Abstract
The regenerative capacity of the liver is essential for recovery from surgical resection or injuries induced by trauma or toxins. During liver regeneration, the concentration of nicotinamide adenine dinucleotide (NAD) falls, at least in part due to metabolic competition for precursors. To test whether NAD availability restricts the rate of liver regeneration, we supplied nicotinamide riboside (NR), an NAD precursor, in the drinking water of mice subjected to partial hepatectomy. NR increased DNA synthesis, mitotic index, and mass restoration in the regenerating livers. Intriguingly, NR also ameliorated the steatosis that normally accompanies liver regeneration. To distinguish the role of hepatocyte NAD levels from any systemic effects of NR, we generated mice overexpressing Nicotinamide phosphoribosyltransferase (Nampt), a rate-limiting enzyme for NAD synthesis, specifically in the liver. Nampt overexpressing mice were mildly hyperglycemic at baseline and, similarly to the mice treated with NR, exhibited enhanced liver regeneration and reduced steatosis following partial hepatectomy. Conversely, mice lacking Nampt in hepatocytes exhibited impaired regenerative capacity that was completely rescued by administering NR.
Conclusion
NAD availability is limiting during liver regeneration and supplementation with precursors such as NR may be therapeutic in settings of acute liver injury.
Keywords: Nicotinamide riboside, NAD metabolism, Nampt, phosphoribosyltransferase, steatosis
Introduction
The liver is one of the few structures that is capable of substantial regeneration in mammals, and can fully re-grow from less than 30% of its original mass1, 2. This regenerative capacity is critical to survival under conditions such as traumatic injury, exposure to hepatotoxins or infectious agents, or surgical resection of a tumor. Recovery of liver function is highly dependent upon increasing energy production necessary for restoring liver mass3. However, the mechanisms that are important for the liver to respond to acute and chronic injury and the biological pathways that govern the ability of the liver to regenerate are not well understood. As such, there is substantial interest in therapeutic approaches that could accelerate the regenerative process and speed the return to normal liver function in patients with severe liver injury.
It has been known for decades that a transient decrease in nicotinamide adenine dinucleotide (NAD) content accompanies the early stages of liver regeneration4. This has generally been attributed to competition with nucleic acid synthesis, which requires many of the same precursors and cofactors, including ATP and phosphoribosylpyrophosphate (PRPP)5, 6. Both of the major routes for ATP generation, glycolysis and mitochondrial respiration require NAD as a cofactor and several classes of enzymes require the molecule as a cosubstrate to execute their signaling functions7. Thus, NAD deficiency in the injured liver might be expected to compromise both energetics and intracellular signaling, either of which could plausibly slow regeneration. Moreover, NAD deficiency in an otherwise healthy liver decreases oxidation of fatty acids and promotes steatosis, which is a characteristic feature of liver regeneration8–11. At present, however, it remains unclear whether the changes in NAD concentration observed in vivo have a meaningful impact on liver regeneration, or whether other intrinsic aspects of the regenerative process ultimately control the pace at which new hepatocytes are produced.
To directly test whether NAD availability is limiting for liver regeneration, we performed partial hepatectomy, a well-established model for liver injury, in mice given the NAD precursor nicotinamide riboside (NR) in the drinking water. To probe the role of NAD metabolism more specifically in hepatocytes, we also employed genetic strategies to overexpress or delete Nicotinamide phosphoribosyltransferase (Nampt), a rate-limiting enzyme for NAD biosynthesis. Our results demonstrate that NAD availability is indeed limiting for liver regeneration and suggest that strategies to increase hepatic NAD content may be protective against liver injuries or toxic insults.
Materials and Methods
Animals, housing and drug treatment
Mice overexpressing Nampt in hepatocytes were obtained by crossing animals carrying an exogenous copy of the Nampt cDNA separated from the CAGGS promoter by a floxed stop cassette that were backcrossed to the C57BL/6J background (as described previously8), with mice carrying Albumin-Cre (JAX strain B6.Cg-Tg(Alb-cre)21Mgn/J, stock number 003574). Littermates lacking Albumin Cre were used as controls. For studies on the adult onset of Nampt overexpression, 8–9 week old male Namptfl/fl and Namptwt/wt mice were injected in the retroorbital plexus with adeno-associated virus expressing either Cre recombinase (AAV-Cre) or enhanced green fluorescent protein (AAV-Gfp) under the control of thyroxine-binding globulin (Tbg) promoter (AAV-Cre). Namptfl/fl mice injected with AAV-Gfp and Namptwt/wt injected with AAV-Cre served as controls. For inducible deletion of Nampt, we employed mice originally created by Oberdan Leo at Université de Liège and backcrossed to the C57/BL6 background and generously provided by Shin-Ichiro Imai at Washington University9, 12. As above, 8–9 week old male mice bearing two floxed alleles were injected retroorbitally with adeno-associated virus expressing either Cre recombinase (AAV-Cre) or enhanced green fluorescent protein (AAV-Gfp) under the control of thyroxine-binding globulin (Tbg) promoter. Fasting blood glucose was measured and partial hepatectomies were performed at >14 days after AAV infection in both the adult onset overexpression and liver-specific knock out cohorts. Animals were housed in groups of 4–5 mice/cage in pathogen-free barrier facility in a 12h light-dark cycle with free access to food and water. For experiments containing only wild type animals, male C57BL/6J mice were ordered directly from Jackson Labs. For nicotinamide riboside (NR) supplementation experiments, NR (Chromadex Inc.) was dissolved in the drinking water at 3.0 mg/ml in light-protected bottles and was changed every 3 days. For fasting and re-feeding experiments, animals were fasted for 16h with access to water ad libitum in cages containing Alpha-dri bedding. All animal work was performed in accordance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee.
Partial hepatectomy
10–14 week old male transgenic mice underwent partial hepatectomy (PHx) according to the protocol of Mitchell and Willenbring10, 11 between 8 AM and noon. Briefly, after isofluorane anesthesia, a ventral midline incision was made. Then the median and left lateral lobes, comprising 70% of the liver, were resected by pedicle ligation. For EdU (5-ethynyl-2′-deoxyuridine) labeling (Molecular probes), mice were injected intraperitoneally with the labeling reagent at a dose of 16mg/kg 12h before sacrifice, except for the 36h time point, in which case EdU was injected 5h before sacrifice to capture the peak of DNA synthesis. Animals were sacrificed at 24h, 36h, 48h and 192h after PHx, as indicated. The resected and regenerating livers were snap frozen in liquid nitrogen, fixed in 4% paraformaldehyde, embedded in OCT medium (Tissue-TEK O.C.T compound, Sakura), or rapidly frozen in pre-chilled metal clamps in liquid nitrogen for NAD and ATP assays. All surgical procedures involving mice were approved by the Institutional Animal Care and Use Committee protocols of the University of Pennsylvania.
Histology, Immunohistochemistry, and Immunofluoresence
Mitotic figures were counted in hematoxylin and eosin stained slides. Paraffin embedded liver sections of 5μm thickness were deparaffinized in Xylene followed by rehydration through a graded ethanol series were stained with Gill 3 hematoxylin (Thermo Scientific) and eosin (Sigma). Mitotically active areas were first screened under lower magnification. For quantification, total mitotic counts in 10 high power fields (40X) in the most mitotically active areas were considered. Representative photomicrographs were taken at 400X magnification using a light microscope coupled with a digital image acquisition system. For immunofluorescent detection of Nampt expression in paraffin sections, antigens were retrieved in R-buffer (Electron Microscopy Sciences Ct # 62706-10) followed by quenching and blocking in Cas-Block (Invitrogen 00-8120) for 20 min. Slides were then incubated overnight at 4°C in humidified chamber with primary rabbit polyclonal anti-NAMPT antibody (Bethyl Laboratories, dilution 1:1000). Goat anti-rabbit IgG (Alexa Fluor 488) was used as secondary antibody at dilution of 1:600 and slides were counter-stained with DAPI (Vector laboratories). For quantification, cells were examined and imaged using a Zeiss Axioplan 2 imaging system under a 20X objective taking both the central and portal venous areas. For Ki67 staining, paraffin embedded liver sections were stained by standard immunoperoxidase method, using antigen retrieval in Pickcell for 10–15 minutes in1X R-Buffer A (Electron Microscopy Sciences Cat # 62706-10). Sections were incubated with rabbit monoclonal primary anti-Ki67 (Vector Labs VP-RM04, 1:200) at 4°C overnight. Biotinylated anti-rabbit secondary antibody (1:600) (Vector Laboratories) was used followed by detection with the Vectastain ABC kit. Slides were counterstained in hematoxylin. Images were captured at 200X magnification using a Nikon E600 bright field microscope.
EdU staining
Paraffin-embedded liver sections were stained for EdU using either Click-iT EdU Alexa Fluor 488 or Alexa Fluor 594 Imaging Kit (Molecular Probes) according to manufacturer’s protocol and 5–6 random areas of each liver were imaged. The fraction of EdU positive hepatocytes was determined by counting manually at 200X in both central vein and portal triad regions and normalizing the DAPI counterstain.
Oil Red O staining
Liver samples frozen in OCT were sectioned at 5 μm thickness and fixed in 10% Neutral Buffered Formalin. Slides were placed in propylene glycol (Sigma) for 2 min and then incubated in Oil Red O (Sigma) for 10 min at 60°C. The slides were then transferred to 85% propylene glycol solution for 1 min and counterstained with hematoxylin (Fisher Scientific). Representative images were captured at 200X magnification using a Nikon E600 bright field microscope coupled with a digital image acquisition system.
Immunoblotting
For Western blot analysis, snap frozen liver pieces were lysed in RIPA buffer supplemented with Halt phosphatase inhibitors (Thermo Scientific) and complete protease inhibitors (Roche) in a tissue lyzer (Qiagen). Tissue lysates were pre-clarified by centrifugation at 15,000 g for 15 minutes at 4°C. After denaturation, samples were resolved on 4–15% SDS-PAGE gels, transferred to PVDF membranes (Milipore) on a wet transfer apparatus (Bio-Rad), and probed using anti-NAMPT (Bethyl Laboratories, PBEF (A300-372A), dilution 1:5000) and HRP conjugated β-actin (Abcam, ab49900) antibodies. Immunoreactive proteins were detected by chemiluminescence. Images were captured in a Bio-Rad imaging station using Super Signal West Femto substrates (Pierce).
Gene-expression analysis
Total RNA was extracted from frozen liver with Trizol (Sigma-Aldrich). 1μg of RNA was reverse transcribed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s recommendations. Real time PCR was performed on an Applied Biosystems 7900HT system with SYBR green master mix (Applied Biosystems). Three technical replicates were obtained for each sample and a relative standard curve method was used to quantify the results obtained using gene-specific primers. TATA box binding protein gene (TBP) and β-actin were used as a housekeeping gene. Primers used for RT-qPCR are listed in Supporting Table S1.
NAD Metabolite Extraction from Liver
NAD was extracted from 50 mg of clamp frozen liver in 0.6 M perchloric acid at 4 °C using a tissue lyzer (Qiagen) with a metal bead set at 20 Hz for 2 min. The insoluble materials were pelleted by at 15,000 g for 10 min at 4 °C and the clear supernatant was diluted to 1:100 in ice-cold 100 mM phosphate buffer, pH 8. NAD was measured by an enzymatic cycling assay in a 96-well format modified from the procedure of Graeff and Lee13 as described previously8. Briefly, the cycling mix was prepared freshly before use, 5ul of NAD standards or diluted tissue extracts were mixed with 95 μl of cycling mixture comprising of (2% ethanol, 100 μg/ml alcohol dehydrogenase, 10 μg/ml diaphorase, 20 mM resazurin, 10 mM flavin mononucleotide, 10 mM nicotinamide, 0.1% BSA in 100 mM phosphate buffer, pH 8.0). The cycling reaction was allowed to proceed for 30 min at room temperature, and the concentration of NAD was determined based on the rate of resorufin accumulation, measured as fluorescence at excitation at 544nm and emission at 590 nm. NADH was similarly extracted from 50 mg of clamp frozen liver tissue in pre chilled extraction buffer (25 mM NH4Ac, 25 mM NaOH, 50% (v/v) acetonitrile) bubbled with nitrogen gas. Alkaline lysates were mixed 1:1 (v/v) with ethanol extraction buffer (250 mM KOH, 50% (v/v) EtOH) and heated at 55 °C for 10 min. Lysate supernatants were diluted 1:50 in ice-cold 100 mM phosphate buffer, pH 8 for NADH measurement by cycling assay.
Hepatic triglyceride assay
25 mg of snap frozen liver tissue was extracted in cell lysis buffer (140mM NaCl, 50mM Tris, pH 7.4, 0.1% Triton-X) using a tissue lyser. Samples were centrifuged for 15 min at 14,000 rpm and triglycerides were assayed with a Triglyceride Assay Kit (Stanbio) using glycerol as a standard.
ATP determination
ATP was measured in neutralized acid extracts of ~ 50 mg of snap frozen liver using ATP determination Kit (Life Technologies) according to the manufacturer’s protocol.
Glucose measurement
Blood glucose values were measured from the tail vein using a OneTouch Ultra glucose analyzer after an overnight (16h) fast or under random fed conditions.
Free fatty acid assay
Plasma non-esterified fatty acid was measured using free fatty acid (FFA) Kit (Sigma-Aldrich, MAK044).
Statistics
All results are expressed as mean ± SEM. Comparisons between two groups were analyzed using unpaired 2-tailed Student’s t test. Comparisons between three or more groups were analyzed using one-way ANOVA in Prism 6 (GraphPad), followed by post-hoc analysis with Student’s t test. For correlation matrices, data were analyzed using Pearson correlation coefficients. Differences between means were defined as significant at P < 0.05.
Results
Nicotinamide riboside promotes liver regeneration
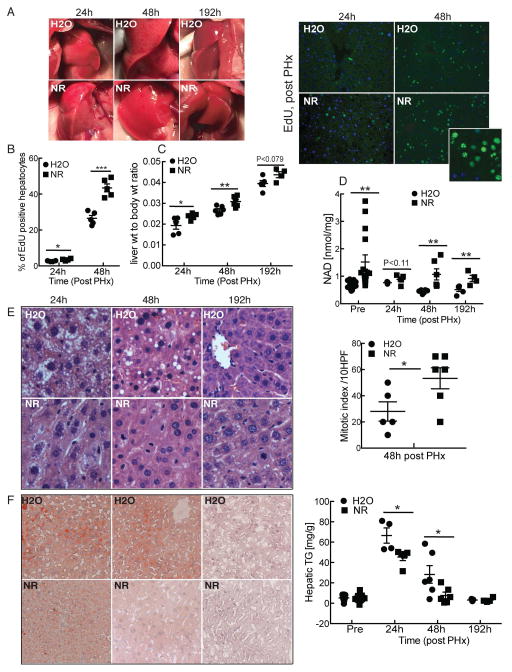
To test whether NAD availability limits the rate of liver regeneration, we supplemented the drinking water of wild-type C57BL/6J mice with NR (3 mg/ml, ~500 mg/kg) beginning 14 days prior to two-thirds partial hepatectomy (PHx). While regenerating livers from control mice were pale with a characteristic mottled pattern at early time points after PHx, those from NR-treated mice were more uniformly colored (Fig. 1A, left) and displayed greatly enhanced hepatocyte proliferation, as assessed by incorporation of EdU into DNA (Fig. 1A, right and B) or Ki-67 immunostaining (Supporting Fig. S1A). Consistently, expression of Cyclin D1 and Cyclin dependent kinase 4 (Cdk4) were increased in NR treated mice at 24h post PHx (Supporting Fig. S1B). Increased hepatocyte replication was reflected by a significant improvement in liver mass at early time points and a trend toward increased mass even at eight days, when regeneration is considered complete14 (Fig. 1C). Notably, we have not observed changes in the size of the liver in control mice given NR, and there was no difference in the weights of the resected lobes between the two groups (Supporting Fig. S1C). In addition, while both groups of mice lost weight after surgery, body weight stabilized more quickly in the NR treated mice than in controls (Supporting Fig. S1D). As expected, NAD content was increased in the livers of NR treated mice both pre (~22%) and post PHx (~42%, at 48h) and remained significantly elevated through 192h (Fig. 1D). In general, NADH content moved in parallel with that of NAD, such that there was no major shift in redox potential (Supporting Fig. S1E).
Figure 1. Nicotinamide riboside promotes liver regeneration.
10–14 week old male C57BL6/J mice were treated with nicotinamide riboside (NR) at a dose of ~500mg/kg/day. After 14 days, animals were subjected to 2/3 partial hepatectomy and analyzed 24, 48 and 192 hours later (n = 6 per group).
(A) Left: Photographs of regenerating livers. Right: EdU incorporation in regenerating livers. Inset shows an enlarged view from an NR-treated liver at 48h post PHx.
(B) Quantification of EdU positive hepatocytes.
(C) Liver to body weight ratio.
(D) Liver NAD content pre and post PHx.
(E) Left: Representative liver sections stained with H&E at 40X showing mitotic figures and micro and macro-vesicular fatty changes. Right: Quantification of mitotic figures across multiple high power fields.
(F) Left: Oil Red O staining to detect neutral lipids. Right: Hepatic triglyceride content in pre and post hepatectomized NR and H2O treated mice.
Error bars represent S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Analysis of H&E stained sections revealed a 48% increase in mitotic activity in the presence of NR (Fig. 1E). Moreover, the fatty changes characteristic of early stages of liver regeneration, while readily apparent in controls, were reduced at 24h post PHX in NR treated mice and nearly absent by 48h. Hepatic steatosis was characterized by the presence of both micro and macro vesicular lipid droplets (Fig. 1F, left) as well as accumulation of hepatic triglycerides (Fig. 1F, right). Glycemia and circulating free fatty acids (FFA) were similar between the two groups, although NR treated mice had very slightly higher glucose levels pre-PHx and a slightly, but significantly lower FFA level immediately following PHx (Supporting Fig. S1F). Thus, NR treatment is sufficient to increase hepatic NAD content and significantly promotes liver regeneration while ameliorating fatty changes.
Hepatocyte-specific Nampt over-expression recapitulates the effects of systemic NR on liver regeneration
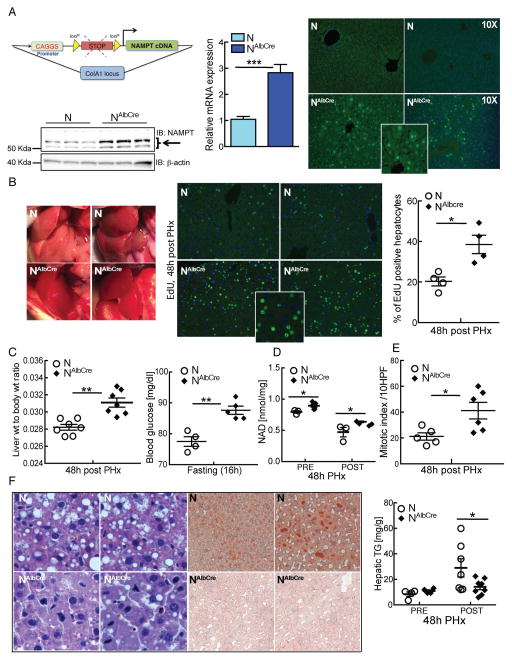
Because oral NR may have systemic effects that influence the rate of liver regeneration, we next wanted to test whether NAD synthesis specifically in hepatocytes is sufficient to recapitulate our observations. We employed a strain of mice that overexpress Nicotinamide phosphoribosyltransferase (Nampt) in a Cre-inducible manner8, crossed to mice expressing Cre under the control of the Albumin promoter (Fig. 2A, left). Nampt overexpression was not uniform (Fig. 2A, right), and was markedly more prominent near the peri portal and central venous areas. By 48 hours following PHx, Nampt-overexpressing livers appeared darker and more uniform in color (Fig. 2B, left) and, as was observed with NR treatment, had dramatically enhanced EdU incorporation (Fig. 2B, right). These changes correlated with an increase in the size of regenerated livers (Fig. 2C, left). Notably, hepatocyte-specific overexpression of Nampt did not affect body or liver weight prior to surgery (Fig. 1C and data not shown), although we noted a mild elevation of fasting glucose levels (Fig. 2C, right). Liver NAD content was significantly elevated both pre- and post-PHx (Fig. 2D). Mitotic indices were increased, and accumulation of lipid droplets and triglyceride content were dramatically decreased (Fig. 2E and F). Accordingly, liver-specific enhancement of NAD biosynthesis recapitulates the key effects of systemic NR treatment on liver regeneration.
Figure 2. Hepatocyte-specific overexpression of Nampt promotes liver regeneration.
Mice carrying a Cre-inducible allele of Nampt in addition to Alb-Cre (denoted as NAlbcre) and littermate controls (N) were subjected to 2/3 partial hepatectomy and analyzed 48 hours later.
(A) Nampt overexpression is driven by the CAGGS promoter, separated from the NAMPT transgene by a STOP cassette flanked with loxP sites. Under the influence of Cre recombinase, the STOP cassette is removed, resulting in overexpression of Nampt. The construct was inserted at the ColA1 locus. Left: Nampt protein and mRNA expression in liver. Right: Immunofluorescence showing overexpression of Nampt in peri-portal and peri-central venous areas of NAlbcre mice. Inset shows an enlarged view of NAlbcre.
(B) Left: Photographs of regenerating livers. Right: Proliferating hepatocytes identified by EdU detected by immunofluorescence (green) and counterstained with DAPI (blue) with quantification of EdU positive hepatocytes (n=4–5/group). Inset shows an enlarged view of NAlbcre.
(C) Left: Liver to body weight ratios. Right: Fasting blood glucose.
(D) Liver NAD content before and after PHx.
(E) Mitotic index as determined by counting mitotic figures in hepatocytes under high power in H&E stained sections.
(F) Left: Representative liver sections stained with H&E. Right: Hepatic lipid content as determined by Oil Red O and triglyceride assay.
Error bars represent S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Adult onset over-expression is sufficient for the beneficial effects of Nampt
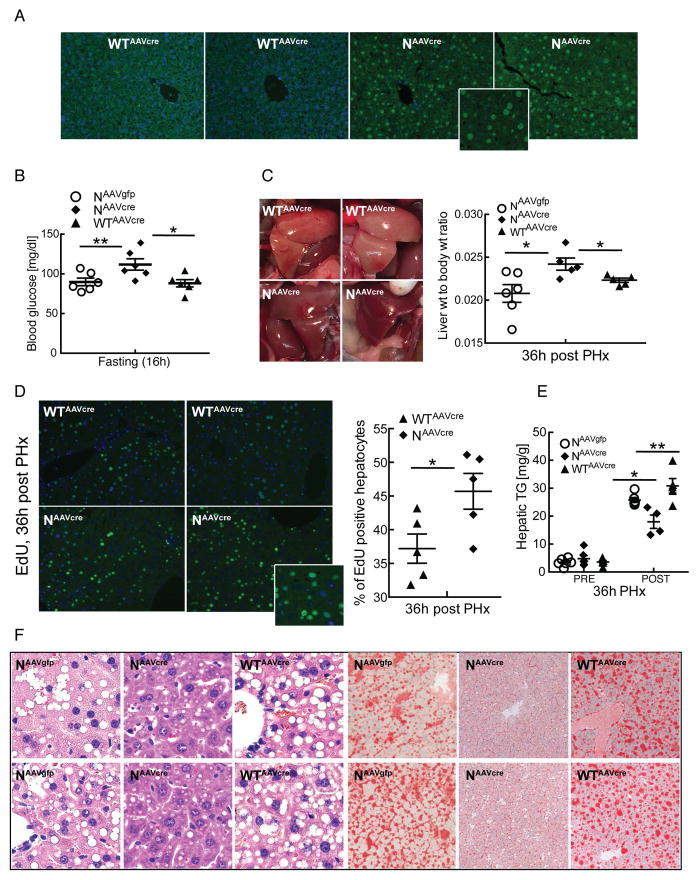
To exclude the possibility that improved regeneration in Nampt-overexpressing mice could be secondary to metabolic adaptations in early life, we performed a second set of experiments in which Cre was delivered via adeno-associated virus (AAV) in adult mice. Nampt overexpressing mice from this experiment were compared to two distinct sets of littermate controls: those carrying the floxed allele and infected with AAV encoding GFP and wild type mice infected with AAV-Cre. Mice that overexpressed Nampt in their livers only during adulthood (Fig. 3A) displayed mildly elevated fasting glucose (Fig. 3B), darker color, increased mass, and enhanced hepatocellular proliferation (Fig. 3C–D), similar to the results obtained in mice with life-long overexpression. Whereas the previous groups of mice were sacrificed at 48 hours post PHx to allow quantification of mitotic figures, the adult onset group was sacrificed at 36 hours to capture the peak of DNA synthesis (Fig. 3D). As in the previous experiments, Nampt overexpression attenuated acute hepatic steatosis and accumulation of hepatic triglycerides (Fig. 3E and F).
Figure 3. Adult onset Nampt over-expression is sufficient for regenerative phenotypes.
Mice carrying a Cre-inducible allele of Nampt infected with AAV-Cre (denoted as NAAVcre) and littermate controls (NAAVgfp or WTAAVcre) were subjected to 2/3 partial hepatectomy and analyzed 36 hours later.
(A) Immunofluorescence showing over-expression of Nampt in peri-portal and peri-central venous areas of NAAVcre mice. Inset shows an enlarged view of NAAVcre.
(B) Fasting blood glucose.
(C) Left: Photographs of regenerating livers. Right: Liver to body weight ratios.
(D) Left: Proliferating hepatocytes identified by EdU detected by immunofluorescence (green) and counterstained with DAPI (blue). Inset shows an enlarged view of NAAVcre. Right: Quantification of EdU-positive hepatocytes (n=5/group).
(E) Hepatic triglyceride content.
(F) Left panels: representative liver sections stained with H&E. Right panels: lipid accumulation by Oil Red O.
Error bars represent S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Hepatocyte-specific deletion of Nampt impairs liver regeneration and is completely rescued by nicotinamide riboside (NR)
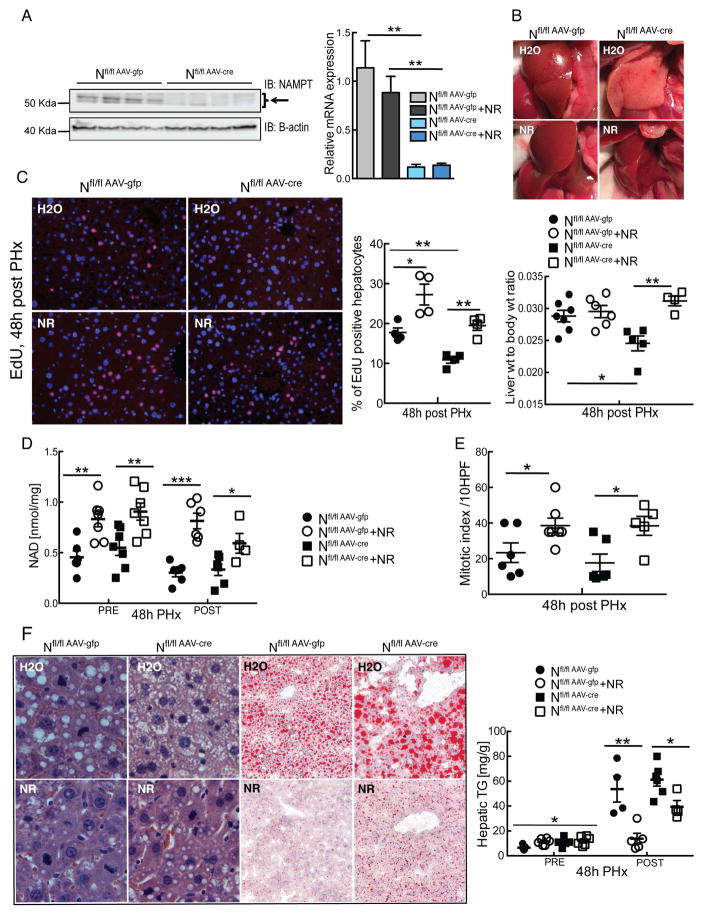
Nampt is secreted from adipose tissue and immune cells, and the extracellular form (eNampt/PBEF/Visfatin) remains catalytically active9, 15, 16. Nampt secretion from primary hepatocytes has also been observed17, and therefore, it remains formally possible that overexpression of Nampt in hepatocytes from our transgenic mice leads to spillover into the circulation that could have NAD-dependent effects in other cell types. Moreover, eNampt has been proposed to have functions that are independent of its catalytic activity18, raising the possibility that NR and Nampt overexpression could work through independent pathways to influence liver regeneration. To address these points, we generated hepatocyte-specific Nampt deficient mice by injecting AAV-Cre into adult animals bearing two floxed alleles for Nampt (Fig. 4A). Littermates carrying the floxed allele that were infected with AAV-GFP served as controls. Five days after infection, half of the mice in each group were placed on drinking water containing NR and treated for an additional 2 weeks prior to PHx. Macroscopically, regenerated livers of Nfl/flAAV-Cre mice were pale with petechial hemorrhage, which was prevented by NR treatment (Fig. 4B). Liver regeneration during the first 48 hours after PHx was markedly compromised in the Nampt KO mice, and restored by NR treatment (Fig. 4C), which effectively increased NAD content in both genotypes (Fig. 4D). Consistently NR treatment largely restored mitotic index in the Nampt KO mice (Fig. 4E), and again there was no change in the weight of the resected lobes, indicating that all livers were normal size prior to injury.
Figure 4. Hepatocyte-specific loss of Nampt impairs regeneration, and is rescued by nicotinamide riboside (NR).
Hepatocyte specific Nampt deficient mice were generated by injecting animals bearing two floxed alleles with an AAV expressing Cre recombinase (AAV-Cre). Littermates infected with AAV-Gfp served as controls. Treatment with NR (~500 mg/kg/day) began 5 days after infection and 2 weeks prior to PHx. Analyses were performed 48 hours after PHx.
(A) Protein and mRNA expression of Nampt in livers.
(B) Photographs of regenerating livers.
(C) Left: Proliferating hepatocytes identified by EdU detected by immunofluorescence (red) and counterstained with DAPI (blue). Middle: Quantification of EdU positive hepatocytes (n=4–5/group). Left: Liver to body weight ratios.
(D) NAD content in livers before and after PHx.
(E) Mitotic index as determined by counting mitotic figures in hepatocytes under high power in H&E stained sections (n= 5–6/group).
(F) Left: Representative liver sections stained with H&E (left panels) and Oil Red O (right panels). Right: Hepatic triglyceride content.
Error bars represent S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Histological examination revealed dramatic hepatic steatosis in Nfl/flAAV-Cre mice, comprising both micro and macro-vesicular lipid droplets (Fig. 4F, left upper panels). In contrast, NR abolished hepatic lipid accumulation in regenerating wild type livers and nearly normalized hepatic fatty changes in the NAMPT deficient regenerating livers (Fig. 4F, left lower panels). Hepatic triglycerides were also reduced by ~75% in Nfl/flAAV-Gfp and ~35% in Nfl/flAAV-Cre mice with NR treatment (Fig. 4F, right). Our results demonstrate that loss of endogenous Nampt expression in hepatocytes impairs liver regeneration and deteriorates lipid metabolism, and that supplementation with an NAD precursor is sufficient to ameliorate these changes.
Liver NAD content strongly correlates with energy status, triglyceride accumulation, and hepatocyte proliferation
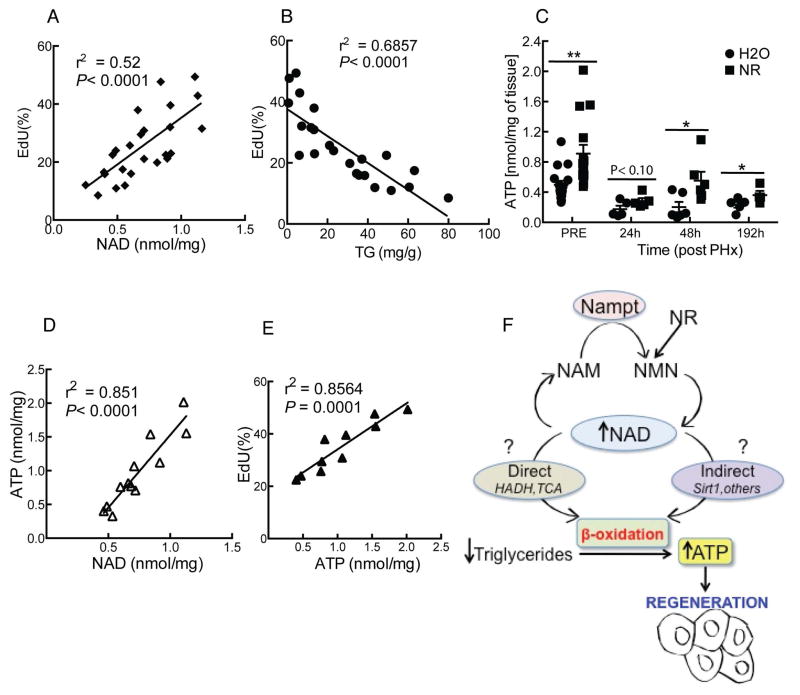
To gain further insight into the relationships between NAD, lipid metabolism and hepatocyte proliferation, we tested whether these correlations hold true at the level of individual animals. Hepatocyte proliferation displays a striking positive correlation with liver NAD content and an inverse correlation with hepatic triglycerides (Fig. 5A and B) We speculated that this could reflect the need for optimal NAD levels to allow oxidation of lipids to provide energy for cell growth and division, either due to the direct requirement for NAD in the 3-hydroxyacyl-CoA dehydrogenase (HADH)-catalyzed step of fatty acid oxidation and the tricarboxylic acid cycle (TCA), or due to its role as a cosubstrate for signaling enzymes such as sirtuins. ATP levels have previously been reported to drop rapidly (within 30 seconds) after PHx independently from energetic demand19. Consistent with the hypothesis that optimal NAD levels are required to facilitate the use of lipids as a fuel source, NR treatment greatly accelerated the recovery of ATP concentration during regeneration (~75% increase at 48h post PHx, Fig. 5C). Moreover, ATP content correlated positively with both NAD and hepatocyte proliferation (Fig. 5D and E), suggesting that NR can alleviate the energetic stress imposed by ongoing regeneration in the liver.
Figure 5. Hepatocyte proliferation exhibits a strong correlation with liver NAD concentration and an inverse relationship with hepatic lipid content.
Data from individual animals were plotted to study the correlations between NAD, lipid metabolism and hepatocyte proliferation.
(A) Positive correlation between NAD concentration and hepatocyte proliferation.
(B) Inverse correlation between hepatocyte proliferation and triglyceride content.
(C) Liver ATP content assessed pre or post hepatectomy in NR treated mice (n=6/group).
(D,E) ATP correlates positively with NAD concentration and hepatocyte proliferation.
(F) Proposed model: Hepatic NAD promotes fatty acid oxidation, thereby generating ATP necessary for hepatocellular growth and regeneration. NAD is directly required in the 3-hydroxyacyl-CoA dehydrogenase (HADH)-catalyzed step of fatty acid oxidation as well as the tricarboxylic acid cycle (TCA), but may also act indirectly via signaling enzymes such as Sirt1, which use NAD as a cosubstrate. NAD concentration can be modulated based on the expression of Nampt, which catalyzes the formation of nicotinamide mononucleotide (NMN) from nicotinamide and phosphoribosylpyrophosphate. Alternatively, NMN can be generated from NR by the action of NR kinase.
Error bars represent S.E.M. *, p < 0.05; **, p < 0.01.
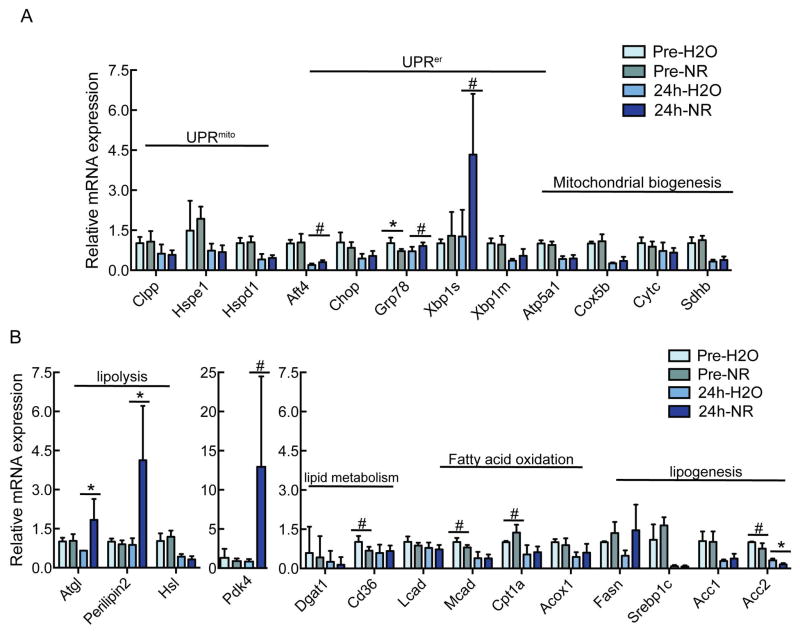
NR enhances expression of lipolytic genes during regeneration
NR has been proposed to alleviate hepatic steatosis via induction of the mitochondrial unfolded protein response (UPRmito) and subsequent induction of genes related to beta oxidation and mitochondrial biogenesis as well as widespread suppression of genes related to lipogenesis20. In contrast, NR treatment does not induce the UPRmito or have a consistent effect on the expression of mitochondrial genes in the regenerating liver (Fig. 6A). Instead, we observed selective induction of adipocyte triglyceride lipase (Atgl) and perilipin 2, and suppression of acetyl-CoA carboxylase 2 (Acc2) in NR-treated regenerating livers (Fig. 6B). Atgl catalyzes the first step in triglyceride breakdown, whereas perilipins coat lipid droplets and regulate fat mobilization. Acc2 is the primary enzyme responsible for producing malonyl-CoA that inhibits transport of fatty acids into the mitochondria21. Thus, the gene expression profile induced by NR in the regenerating liver is consistent with enhanced breakdown of neutral lipids and import of the resulting fatty acids to mitochondria. Interestingly, hepatocyte-specific overexpression of Nampt was also sufficient to induce Atgl and perilipin 2 expression, suggesting that this is a tissue autonomous effect (Supporting Fig. S2).
Figure 6. Increased expression of lipolytic genes in NR treated mice.
10–14 week old male C57BL6/J mice were treated with NR at a dose of ~500mg/kg/day. After 2 weeks, mice were subjected to 2/3 partial hepatectomy. Hepatic gene expression was determined either pre PHx or 24h post PHx.
(A) Expression of genes related to the unfolded protein response in the mitochondria (UPRmito) or endoplasmic reticulum (UPRer), or to mitochondrial biogenesis.
(B) Expression of genes related to lipid metabolism.
Expression of Atgl, Hsl, Mcad, Acox1, Fasn, Srebp1c, Acc1, Acc2, Atf4, Xbp1m, Hspd1, Atp5a1, Sdhb and Cox5b were significantly different (p < 0.05) in regenerating liver as compared to pre-PHx livers.
Error bars represent S.E.M. #, p < 0.1; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
We have shown for the first time that a supplemental NAD precursor can improve the outcome in an experimental model of liver injury. Consistently, recovery from partial hepatectomy is also influenced by the expression level of Nampt in hepatocytes, and poor regeneration in mice lacking Nampt is completely rescued by nicotinamide riboside.
Previous studies have attributed the lower NAD concentration in regenerating livers to competition for common metabolic precursors and cofactors that are also required for synthesis of nucleic acids4–6, 22, 23. NR is taken up by cells and phosphorylated to NMN, thereby sparing one molecule of PRPP (with a net gain of one high-energy phosphate bond from ATP) as compared to conventional synthesis from nicotinamide. Genetically altering Nampt expression, however, does not introduce new metabolic precursors and yet is able to replicate the effect of NR. This suggests at two possible explanations: 1) The expansion or contraction of the hepatocyte NAD pool prior to injury might be sufficient to confer the corresponding increase or decrease in regenerative capacity. This seems plausible given that the transient loss of NAD during regeneration occurs prior to the first cell division, and thus a modest increase in pre-formed NAD might insulate a hepatocyte from this effect. 2) The natural course of regeneration from severe injuries might be inappropriately skewed toward nucleic acid synthesis at the expense of NAD, such that tipping the balance back toward NAD has a favorable effect overall. In support of the latter hypothesis, it was recently shown that supplemental nicotinamide is protective against acetaminophen-induced liver toxicity, even when administered after the injury24. In this vein, it may also be interesting to examine the activity of nicotinamide N-methyl transferase (NNMT), which can limit the production of NAD by competing for nicotinamide25. Given that NAD is required for ATP synthesis and ATP is required to make NAD, the potential for an intractable energetic crisis can be envisioned if NAD levels are allowed to dip too low. Indeed, this appears to be the major mechanism of action for cancer drugs that target Nampt activity26.
Transient hepatic steatosis is a characteristic feature of liver regeneration27–29. In other experimental settings, Nampt has been suggested to confer resistance to hepatic steatosis via NAD synthesis30 and it was recently shown that NR supplementation confers protection against steatosis in mice fed a high fat/high sucrose diet20, 31. These findings are in good agreement with our observation that steatosis is almost completely absent at 48 hours after PHx in mice given NR or overexpressing Nampt in hepatocytes. However, it will be important to determine whether these observations reflect a common underlying mechanism. Much of the triglyceride accumulation following PHx likely results from increased esterification of FFA32, which are released by adipose tissue in response to a fall in insulin. Thus, a molecule that preserves glucose (and consequently, insulin) levels might decrease fat accumulation in the liver by preventing FFA release from adipose tissue. Our data suggest that this mechanism plays only a minor role in the prevention of steatosis by NR, since circulating glucose and FFA levels are similar throughout most of the experiment (Supporting Fig. S1F). Moreover, some steatosis was visible in NR-treated mice at 24 hours post-PHx, supporting the possibility that lipids accumulate normally during regeneration in the presence of enhanced NAD synthesis, but are cleared more quickly. This would be consistent with a model where lipids are processed by the regenerating liver in an NAD-sensitive manner to provide a source of energy while sparing glucose for output into the blood stream or use as a metabolic precursor. In the absence of sufficient NAD concentration, lipid oxidation and consequently ATP levels would fall, halting cell proliferation.
While increasing NAD availability might improve regeneration solely through its role as a cofactor for enzymes directly involved in ATP production and/or by sparing precursors for nucleic acid synthesis, NAD could also act via its role as a cosubstrate for signaling enzymes, including the sirtuins. Importantly, steady-state measurements of NAD do not necessarily reflect changes in flux through such enzymes, since decreased synthesis of NAD cannot be distinguished from increased utilization. However, Nampt is known to influence the deacetylase activity of the sirtuin 1 (Sirt1)16, 33, 34, and the ability of NR to ameliorate steatosis in the context of a high fat diet requires the induction of a Sirt1-dependent transcriptional program to support β-oxidation20. While we detected only a limited overlap with this transcriptional program, our profiling revealed the induction of Atgl and perilipin 2, and suppression of Acc2 in NR-treated regenerating livers, all of which catalyze key steps in the breakdown of triglycerides and mitochondrial import of the liberated fatty acids. Whole-body disruption of perilipin 2 has been shown to reduce hepatic lipid accumulation and weight gain in mice fed a high fat diet35,36, which is contrary to our observation that increased expression correlates with decreased triglyceride content in regenerating livers. However, perilipin 2 can have complex and context-dependent effect on hepatic lipid metabolism37,38, and the effects of constitutive deletion may be at least partially related to reduced food intake and increased energy expenditure, rather than to primary effects on hepatic lipid storage39. Indeed, it has recently been shown that perilipin 2 plays an unexpected positive role in adipocyte lipolysis40,41 and promotes lipid oxidation via chaperone-mediated autophagy in mouse hepatocytes42. It will be intriguing to test whether the induction that we observe in regenerating hepatocytes has similar consequences. Overall, our data support a model where steatosis is counteracted by increased lipolysis and fatty acid utilization when NAD levels are maintained.
Recent studies have suggested roles for Sirt1 in hepatic lipid metabolism43 and liver regeneration44, 45. Jin et al showed that old mice with impaired liver regeneration express low hepatic Sirt1, and that ectopically expressing Sirt1 restores hepatocyte proliferation in response to partial hepatectomy in old mice whereas knockdown of Sirt1 impairs regeneration in young mice45. In contrast, Garcia-Rodriguez et al showed that young mice overexpressing Sirt1 have increased mortality following partial hepatectomy due to defects in bile acid regulation44. Tao et al showed that Nampt expression reduces hepatocyte triglyceride levels in uninjured livers, and that the effect is correlated with increased Sirt1 activity as well as phenocopied by overexpressing Sirt130. Accordingly, the reduction in steatosis that we observe with enhanced NAD regeneration may reflect a Sirt1-dependent effect on lipid metabolism. In addition, resveratrol, a small polyphenol that activates Sirt1, has been shown to improve hepatocyte survival in injury models, but also to slow replication46. Thus, it remains unclear whether Sirt1 activation per se could account for the beneficial effects of enhanced NAD synthesis on liver regeneration, and it will be intriguing to test the requirement for Sirt1 using knockout models.
Overall, we present the first evidence that NAD metabolism can be modulated to promote recovery from liver injury. As our findings suggest that NAD precursors may be therapeutic in a variety of settings requiring liver regeneration, further investigation into downstream mechanisms and optimal delivery strategies is warranted.
Supplementary Material
Acknowledgments
Funding:
The work was supported by grants from the National Institutes of Health (R01 AG043483 and R01 DK098656 to J.A.B).
We thank all members of the Baur lab for constructive feedback and suggestions, and especially D. Frederick and Q. Chu for advice and technical assistance. We thank Dr Klaus Kaestner for critical reading of manuscript and Dr Abir Mukherjee, Temple University Hospital, for histopathological evaluation. This work was supported by grants from the National Institutes of Health (R01-AG043483 and R01-DK098656 to J.A.B). We thank the University of Pennsylvania Diabetes Research Center (DRC) for the use of the Radioimmunoassy/Biomarkers and Mouse Metabolic Phenotyping, and Metabolism Cores (P30-DK19525) and the Molecular Pathology & Imaging Core of the Penn Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) for processing tissues for histology.
Footnotes
Conflicts of interest:
R.W.D. is an employee and stockholder of Chromadex Inc., which manufactures and distributes NR. The remaining authors declare no conflicts of interest.
Authors Contributions:
SM and JAB conceived the experiments. SM, KC, AM, JN, JGD and BA performed experiments. SM and JAB analyzed data and wrote the manuscript. RWD provided constructive feedback, advice and NR.
References
- 1.Fan ST, Lo CM, Liu CL, et al. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336–40. doi: 10.1001/archsurg.135.3.336. [DOI] [PubMed] [Google Scholar]
- 2.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–81. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris GM, Clark JB. Nicotinamide nucleotide synthesis in regenerating rat liver. Biochem J. 1971;121:655–62. doi: 10.1042/bj1210655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JB, Greenbaum AL, McLean P. The concentration and biosynthesis of nicotinamide nucleotides in the livers of rats treated with carcinogens. Biochem J. 1966;98:546–56. doi: 10.1042/bj0980546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark JB, Pinder S. Control of the steady-state concentrations of the nicotinamide nucleotides in rat liver. Biochem J. 1969;114:321–30. doi: 10.1042/bj1140321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menyhart J, Grof J. Reinvestigation of the anaerobic glycolytic potential of the regenerating rat liver. Acta Physiol Acad Sci Hung. 1972;42:129–36. [PubMed] [Google Scholar]
- 8.Frederick DW, Davis JG, Davila A, Jr, et al. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290:1546–58. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon MJ, Yoshida M, Johnson S, et al. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015;21:706–17. doi: 10.1016/j.cmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–70. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell C, Willenbring H. Addendum: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2014:9. doi: 10.1038/nprot.2014.122. [DOI] [PubMed] [Google Scholar]
- 12.Rongvaux A, Galli M, Denanglaire S, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–95. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 13.Graeff R, Lee HC. A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem J. 2002;361:379–84. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borude P, Edwards G, Walesky C, et al. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56:2344–52. doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci (Landmark Ed) 2009;14:2983–95. doi: 10.2741/3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garten A, Petzold S, Korner A, et al. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–8. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garten A, Petzold S, Barnikol-Oettler A, et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem Biophys Res Commun. 2010;391:376–81. doi: 10.1016/j.bbrc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 18.Soncini D, Caffa I, Zoppoli G, et al. Nicotinamide phosphoribosyltransferase promotes epithelial-to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J Biol Chem. 2014;289:34189–204. doi: 10.1074/jbc.M114.594721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crumm S, Cofan M, Juskeviciute E, et al. Adenine nucleotide changes in the remnant liver: An early signal for regeneration after partial hepatectomy. Hepatology. 2008;48:898–908. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gariani K, Menzies KJ, Ryu D, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–43. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burzio L, Koide SS. A functional role of polyADPR in DNA synthesis. Biochem Biophys Res Commun. 1970;40:1013–20. doi: 10.1016/0006-291x(70)90894-6. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino J, Kuhne U, Kroger H. Methylation of nicotinamide in rat liver cytosol and its correlation with hepatocellular proliferation. Biochim Biophys Acta. 1982;719:518–26. doi: 10.1016/0304-4165(82)90241-0. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Zhang L, Jiang R, et al. Protective effects of nicotinamide against acetaminophen-induced acute liver injury. Int Immunopharmacol. 2012;14:530–7. doi: 10.1016/j.intimp.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Kraus D, Yang Q, Kong D, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–62. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–42. [PubMed] [Google Scholar]
- 27.Pauta M, Rotllan N, Fernandez-Hernando A, et al. Akt-mediated FoxO1 inhibition is required for liver regeneration. Hepatology. 2016;63:1660–74. doi: 10.1002/hep.28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudnick DA. Trimming the fat from liver regeneration. Hepatology. 2005;42:1001–3. doi: 10.1002/hep.20931. [DOI] [PubMed] [Google Scholar]
- 29.Rudnick DA, Davidson NO. Functional Relationships between Lipid Metabolism and Liver Regeneration. Int J Hepatol. 2012;2012:549241. doi: 10.1155/2012/549241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao R, Wei D, Gao H, et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem. 2011;286:14681–90. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canto C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–47. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein TA, Burns GP, Tropp BE, et al. Hepatic fat accumulation during liver regeneration. J Surg Res. 1985;39:338–43. doi: 10.1016/0022-4804(85)90112-x. [DOI] [PubMed] [Google Scholar]
- 33.Garten A, Schuster S, Penke M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–46. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 34.Dahl TB, Holm S, Aukrust P, et al. Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu Rev Nutr. 2012;32:229–43. doi: 10.1146/annurev-nutr-071811-150746. [DOI] [PubMed] [Google Scholar]
- 35.Chang BH, Li L, Paul A, et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–76. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai Y, Varela GM, Jackson MB, et al. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–54. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y, Boyle S, Varela GM, et al. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol Genomics. 2012;44:1125–31. doi: 10.1152/physiolgenomics.00045.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najt CP, Senthivinayagam S, Aljazi MB, et al. Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2016;310:G726–38. doi: 10.1152/ajpgi.00436.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McManaman JL, Bales ES, Orlicky DJ, et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 2013;54:1346–59. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S, Yang W, Kozusko K, et al. Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis. Proc Natl Acad Sci U S A. 2014;111:9163–8. doi: 10.1073/pnas.1318791111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi Y, Shinoda A, Kamada H, et al. Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci Rep. 2016;6:20975. doi: 10.1038/srep20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–70. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purushotham A, Schug TT, Xu Q, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Rodriguez JL, Barbier-Torres L, Fernandez-Alvarez S, et al. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology. 2014;59:1972–83. doi: 10.1002/hep.26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin J, Iakova P, Jiang Y, et al. The reduction of SIRT1 in livers of old mice leads to impaired body homeostasis and to inhibition of liver proliferation. Hepatology. 2011;54:989–98. doi: 10.1002/hep.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Jiang Y, Fan X, et al. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol Lett. 2015;236:82–9. doi: 10.1016/j.toxlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.