
Primary bovine chondrocytes are useful as a model system because we can obtain a large number of cells for experiments and they represent permanent cartilage; however, they are resistant to most transfection protocols making loss of function experiments difficult. To determine if Sox9 is required to mediate TGF-β’s effects on Papss2 expression we used the ATDC5 chondrogenic cell line [32]. Cells were transfected with siRNA to Sox9 or Smad2/3. Knock-down was confirmed by qPCR and Western blot (Figure 4A, D; Table S4A). Prg4 mRNA is not detectable in ATDC5 cells in the absence of TGF-β so expression of Acan mRNA was used as a functional control for Sox9 and Smad2/3 knock-down [40, 41]. Acan mRNA expression was significantly down-regulated by loss of each of these transcription factors (Figure 4B, E; Table S4B, E). As expected, Papss2 was up-regulated by TGF-β1 in cells containing the NS-siRNA (Figure 4C, F; Table S4C, F). Up-regulation of Papss2 by TGF-β1 was significantly reduced (~60%) in cells with knock-down of Sox9 relative to cells containing NS-siRNA (Figure 4C; Table S4C) suggesting Sox9 is required for full induction of Papss2 by TGF-β. The remaining induction of Papss2 mRNA could be due to either incomplete knock-down of Sox9 or the presence of another unknown regulatory factor. It is likely that many transcription factors mediate Papss2 expression and Sox9 is not the sole mediator. Up-regulation of Papss2 by TGF-β in the presence of Smad2/3 siRNA was not statistically different than that in NS siRNA containing controls. Together the results suggest that SOX9 is sufficient to regulate PAPSS2 and necessary for TGF-β-mediated regulation of PAPSS2.
Figure 4. Knock-down of Sox9 attenuates TGF-β-mediated regulation of Papss2.
ATDC5 cells were transfected with Sox9 siRNA, Smad2/3 siRNA, or control non-specific (NS) siRNA. Sox9 mRNA expression was significantly reduced in cells expressing Sox9 siRNA (A). Acan mRNA was significantly reduced in the presence of Sox9 siRNA (B). Cells containing NS siRNA or Sox9 siRNA were treated with TGF-β1 and expression of Papss2 mRNA was measured by qPCR (C). In A–C * = p < 0.05 REST, n = 5. Western blots showed Smad2/3 protein levels were reduced in the presence of Smad2/3 siRNA, α-Tubulin was used as a loading control (n = 6) (D). Acan mRNA was significantly down-regulated in the Smad2/3 siRNA-transfected cells compared to cells containing NS siRNA (REST, * = p < 0.05, n = 2) (E). Cells containing NS or Smad2/3 siRNA siRNA were treated with TGF-β1 and Papss2 mRNA was, measured by qPCR (REST, * = p <0.05, n.s = not-significant n = 4) (F).
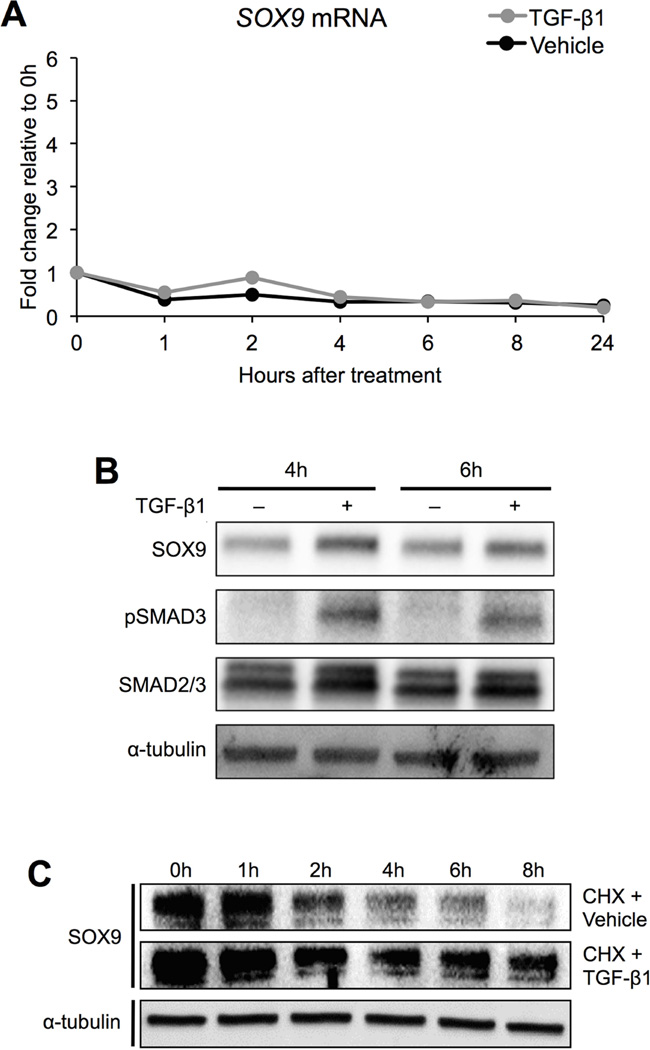
TGF-β stabilizes SOX9 protein in bovine chondrocytes
Since the results indicated that SOX9 is an important regulator of PAPSS2, we tested the hypothesis that TGF-β regulates SOX9 expression. We first determined whether or not treatment with TGF-β regulated SOX9 mRNA levels in bovine chondrocyte cultures. mRNA was isolated from chondrocytes treated with TGF-β1 or vehicle at varying time points, and relative levels of SOX9 mRNA were determined using qPCR (Figure 5A, Table S5). There were no significant changes in SOX9 mRNA levels at any time point tested. Next, we determined if TGF-β regulated SOX9 protein levels. Protein was isolated from vehicle- and TGF-β1-treated cells after 4 and 6 hours of treatment and used in Western blot analysis. Even though SOX9 mRNA was not regulated by treatment with TGF-β1, SOX9 protein levels were increased with TGF-β1 relative to controls at the time points tested (Figure 5B). In addition, there was an increase in the amount of phosphorylated SMAD3 protein relative to the total amount of SMAD2/3 protein after TGF-β1 treatment, confirming that the SMAD signaling pathway had been activated (Figure 5B). Based on these results, we hypothesized that TGF-β regulated SOX9 post-translationally, perhaps by increasing the stability of SOX9 protein. To test this hypothesis, new protein synthesis was inhibited by adding cycloheximide (50 µg/mL) to cells for 1 hour and subsequently treating the cells with either TGF-β1 or vehicle for 0, 1, 2, 4, 6, and 8 hours. In vehicle-treated cultures, SOX9 protein levels dropped off between 2 and 4 hours; however, in TGF-β1-treated cultures, SOX9 protein was maintained (Figure 5C). These data indicate that TGF-β likely regulates SOX9 protein levels via protein stabilization.
Figure 5. TGF-β1 stabilizes SOX9 protein in bovine chondrocytes.
In A, mRNA was collected from bovine chondrocytes treated with vehicle or TGF-β1 for the indicated amounts of time. SOX9 mRNA levels were determined by qPCR (REST, no statistically significant differences, n = 5). In B, protein was collected from bovine chondrocytes that were treated with either vehicle or TGF-β1 for 4 or 6 hours. SOX9, pSMAD3, and SMAD2/3 protein levels were determined by Western blot (n = 3). α-Tubulin was used as a loading control. In C, new protein synthesis was inhibited with cycloheximide. Cells were subsequently treated with either vehicle or TGF-β1 for up to 8 hours. Protein was isolated at specified time points, and the levels of SOX9 protein were determined by Western blot (n = 3). pSMAD3 = phosphorylated SMAD3, CHX = cycloheximide.
Discussion
The present study focused on a downstream target of TGF-β signaling, PAPSS2, which is required for proper sulfation of cartilage matrix. In this study, we showed that TGF-β regulates PAPSS2 mRNA over time, TGF-β stabilizes SOX9 protein, SOX9 is sufficient to regulate PAPSS2 mRNA, and SOX9 cooperates with and is required for TGF-β-mediated regulation of PAPSS2 mRNA. In contrast, SOX9 was not sufficient to regulate PRG4, another TGF-β-regulated cartilage gene.
Previously, it was shown that over-expression of SOX9 in human articular chondrocytes in vitro resulted in an increase in sulfate incorporation into the matrix [26]. The mechanism whereby SOX9 increased matrix sulfation was not determined, but over-expression of SOX9 did not change mRNA levels for various genes encoding sulfotransferases [26]. The study did not assess mRNA levels for PAPSS2. However, a microarray screen that compared limb buds from Sox9-3’ eGFP knock-in embryos to limb buds from Sox9-eGFP/eGFP null chimeric embryos showed that Papss2 was the most differentially regulated gene of all enzymatic genes that were assessed [42]. Based on our present study, we hypothesize that SOX9 regulated sulfation in these cases via transcriptional regulation of Papss2. A new hypothesis for the role of SOX9 in cartilage is that the maintenance of matrix sulfation and stability of articular cartilage mediated by SOX9 is at least in part due to regulation of PAPSS2.
In contrast, a previously known target of TGF-β in cartilage, PRG4, was not up-regulated by SOX9 in our study. SMAD3 was sufficient to regulate PRG4 mRNA, and others have shown that SMAD3 is required for full induction of PRG4 mRNA by TGF-β [23]. The fact that TGF-β and SMAD3 regulated both PAPSS2 and PRG4 but SOX9 regulated only PAPSS2 suggests that cartilage genes are regulated through distinct and specific mechanisms, including SOX9-dependent and -independent mechanisms. This specificity was seen in a previous study where Agc1CreERT2;Sox9flox/flox mice injected with tamoxifen exhibited no changes in PRG4 protein expression in the articular cartilage but exhibited a reduction in Safranin-O staining, which stains sulfated proteoglycans [40]. Previously, it was shown that SOX9 and SMAD3 cooperate to regulate the expression of COL2A1 in human mesenchymal stem cells [43]. Although SOX9-expressing cells treated with TGF-β had higher levels of PAPSS2 mRNA than cells treated with SOX9 or TGF-β alone, we did not detect the same cooperation of SOX9 and SMAD3 in regulation of PAPSS2. The results suggest that a different TGF-β-regulated protein may be involved in regulation of PAPSS2 and that various cartilage genes have distinct modes of regulation. Such specificity may be beneficial when searching for targets that could be utilized to modulate PAPSS2 expression while avoiding potentially negative effects.
Recently, a large ChIP-Seq analysis of SOX9 binding sites in murine rib chondrocytes was published [39]. The ChIP-Seq screen identified three highly ranked SOX9 binding sites within the first intron of the PAPSS2 gene. The clustering pattern of the binding sites suggested that these could be so-called super-enhancers to which SOX9 binds. Super-enhancers are thought to drive genes that are essential for the identity of specific cell types [44], in this case chondrocytes. Additional work must be performed to fully characterize the function of the PAPSS2 elements to which SOX9 binds. The study did not identify any SOX9 binding sites associated with PRG4, supporting the finding of the present study that SOX9 does not up-regulate PRG4.
The present study showed that TGF-β stabilized SOX9 protein, but the molecular events that mediate stabilization are unknown. TGF-β has been shown to regulate stability of hypoxia inducible factor 1 alpha (HIF-1α) through transcriptional inhibition of prolyl hydroxylase 2 (PHD2). Specifically, PHD2 hydroxylates proline residues within the oxygen-dependent degradation domain of HIF-1α, thus stabilizing HIF-1α [45]. Furthermore, cyclin-dependent kinase inhibitor p21 can be stabilized by TGF-β through phosphorylation, a post-translational modification [46]. SOX9 has been shown to undergo a range of post-translational modifications, including phosphorylation, sumoylation, ubiquitination, acetylation, and neddylation. Determining whether TGF-β regulates the stability of SOX9 through post-translational modifications and how such modifications may be involved in regulating PAPSS2 may help further elucidate a molecular mechanism important in chondroprotection.
In summary, the present study suggests that SOX9 mediates regulation of PAPSS2 by TGF-β. In contrast, PRG4, another TGF-β stimulated gene, was not regulated SOX9 and appears to be regulated by the canonical TGF-β/SMAD pathway. In addition, TGF-β1 stabilized SOX9 protein, which introduces a potentially new pathway for TGF-β signaling in cartilage.
Supplementary Material
Acknowledgments
Sanger sequencing to verify the sequence of DNA plasmids was done through the Heflin Center Genomic Core Facility at UAB. The SMAD3 plasmid used to generate the Ad-SMAD3 virus, pRK5F SMAD3, was a gift from Rik Derynck (Addgene plasmid # 12625). The SOX9 plasmid used to generate the Ad-SOX9 virus was generously provided by Dr. Veronique Lefebvre at the Cleveland Clinic Lerner Research Institute.
Declaration of funding and role of the funding source
The study was funded by R01 AR062507 to RS; HHMI 56005705 (PI Patel) and T32 AR069516 (PI Bridges) to RDC; T90 DE022736 (PI MacDougall) to GC: and F32 AR061246 to JP. The study sponsors had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors contributed to the conception and design of the study or the acquisition, analysis and interpretation of the data. All authors contributed to drafting the manuscript or revising for intellectual content. All authors approved the final version of the manuscript. All authors take responsibility for the integrity of the work.
Competing interest statement
There are no conflicts to declare.
References
- 1.Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263(26):12828–12831. [PubMed] [Google Scholar]
- 2.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71(2):279–290. [PubMed] [Google Scholar]
- 4.Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun. 2000;274(2):467–471. doi: 10.1006/bbrc.2000.3167. [DOI] [PubMed] [Google Scholar]
- 5.Ramaswamy G, Sohn P, Eberhardt A, Serra R. Altered responsiveness to TGF-beta results in reduced Papss2 expression and alterations in the biomechanical properties of mouse articular cartilage. Arthritis Res Ther. 2012;14(2):R49. doi: 10.1186/ar3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56(7):2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 7.Coles JM, Zhang L, Blum JJ, Warman ML, Jay GD, Guilak F, Zauscher S. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62(6):1666–1674. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, Melrose J, Sonnabend DH, Flannery CR, Little CB. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8(2):R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, Jiang MM, Yustein J, Gannon F, Lee BH. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugahara K, Schwartz NB. Defect in 3'-phosphoadenosine 5'-phosphosulfate synthesis in brachymorphic mice. I. Characterization of the defect. Arch Biochem Biophys. 1982;214(2):589–601. doi: 10.1016/0003-9861(82)90064-9. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen CD, Boles JW. Sulfation and sulfotransferases 5: the importance of 3'-phosphoadenosine 5'-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997;11(6):404–418. doi: 10.1096/fasebj.11.6.9194521. [DOI] [PubMed] [Google Scholar]
- 12.Cho YR, Lee SJ, Jeon HB, Park ZY, Chun JS, Yoo YJ. Under-sulfation by PAPS synthetase inhibition modulates the expression of ECM molecules during chondrogenesis. Biochem Biophys Res Commun. 2004;323(3):769–775. doi: 10.1016/j.bbrc.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 13.Stelzer C, Brimmer A, Hermanns P, Zabel B, Dietz UH. Expression profile of Papss2 (3'-phosphoadenosine 5'-phosphosulfate synthase 2) during cartilage formation and skeletal development in the mouse embryo. Dev Dyn. 2007;236(5):1313–1318. doi: 10.1002/dvdy.21137. [DOI] [PubMed] [Google Scholar]
- 14.Faiyaz ul Haque M, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, Superti-Furga A, Haque S, Abbas H, Ahmad W, Ahmad M, Cohn DH. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat Genet. 1998;20(2):157–162. doi: 10.1038/2458. [DOI] [PubMed] [Google Scholar]
- 15.Sugahara K, Schwartz NB. Defect in 3'-phosphoadenosine 5'-phosphosulfate formation in brachymorphic mice. Proc Natl Acad Sci U S A. 1979;76(12):6615–6618. doi: 10.1073/pnas.76.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentation and osteoarthritis. Journal of Cell Biology. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276(1):124–142. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Ford-Hutchinson AF, Ali Z, Seerattan RA, Cooper DM, Hallgrimsson B, Salo PT, Jirik FR. Degenerative knee joint disease in mice lacking 3'-phosphoadenosine 5'-phosphosulfate synthetase 2 (Papss2) activity: a putative model of human PAPSS2 deficiency-associated arthrosis. Osteoarthritis Cartilage. 2005;13(5):418–425. doi: 10.1016/j.joca.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Mabuchi A, Fukuda A, Hiraoka H, Kawakami A, Yamamoto S, Machida H, Takatori Y, Kawaguchi H, Nakamura K, Ikegawa S. Identification of sequence polymorphisms in two sulfation-related genes, PAPSS2 and SLC26A2, and an association analysis with knee osteoarthritis. J Hum Genet. 2001;46(9):538–543. doi: 10.1007/s100380170036. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pateder DB, Ferguson CM, Ionescu AM, Schwarz EM, Rosier RN, Puzas JE, O'Keefe RJ. PTHrP expression in chick sternal chondrocytes is regulated by TGF-beta through Smad-mediated signaling. J Cell Physiol. 2001;188(3):343–351. doi: 10.1002/jcp.1118. [DOI] [PubMed] [Google Scholar]
- 22.Chimal-Monroy J, Diaz de Leon L. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41(1):91–102. [PubMed] [Google Scholar]
- 23.DuRaine GD, Chan SM, Reddi AH. Effects of TGF-beta1 on alternative splicing of Superficial Zone Protein in articular cartilage cultures. Osteoarthritis Cartilage. 2011;19(1):103–110. doi: 10.1016/j.joca.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Amano K, Hata K, Sugita A, Takigawa Y, Ono K, Wakabayashi M, Kogo M, Nishimura R, Yoneda T. Sox9 family members negatively regulate maturation and calcification of chondrocytes through up-regulation of parathyroid hormone-related protein. Mol Biol Cell. 2009;20(21):4541–4551. doi: 10.1091/mbc.E09-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardingham TE, Oldershaw RA, Tew SR. Cartilage, SOX9 and Notch signals in chondrogenesis. J Anat. 2006;209(4):469–480. doi: 10.1111/j.1469-7580.2006.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tew SR, Pothacharoen P, Katopodi T, Hardingham TE. SOX9 transduction increases chondroitin sulfate synthesis in cultured human articular chondrocytes without altering glycosyltransferase and sulfotransferase transcription. Biochem J. 2008;414(2):231–236. doi: 10.1042/BJ20080262. [DOI] [PubMed] [Google Scholar]
- 27.Tew SR, Li Y, Pothacharoen P, Tweats LM, Hawkins RE, Hardingham TE. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2005;13(1):80–89. doi: 10.1016/j.joca.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Kondo M, Yamaoka K, Sonomoto K, Fukuyo S, Oshita K, Okada Y, Tanaka Y. IL-17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8(11):e79463. doi: 10.1371/journal.pone.0079463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Vukicevic S, Sampath TK, Luyten FP. Bovine articular chondrocytes do not undergo hypertrophy when cultured in the presence of serum and osteogenic protein-1. Biochem Biophys Res Commun. 1993;197(3):1253–1259. doi: 10.1006/bbrc.1993.2612. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30(2):109–116. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhai Z, Yao Y, Wang Y. Importance of suitable reference gene selection for quantitative RT-PCR during ATDC5 cells chondrocyte differentiation. PLoS One. 2013;8(5):e64786. doi: 10.1371/journal.pone.0064786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian AR, Kaufmann M, Morgenstern B. DIALIGN-TX: greedy and progressive approaches for segment-based multiple sequence alignment. Algorithms Mol Biol. 2008;3:6. doi: 10.1186/1748-7188-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. http://www.genomatix.de/solutions/genomatix-software-suite.html. [Google Scholar]
- 37.Genzer MA, Bridgewater LC. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35(4):1178–1186. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentsendorj O, Nagy A, Sinko I, Daraba A, Barta E, Kiss I. Highly conserved proximal promoter element harbouring paired Sox9-binding sites contributes to the tissue- and developmental stage-specific activity of the matrilin-1 gene. Biochem J. 2005;389(Pt 3):705–716. doi: 10.1042/BJ20050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohba S, He X, Hojo H, McMahon AP. Distinct Transcriptional Programs Underlie Sox9 Regulation of the Mammalian Chondrocyte. Cell Rep. 2015;12(2):229–243. doi: 10.1016/j.celrep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27(12):2511–2525. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Yamamoto K, He X, Otsuki B, Kim Y, Murao H, Soeda T, Tsumaki N, Deng JM, Zhang Z, Behringer RR, Crombrugghe B, Postlethwait JH, Warman ML, Nakamura T, Akiyama H. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat Commun. 2011;2:251. doi: 10.1038/ncomms1242. PMCID: 4040945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280(9):8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 44.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281(34):24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 46.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277(33):29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.