Abstract
Many organisms respond to noxious stimuli with defensive maneuvers. This is noted in the hornworm, Manduca sexta, as a defensive strike response. After tissue damage, organisms typically display sensitized responses to both noxious or normally innocuous stimuli. To further understand this phenomenon, we used novel in situ and in vitro preparations based on paired extracellular nerve recordings and videography to identify central and peripheral nerves responsible for nociception and sensitization of the defensive behavior in M. sexta. In addition, we used the in vivo defensive strike response threshold assayed with von Frey filaments to examine the roles N-methyl-D-aspartate receptor (NMDAR) and hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels play in this nociceptive sensitization using the inhibitors MK-801 and AP5 (NMDAR), and ivabradine and ZD7288 (HCN). Using our new preparations, we found that afferent activity evoked by noxious pinch in these preparations was conveyed to central ganglia by axons in the anterior- and lateral-dorsal nerve branches, and that sensitization induced by tissue damage was mediated centrally. Furthermore, sensitization was blocked by all inhibitors tested except the inactive isomer L-AP5, and reversed by ivabradine both in vivo and in vitro. Our findings suggest that M. sexta’s sensitization occurs through central signal amplification. Due to the relatively natural sensitization method and conserved molecular actions, we suggest that M. sexta may be a valuable model for studying the electrophysiological properties of nociceptive sensitization and potentially related conditions such as allodynia and hyperalgesia in a comparative setting that offers unique experimental advantages.
Keywords: Nociception, Invertebrate, Sensitization, Hyperpolarization Activated Cyclic Nucleotide Gated Ion Channel (HCN), NMDA receptor, Manduca sexta, Electrophysiology, RRID:SCR_000903, RRID:SCR_003257
Graphical Abstract
Using novel electrophysiological preparations we show that nociceptive sensitization in M. sexta is driven centrally, demonstrated here by sample recordings and force response curve where less force applied (black bar) was required to elicit elevated firing rate response in the central connective nerve after sensitization by a noxious pinch.
Introduction
Organisms typically exhibit defensive behaviors, termed nocifensive behaviors, when presented with a noxious stimulus. These behaviors provide a useful metric to quantify changes in the sensitivity of nociception. After tissue damage, the frequency of nocifensive behaviors can increase and the activation threshold can decrease (e.g. Walters et al., 1994). This sensitization is a type of non-associative learning, akin to clinically relevant conditions such as hyperalgesia and allodynia (Zigmond et al., 1999; Walters et al., 1994).
Many nociception studies focus on invertebrate models, which have been indispensable tools for studying the electrophysiological properties of both nociception and sensitization (Walters et al., 1994; Tobin and Bargmann, 2004; Kandel, 2012), but the majority of invertebrate studies have been on aquatic organisms. Only recently have studies been expanded to terrestrial invertebrates such as Drosophila melanogaster (Tracey et al., 2003; Hwang et al. 2007; Im and Galko, 2012; Im et al., 2015) and the hornworm, Manduca sexta (Waldrop and Levine, 1992; Walters et al., 2001; van Griethuijsen et al., 2013; van Griethuijsen and Trimmer, 2014; McMackin et al., 2016). Studies in D. melanogaster have revealed conservation of nociceptive signaling (Tracey et al., 2003; Hwang et al. 2007; Im and Galko, 2012; Im et al., 2015), and it has been suggested that they may be useful models for studying medical conditions (Neely et al., 2011; Babcock et al., 2011). Paralleling this, Walters et al. (2001) first characterized tissue damage-induced sensitization of a nocifensive strike response (a rapid bending of the head to the stimulated site) in M. sexta, and found that the number of strikes increased after delivery of a noxious stimulus. McMackin et al. (2016) described a reduction in the strike threshold as well, in response to the same noxious stimulus and quantified this defensive behavior in vivo using a variety of methods with von Frey monofilaments (e.g up and down and simplified up and down methods) previously established in rodents (Dixon and Mood, 1948; Dixon, 1965; Chaplan et al., 1994; Bonin et al., 2014). Building on this work, our goal was to develop electrophysiological preparations to study sensitization and nociception in M. sexta to complement studies in the leech, Hirudo medicinalis (Ehrlich et al., 1992; Sahley et al., 1994), Aplysia californica (Walters et al., 1983; Clatworthy et al., 1993), and most recently in D. melanogaster (Im and Galko, 2012; Im et al., 2015).
One common theme in the induction of sensitization, first described and later reviewed by Kandel (2012), is the role played by cyclic nucleotides and associated kinases. In addition, a growing body of evidence indicates that direct activation of ion channels by cyclic nucleotides also contributes to nociceptive sensitization (Beaumont and Zucker, 2000; Robinson and Siegelbaum, 2003; Chaplan et al., 2003; Jiang et al., 2008a, 2008b; Biel et al., 2009; Emery et al., 2011; Young et al., 2014). In particular, hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels are non-selective cation channels that interact directly with molecules such as cAMP or cGMP. These nucleotides shift the channels’ voltage dependence, opening the channels at resting membrane potentials to generate a depolarizing current known as Ih (syn. IHCN, If “funny”, Iq “queer”). These changes can cause the cell to reach threshold, triggering action potentials (Beaumont and Zucker, 2000; Craven and Zagotta, 2006; Jiang et al., 2008a, 2008b; Biel et al., 2009). If HCN channels prove to play a significant role in pain sensitization, it opens the door to development of a novel class of analgesics (Chaplan et al., 2003; Beil et al., 2009; Heine et al., 2011; Emery et al., 2011; Young et al., 2014). For instance, ivabradine selectively inhibits HCN channels and is used clinically as an antiarrhythmic and has shown promise as an analgesic in some pre-clinical models of nociception (Thollon et al., 2007; Jiang et al., 2008a, 2008b; Biel et al., 2009; Takasu et al., 2010; Emery et al., 2011; Noh et. al., 2014; Young et al., 2014).
Given the simple in vivo assay available to quantify nociceptive responses in M. sexta (McMackin et al., 2016), the relative simplicity of M. sexta’s nervous system, and its lasting viability in simple un-oxygenated saline (Waldrop and Levine, 1989 and 1992; van Griethuijsen et al., 2013; van Griethuijsen and Trimmer, 2014), we sought to develop an in vitro electrophysiological preparation to characterize nociception and nociceptive sensitization in this terrestrial invertebrate. To this end, we have shown that, in contrast to many other models in mammals and molluscs, nociceptive sensitization of the defensive strike response in M. sexta is associated primarily with central rather than peripheral neural hyperactivity. In addition, preliminary data suggest a dependence upon NMDA receptors and HCN channels.
Materials and Methods
Animal Handling
Animals were raised in individual plastic cups, on an artificial diet of wheat germ (MP Biomedicals, Burlingame, CA, USA) with a 17h:7h, 27°C:25°C light:dark cycle (Wells et al., 2006). 505 animals of either sex were used on the first day of their fifth larval stage and typically weighed between 1.5–2.7 g. Animals receiving injections were further restricted to weights between 1.7–2.4 g to better control dosing. Control animals were injected with H2O (vehicle). Test solutions consisted of (i) selective HCN channel inhibitors ivabradine-hydrochloride (Sigma-Aldrich St. Louis, MO, USA) or ZD7288 (Tocris Biosciences Bristol, UK), or (ii) NMDAR inhibitors MK-801 (Tocris Biosciences Bristol, UK) or D-AP5 (Tocris Biosciences Bristol, UK), and (iii) the inactive enantiomer L-AP5 (Tocris Biosciences Bristol, UK), all dissolved in H2O. Solution was injected into the body cavity through the dorsal side of the fifth abdominal segment (A5) with a shallow angle to avoid puncturing the gut of the animal. The volume for injection was calculated for each animal based on its body mass and varied between 1.7μL and 7.2 μL (1 or 3 μL per gram). Based on dye injections of similar volumes we estimate that the solution was rapidly pumped throughout the body by the aorta within seconds to a minute (Tabuena, unpublished observation). This duration was much shorter than the 10 min period between injection and the first test or pinch (see “Noxious Stimulus – Pinch”) and allowed ample time for drug circulation. For one in vitro preparation, animals received ivabradine via the bath recording solution, which was supplemented to a concentration of 160 nM. This concentration is comparable to a dosage of 30 ng/g based on 35%–40% hemolymph to body mass ratio (g/g) for M. sexta (Cymborowski et al., 1982).
Electrophysiology
The recordings for all electrophysiological experiments were made in an approximately 15 mL bath at room temperature (15–17 °C) physiological saline solution described by Trimmer and Weeks (1989), modified from Miyazaki (1980), containing 140 mM NaCl, 5 mM KCl, 4 mM CaCl2, 28 mM Glucose, and 5 mM HEPES. The pH was adjusted to 7.4 using 1 M NaOH. Voltage recordings were amplified and filtered using a differential AC amplifier (Model 1800, A–M Systems; Carlsborg, WA, USA) using one or two channels. The low and high frequency cutoff filters were set to 10 Hz and 10 kHz, respectively. The voltage output was sampled at 25 kHz using a Cambridge Electronic Design Micro mk-II converter and accompanying Spike2 v5.21 software (CED Systems; Cambridge, UK, RRID:SCR_000903), and stored on a PC for analysis. The signal was further processed offline with Spike2 software to reduce electrical noise and remove DC offset voltages. Spikes were identified using the Spike2 software with a minimum threshold of 3–5 μV based on individual recording quality, and with a spike duration of 1–2 ms. For each nerve recording, the spike frequency of all units was averaged in a 250 ms moving window for the course of the recording. Because the firing response was short with variable duration, the peak of the moving average during stimulation (i.e. the maximum burst rate for each stimulation) was taken as the response for each stimulation. The firing frequency without stimulation was also taken in this manner using a time period just prior to stimulation.
Tissue Preparation
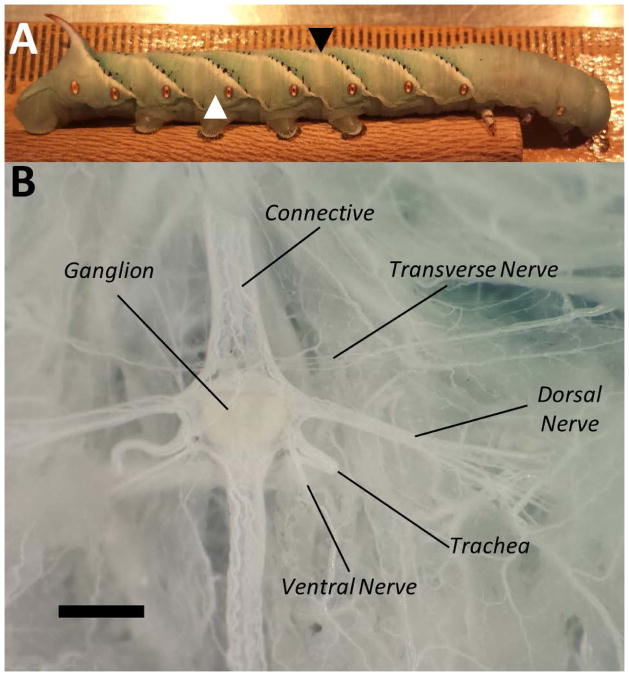
Dissections for electrophysiological experiments were performed after the animals were anesthetized by being placed in a −20 °C freezer for 7–10 min. Successful anesthetization was suggested by lack of muscle tone, immobility, and lack of response to intense physical stimuli such as dissection for at least 5–10 min after removal from −20 °C. When animal movement was to be monitored in the in situ preparation, the animal was ligated with thread in between A3 and A4 (Fig. 1A, black arrow; see “In Situ Preparation”) and access to the ventral nerve cord and individual ganglia was gained by an incision along the dorsal midline beginning at A8 and continuing to A4. The gut of the animal was removed from the area accessible through the dorsal incision. The preparation was allowed to stabilize for 5–7 min after electrode placement, prior to testing, unless stated otherwise.
Figure 1.
Manduca sexta Anatomy. A: Whole animal with solid black arrowhead (between segments A3 and A4) indicating the site of radial ligation for defensive strike correlation, and white arrowhead (segment A5) indicating the site of pinch and von Frey stimulation for all experiments unless otherwise noted. Each hash mark on the ruler in the background represents 1 mm. Anterior is to the right. B: Micrograph of the ganglion from abdominal segment A5. The connective contains central axons that allow the segments to communicate with each other. The transverse, dorsal, and ventral nerves contain the peripheral sensory and motor axons. Scale bar represents 0.5 mm.
In Situ Preparation
After anesthetization, the body of the animal was tightly ligated with thread at the junction of A3 and A4 (Fig. 1A, black arrowhead) to maintain body cavity pressure and preserve movement post dissection, but not so tight as to damage the connective. Tight ligations for shorter durations have been shown to not damage the connective (Fuse and Truman, 2002). Recordings from the connective between A5 and A6 were made in an en-passant configuration. Once the animal recovered from cold anesthesia and began to move and react to external stimuli, we used forceps to pinch the anterior body wall at A3 or A4 to elicit a strike response. A pinch, as opposed to von Frey filaments, was used in this preparation because the filament was not always reliable in producing a strike, perhaps because of altered sensory input during or after the dissection. Observed activity in the interganglionic connective was evoked by peripheral sensory input and central neurons and was not an experimental artifact (e.g., from movement of the preparation) because all evoked activity in the connective was eliminated by severing the connective between recording site and the striking portion of the animal. Throughout this procedure we used a Samsung SGH-I337 camera to video record the animal’s movements. Time periods during the recording were referenced to the period of movement rather than the stimulation itself, as induction of behavioral response occasionally required multiple stimuli or appeared before complete delivery of the stimulus (before the forceps could be completely closed) making it an unreliable reference point. The variability in stimuli to induce the behavior may have been due to artifacts of this dissection method, resulting in some level of sensitization. Thus we were only concerned with correlating behaviors with electrical activity during periods of movement. To quantify the neural response, we measured the firing frequency in discrete 200 ms bins. We defined the time periods as (i) early pre-strike period 5 s prior to beginning of movement, (ii) late pre-strike 600 ms prior to movement, (iii) mid-strike, the period between the start and stop of gross movement, and (iv) post-strike period, 5 s after the cessation of movement (see also Fig. 2).
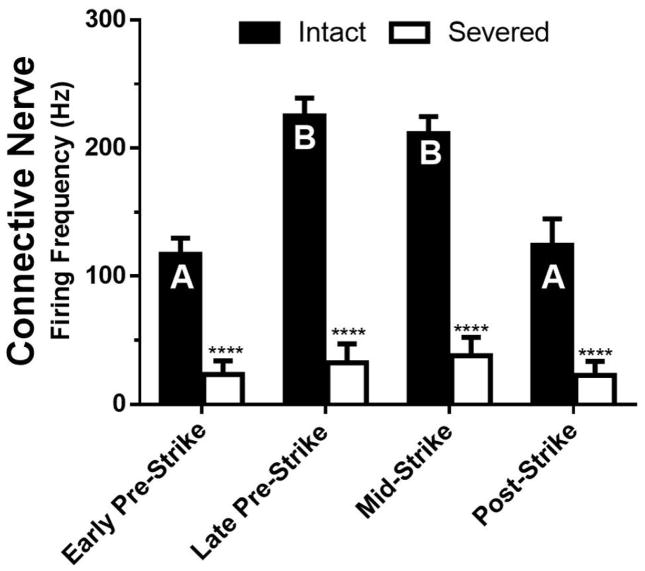
Figure 2.
Firing in the Connective Precedes the Defensive Strike. Plot of the average firing frequency before, during, and after the defensive strike, when recording from the intact nerve (black bars) and after the nerve was been severed (white bars) from the striking portion of the animal. Bars with dissimilar letters denote significant differences within the intact preparations (P < 0.0002), while asterisks indicate significant differences between intact and severed groups at each time point (P < 0.0001) determined by a 2-way ANOVA with Holm-Sidak multiple comparison test (n = 8). (See also Supplemental Video 1).
Ganglion-Body Wall Preparation
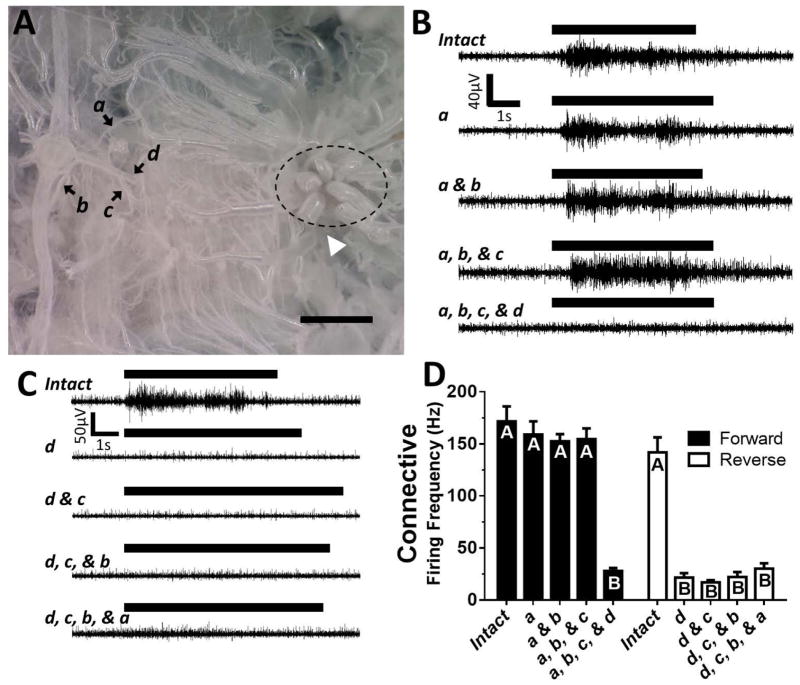
An incision was made along the dorsal midline from A8 to the head. The gut was removed and the animal was pinned dorsal side up to reveal the ventral nerve cord. The fifth ganglion was isolated from the rest of the ventral nerve cord by severing the connectives anterior and posterior to the ganglion. In addition, left peripheral nerves - transverse, dorsal, and ventral – were also severed to leave only the ganglion and connections to the right side of body wall intact (Fig. 3A). Recordings were made from the anterior connective with a suction electrode. We then applied force to the body wall slightly posterior to the spiracle (Fig. 3A, white arrowhead) using a 9.8 mN von Frey filament placed in a micromanipulator, and recorded the activity in the connective. We severed the right transverse, dorsal, and ventral nerves, and sub-branches in a systematic fashion with mechanical stimulation in between each cut, to observe when activity was lost and if additional cuts reduced the signal further.
Figure 3.
Dorsal Nerve Branches Convey Afferent Information from Noxious Pinch and Test Stimuli Used in this Study. A: Micrograph of the isolated ganglion and body wall with right peripheral nerves and sub branches emanating from the body wall. Black arrows denote the various cuts: ‘a’ (transverse nerve), ‘b’ (ventral nerve), ‘c’ (posterior-dorsal nerve branch), and ‘d’ (anterior- and lateral-dorsal nerve branches). White arrowhead indicates the site of mechanical stimulation, just posterior to spiracle (dashed circle). Scale bar represents 1 mm. A significant portion of fat body and trachea has been removed in this preparation for clarity B: A sample recording from the connective during stimulation with cuts in the forward direction (Intact→a→b→c→d). C: Sample recordings from cuts made in reverse order (Intact→d→c→b→a). The black bars above all traces represent the time during which 9.8 mN of force was applied to the body wall. D: Average burst firing frequency during stimulation for all animals tested. Different letters indicate significant differences (P < 0.0001) between groups determined by a one-way ANOVA with a Holm-Sidak multiple comparison test.
Noxious Stimulus - Pinch
To induce tissue damage and sensitize the larvae, the body wall of the A5 segment slightly posterior to the spiracle (Fig. 1A, white arrowhead) was pinched with metal forceps (Dumont #5 forceps; Fine Science Tools, Foster City, CA) for five seconds. The area pinched was approximately 2 × 2 mm. The force was not recorded, but animals that did not exhibit the defensive strike for the majority of the pinch were either pinched one more time or excluded from the studies. The cuticle was not punctured, as assessed by a lack of appearance of hemolymph after the pinch. Animals with broken cuticle after the pinch were excluded from the study. Animals were then allowed to recover for 30 min undisturbed at room temperature before testing, unless otherwise stated. Sensitization has previously been noted as early as 5 min after pinch (Walters et al., 2001) and as long as 19 h (McMackin et al., 2016), thus 30 min was used to allow time for potential effects of animal handling (pinch or in some cases injection) to subside.
Force-Response Curve
Once the dorsal nerve was identified as the peripheral input after noxious stimulation, we further reduced the ganglion-body wall preparation to only leave the dorsal nerve intact to the body wall and ganglion. In addition, dissection in this preparation was designed to avoid the receptive fields being measured to minimize artifacts of dissection on sensitization. The cut end of the ascending ganglionic connective was suctioned with an electrode, and the dorsal nerve was recorded in an en-passant configuration. Pressure was applied using von Frey filaments to the interior of the animal slightly posterior to the right A5 spiracle (as previously shown in Fig. 3A) to create a force-response curve. This was done for the filaments delivering 0.078 mN, 0.686 mN, 9.807 mN, 98.067 mN and 980.665 mN, in that order. The forces were separated by full log increments to yield a curve with high resolution, while minimizing the potential loss in activity after repeated stimulation with forces too near in magnitude (wind-down). This process usually took less than 5 min. The firing rate response for each animal and each nerve was individually fit using the modified three parameter Hill equation: f(Force) = fmin + (fmax − fmin)/(1 + 10^(log(Force50) − log(Force)), where f (Force) was the firing rate (Hz) during stimulation with a particular force in mN (Force). fmin and fmax represented the maximum and minimum fit firing rates (Hz) respectively, and the Force50 represented the stimulation force (mN) yielding the half maximal increase in firing rate.
To compare the rate of incremental depression, wind-down, after repeated stimulation with a single force, the burst frequency for pinched and non-pinched animals was fit with the exponential decay equation: f(s) = (f1 − fp)/(exp(10log(k)+log(s)) + fp, where f(s), f1, fp, k, and s denote: burst firing frequency (Hz) at stimulation number s, firing frequency (Hz) upon first stimulation, plateau firing frequency (Hz), decay rate, and stimulation number, respectively.
In Vivo Strike Threshold
Animals were assayed using the “up and down” method (Dixon and Mood, 1948; Dixon, 1965; Chaplan et al., 1994) as modified by McMackin et al. (2016) for M. sexta. In brief, we used an array of von Frey filaments to apply mechanical stimulation to the animal which delivered forces of 0.078, 0.196, 0.392, 0.686, 1.569, 3.922, 9.804, 19.608, 39.216, or 98.039 mN. We determined the threshold force in mN for each animal, using the modified equation described by McMackin et al. (2016): Threshold = 9.80665 • 10Xf + kd, where Xf was the final force used in log(g), k is the statistic tabulated in Dixon and Mood (1948), and d = 0.344 (the average difference in log(g) between each filament). A baseline response was determined, and less than 1 min following the baseline test, animals were pinched to incur tissue damage. Then animals were left undisturbed for 30 min, after which the animals were tested again to determine any changes in the strike response threshold. In what we termed the “prevention” method, animals were injected as described above 10 min before baseline testing and pinching. (This amounted to 40 min prior to the post-pinch test). In our “intervention” method the injection occurred 10 min after baseline and pinch (20 min prior to the post-pinch test). Effects of in vivo drug application were fit using the Hill equation: Threshold(Dose) = Thresholdmin + (Thresholdmax − Thresholdmin)/(1 + (IC50/Dose)HillSlope), where Threshold(Dose), Thresholdmin, and Thresholdmax represent the threshold forces (mN) to elicit a defensive strike determined using the up and down method, given a dose of ivabradine or MK-801 (Dose), the minimum observed and maximum observed, respectively. The IC50 represents the dose at which the half maximal effect of either drug was seen and is given in units of in ng/g (drug/animal weight).
Results
Strike response is correlated with nerve activity
M. sexta has a very distinctive defensive strike response, that has been well described by Walters et al. (2001) and van Griethuijsen et al. (2013). In brief, the head swings directly at the site of the noxious stimulus, with a rapid bending motion. After noxious stimulation such as a sharp pinch, M. sexta becomes sensitized, where it strikes more often in response to innocuous stimuli (Walters et al., 2001; Merchasin, 2009) and at a lower force threshold (McMackin et al., 2016). To determine an electrophysiological correlate of the defensive strike, we compared the firing activity in the connective with the animal’s attempts to strike (Supplemental Video 1). The video shows the dissected animal striking in response to a noxious pinch anterior to the recording and nerve sectioning site, with the synced audio track conveying connective activity with each spike represented by a single click. The activity noted is likely both ascending and descending to coordinate whole body movement and most likely contains a mix of sensory-, motor- and inter-neurons (Levine and Truman, 1985; Waldrop and Levine, 1989 and 1992; Zayas et al., 2000). The average firing frequency for the four time periods early-pre strike, late pre-strike, mid-strike, and post-strike (5 s prior, 600 ms prior, during, and 5 s after strike respectively) are plotted in Figure 2 (black bars). Electrical activity was significantly higher during the late pre-strike and mid-strike, compared to early pre-strike and post-strike levels. We found that during periods of rest the connective was relatively silent (Fig. 2; early pre-strike and post-strike), however approximately 600 ms before the strike began (Fig. 2; late pre-strike) the connective showed a dramatic increase in firing activity which was sustained throughout the defensive strike (Fig. 2; mid-strike). Within the 600 ms pre-strike period, increased firing frequency was noted for all three 200 ms bins (data not shown). This is an interesting contrast to the work by Walters et al. (2001) where the response to stimulation occurred within 100 ms. It is likely that in the current partially dissected preparation, competing sensory information delayed the whole animal reaction to individual stimuli. For instance, early activity with the introduction of the forceps, prior to completion of the pinch, may have contributed to an apparently elongated delay. There was minimal electrical activity during a strike when recording from the nerve after severing the connection between the ganglion being recorded to the striking animal (Fig. 2; white bars), suggesting that the activity during the strike was not an experimental artifact (e.g., from movement of the preparation).
Using the central connective activity as a marker, we then sought to determine the path of the peripheral signal generating firing during a noxious touch. We isolated the A5 ganglion such that it was only attached via peripheral nerves to the body wall. Figure 3A shows a micrograph of the ganglion with one side of peripheral nerves still intact and projecting from the body wall. In this preparation we recorded from the anterior connective while applying a 9.8 mN von Frey filament to the interior of the body wall (white arrowhead). The connective response remained robust (Fig. 3B–D, ‘Intact’) despite being disconnected from neighboring ganglia, the anterior of which (A4) contains motor neurons innervating the A5 segment (Levine and Truman, 1985). A series of cuts were made to sever the peripheral nerves (Fig. 3A, black arrows). Sample recordings from the connective are shown after each cut (Fig. 3B,C) and average firing frequencies are plotted in Figure 3D. Loss of activity was only seen when the anterior and lateral branches of the dorsal nerve were cut, whether the cut was last (Fig. 3B,D; n = 12) or first (Fig. 3C,D; n = 11). In addition, no further loss of signal was observed if more were cut. We did not resolve whether anterior or lateral branches, or both, are necessary, because the junction of these two nerves is deep in the muscle tissue and we did not want to disturb the preparation by dissecting further. To confirm our hypothesis in vivo, we severed the dorsal nerve on one side of A6 in three otherwise intact animals through a 1–2 mm incision on the ventral side of the animal. The animals were allowed to recover for half an hour. After recovery, none of the animals responded to pinches to segment A6 on the denervated segment side. In contrast all three animals responded with strikes when pinched at innervated segments near the cut nerve, sides of segments A5 and A7 ipsilateral to the surgery, or contralateral sides of segments A5, A6, and A7. (3 pinches per segment per side; data not shown).
Sensitization occurs centrally
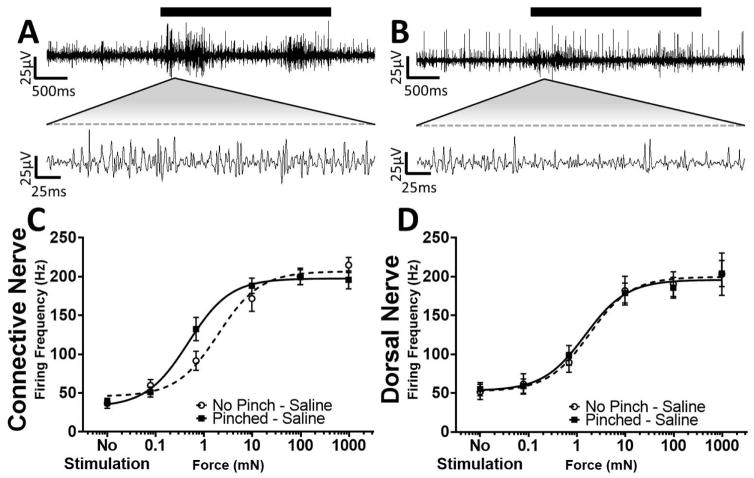
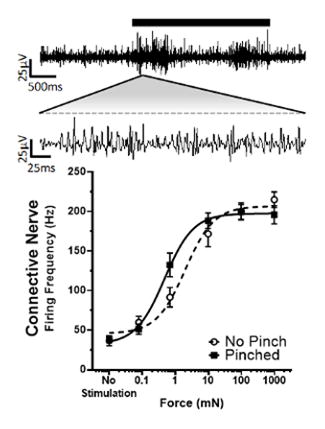
With the central and peripheral nerves identified, we used the isolated ganglion preparation with only the dorsal nerve connected to the body wall to quantify the in vitro response to increasing forces applied to the body wall in animals that were or were not sensitized by a pinch at the segment to be recorded, 30 min prior to dissection. Sample traces of the simultaneous recordings of the anterior connective and right dorsal nerves are shown at two time scales in Figures 4A and 4B, respectively. The average burst firing rates for both the connective (Fig. 4C) and dorsal (Fig. 4D) nerve after sequentially increasing von Frey stimulation were calculated for the control (non-pinched) and sensitized (pinched) animals. The response from each nerve was fit with a Hill equation and showed a robust sigmoidal relationship, as force was increased, the firing frequency increased. Hill fit parameters were compared with a 1-way ANOVA and Holm-Sidak multiple comparison test. There was no significant difference in the Force50, minimum frequency, or maximum frequency when recording from the dorsal nerve after the body wall was pinched (Fig. 4D, P > 0.88, n = 15–21), and no increase in central or peripheral spontaneous activity was noted. However, we cannot rule out changes in afferents with smaller spikes than our recording conditions can resolve. In contrast, when recording from the central connective, there was a significant reduction in the Force50 after the body wall was pinched (control: 1.95 mN; n = 21 vs. pinched: 0.54 mN; n = 26; P = 0.0101; Fig. 4C). That is, the force response curve was shifted to the left, indicating that less force was required to induce a given firing frequency. Given that we were able to measurably induce sensitization by pinch, even though dissection, despite anesthetization, may have induced some sensitization, these findings indicate a robust central sensitization response.
Figure 4.
Pinch Induces Central Sensitization. A, B: Sample traces at two scales from a single animal recorded simultaneously, from the connective and dorsal nerve, respectively. A strong increase in firing rate is noted upon von Frey stimulation (black bar). C, D: Hill fit of the burst firing frequency in the connective and dorsal nerve, respectively, with respect to the force applied to the body wall for animals that were non-pinched (black dashed lines) or pinched (black solid lines).
Central and Peripheral Incremental Depression
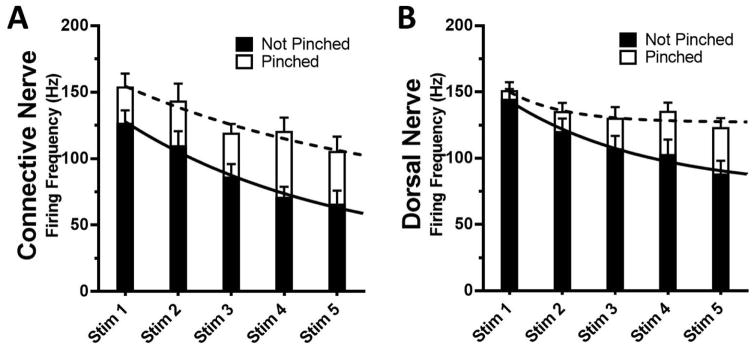
Repeated stimuli often lead to behavioral habituation or can cause incremental depression (wind-down) of the discharge of neurons (Clatworthy and Walters, 1993). For this reason, we looked at the burst firing frequency in the connective and dorsal nerve during five sequential stimulations using a constant force of 3.9mN, which was fit with an exponential decay equation (Fig. 5). When compared using a 2-way ANOVA with repeated measures, the firing rate from the connective (Fig. 5A) showed significant main effect differences for stimulation number (P < 0.0001) and pinch (P = 0.005), but insignificant interaction (P = 0.549). This indicates that there was significantly elevated firing after a pinch, with a significant decay after multiple stimuli, but the rate of decay was similar to that seen in control (non-pinched) preparations. In contrast, firing activity from the dorsal nerve (Fig. 5B) showed significant main effect differences for stimulation number (P < 0.0001) and pinch (P = 0.046) as well as interaction (P = 0.019), indicating a significant pinch-dependent resistance to incremental depression. Thus, although the initial pinch did not have a significant effect on firing frequency, the decay after multiple stimuli was significantly less compared to non-pinched controls.
Figure 5.
Sensitized Dorsal Nerve is Resistant to Incremental Depression. A, B: The burst firing rates for each of five stimulations (Stim 1–5) for Pinched and Not Pinched animals when recording from the connective and dorsal nerve respectively (n = 16–20). Exponential decay lines are overlaid for not pinched (solid) and pinched (dashed lines).
Pharmacological Inhibition of Central Sensitization
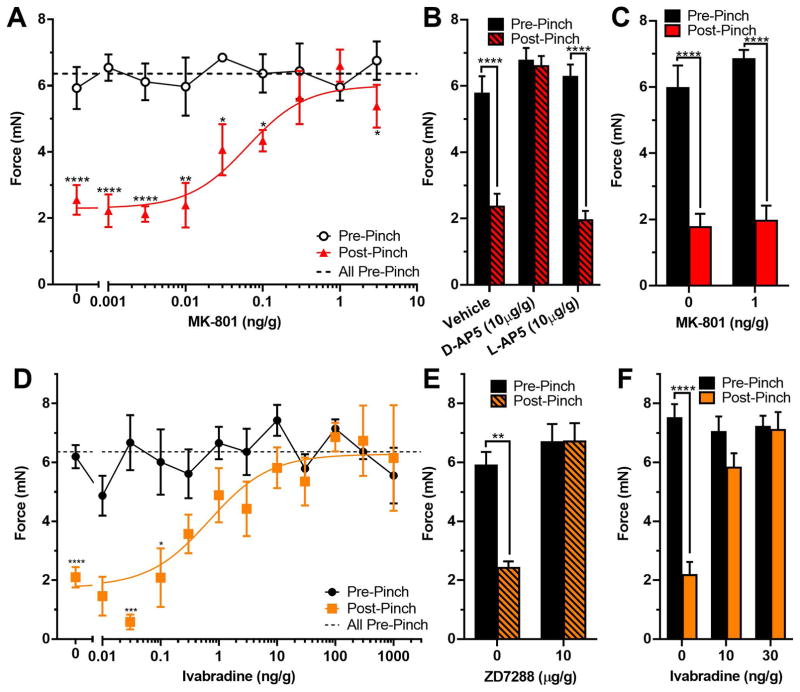
As previously shown by McMackin et al. (2016) animals assayed using the up and down method showed a significant decrease in the force required to elicit a strike after the animal was pinched (6.9 ± 0.3 mN vs. 3.4 ± 0.2 mN respectively; n = 26; P < 0.0001 by two tailed student’s t-test; data not shown). To determine if sensitization in our model occurs by activation of NMDAR (via strengthened synaptic transmission or modulation of postsynaptic excitability), we repeated the up and down method in the presence of multiple concentrations of the NMDAR antagonist MK-801 (Wong et al, 1986) injected 10 min prior to baseline testing (prevention dosing). MK-801 was effective in attenuating sensitization of the strike response with an IC50 of 0.063 ng/g, (Fig. 6A; estimated hemolymph concentration of 0.5 nM). We further tested the role of NMDAR function in sensitization with prevention dosing of 10 μg/g (~126 μM) of another inhibitor D-AP5 (Fig. 6B). D-AP5 prevented sensitization, and the inactive isomer, L-AP5, did not. Responses to L-AP5 were similar to animals receiving vehicle. MK-801 was also tested in an “intervention” style dosing, injection 10 min after a pinch, which had no effect on sensitization (Fig. 6C).
Figure 6.
Sensitization of the Strike Response is Attenuated by NMDAR and HCN Channel Inhibitors. A, B: ‘Prevention’ style dose response plots of MK-801 (n = 3–12) and AP5 (n = 5–9), respectively. The Threshold is displayed 10 min after injection of drug (Pre-Pinch), and 30min after pinch (Post-Pinch). C: ‘Intervention’ style dosing of MK-801 injected 10 min after the pinch (n = 8–9). D, E: ‘Prevention’ style dose response plots of Ivabradine (n = 4–22), and ZD7288 (n = 6–9) respectively. F: ‘Intervention’ style dosing of ivabradine injected 10 min after the pinch (n = 12–18). In all panels pharmacological effects were compared with using 2-Way ANOVAs with Holm-Sidak multiple comparison tests where “*”, “**”, “***”, and “****” denote P < 0.05, P < 0.01, P < 0.001 and, P < 0.0001 respectively between pre- and post-pinch. In A and D the dashed black line (All Pre-Pinch) represents the mean pre-pinch Thresholds of all animals regardless of dose.
We also assessed the actions of the HCN channel inhibitor, ivabradine, on sensitization, using the up and down method. Figure 6D plots the average threshold force to elicit a strike before a pinch (black line) and 30 min after a pinch for animals that were injected with vehicle or varying doses of ivabradine 10 min prior to baseline test, “prevention,” yielding an IC50 of 0.686 ng/g (~4.2 nM). Sensitization was also blocked with the alternate HCN channel inhibitor ZD7288 at 10μg/g (~82 μM) using “prevention” dosing (Fig. 6E). In addition, the effects of ivabradine were examined when injected 10 min after pinch in an “intervention” style of dosing (Fig. 6F). There was a significant difference between pre- and post-pinch thresholds with vehicle, which was blocked by ivabradine at both 10 and 30 ng/g doses.
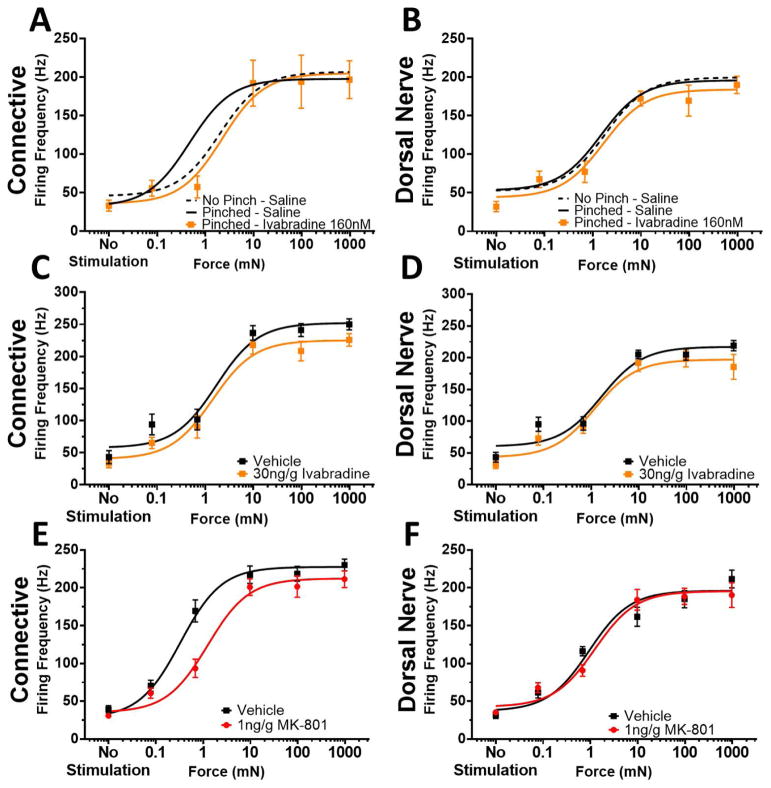
As with the in vivo data, bath application of 160nM ivabradine to pinched in vitro preparations shifted the response curve back to non-pinched levels, when recording centrally via the connective (Fig. 7A). The force required to elicit fifty percent of the maximum frequency was 2.17 mN and did not differ from non-pinched controls (P > 0.8; 1-way ANOVA with Holm-Sidak multiple comparison test), but was significantly higher than pinched animals (P = 0.0386). That is, ivabradine significantly shifted the Force50 to the right, such that the force required to elicit a response was higher, resembling non-pinched animals. Ivabradine did not alter the dorsal nerve responses (Fig. 7B; P > 0.8 for all comparisons; 1-way ANOVA with Holm-Sidak multiple comparison test). In contrast, if ivabradine was injected into intact pinched animals prior to dissection, but not introduced into the bathing medium, it had no significant effect compared to pinched animals with vehicle injection, whether recorded from the connective (Fig. 7C; P > 0.9; student’s t-test) or the dorsal nerve (Fig. 7D; P > 0.5; student’s t-test). However, MK-801 injected prior to pinch, but not present in the bath solution, was effective in preventing induction of sensitization centrally with Force50 equal to 1.24 mN for MK-801 and 0.33 mN for vehicle control (Fig. 7E; P < 0.001, student’s t-test). Again, there was no effect peripherally (Fig. 7F).
Figure 7.
Central Sensitization is Reversed by Ivabradine and Prevented by MK-801. A, B: Force response curves of the burst firing frequency in the connective and dorsal nerve, respectively, with bath-applied ivabradine (orange). Curves of pinched and non-pinched controls from Figure 4 are replotted for clarity (n = 8 for both). C, D: Force response curves of the burst firing frequencies in the connective and dorsal nerve, respectively, while stimulating the body wall when 30 ng/g ivabradine or vehicle (H2O) was injected prior to dissection, and not presented in the bath solution (n = 14–17). E, F: Force response curves of firing frequency in connective and dorsal nerve, respectively, in animals injected with MK-801 or vehicle (H2O) prior to pinching (n = 16 for both).
Discussion
Nociception and the Defensive Strike in M. sexta
We have developed an in vitro electrophysiological preparation to complement the in vivo defensive strike assay (McMackin et al., 2016). Using these two assays, we have characterized aspects of nociceptive sensitization in M. sexta. To our knowledge this work represents the first electrophysiological correlate of nociceptive sensitization in any arthropod to natural stimuli as opposed to artificial methods such as electric shock. Compared to other insect models, M. sexta’s nervous system size and robust physiology in surgically reduced preparations provide a unique advantage to examine the organism’s electrophysiological properties in response to more natural physical stimuli. In addition, we have shown that it is a valuable model to easily test pharmacological properties of insect neurophysiology.
We have correlated elevated firing frequency in a semi-intact preparation with the defensive strike (Fig. 2). Elevated firing frequency is noted a short time before and during the defensive strike, but not during rest or with small movements. This strongly suggests that this firing can be used as a reliable surrogate for the defensive strike in more reduced preparations to evaluate nociception in M. sexta, and that some of the recorded axons are likely to activate neural systems that generate the strike response. Furthermore, the dramatic loss in firing activity in the connective and in the behavioral response when and only when the dorsal nerve is severed strongly suggests that the mechanosensory information driving the defensive strike under our experimental conditions flows from the body wall through this nerve to the ganglion. This is consistent with previous literature on the multi-dendritic complex in M. sexta (Grueber et al., 2001). It has previously been suggested that nociception and the initiation of the strike occur in this network of sensory cells originating on the body wall, whose axons extend to the ganglia through the dorsal nerve (Levine et al., 1985; Grueber and Truman, 1999; Grueber et al., 2001; van Griethuijsen et al., 2013; for a review see van Griethuijsen and Trimmer, 2014). Drosophila also shows a homologous complex of neurons (Grueber et al., 2012) indicating that further examination of this system in conjunction with other model organisms - where genetic manipulation is possible but electrophysiological study is less amenable - may yield insight into nociceptive sensitization as a conserved process. For instance, in Drosophila the multi-dendritic neurons are involved in nocifensive behavior and some express TRPA (painless), a channel required for thermal nociception (Tracey et al., 2003; Hwang et al., 2007; Neely et al., 2011; see also Shimono et al., 2009), which has been shown to contribute to nociceptive sensitization in mammals as well (Lennertz et al., 2012). Furthermore, aspects of nociceptive cytokine signaling have also been shown to be conserved between Drosophila and mammals (Babcock et al., 2009; Im et al., 2011; Im et al., 2015). It will be interesting to determine if this is the case in M. sexta as well in future experiments.
Central and Peripheral Sensitization
Previous studies by Walters et al. (2001) and Merchasin (2009) showed that sensitization of the defensive strike was generalized across the whole animal regardless of the distance between sensitizing and testing stimuli. Paired with this behavioral observation, our in vitro findings of central sensitization suggest that the changes in neuronal response that are responsible for sensitization of the defensive strike occur either by increased central excitability or enhanced synaptic transmission. The increased central network activity is consistent with Walters’ (2001) model of a generalized arousal rather than site-specific sensitization. Sensitization in M. sexta appears to be predominantly central, although we cannot discount the possibility that peripheral changes in activity were too small to resolve via our extracellular recording methods. Interestingly this differs from Drosophila in the setting of ultraviolet induced sensitization, where the peripheral nerves are hyperexcitable (Im et al., 2015). This may stem from differences in damage delivery or injury progression, 30 min in this study vs. hours in Drosophila (Babcock and Galko, 2009). Nevertheless, in our model, absence of confounding factors by prominent peripheral sensitization normally present in other model systems (eg. Aplysia, mammals, squid, and Drosophila; Ji et al., 2003; Treede et al., 1992; Crook et al., 2013; Im et al., 2015), may provide new insights into central regulation of nociception, a significant contributor to clinical pain (Latremoliere and Woolf, 2009).
Interestingly, activity in the connective and dorsal nerve also winds down, and after a pinch this activity shows less wind down in the peripheral nerve than in the central connective. However, the resistance to peripheral wind-down after pinch is not reflected centrally in the connective. This is surprising as one might expect increased peripheral activity to have an effect centrally which was not observed. This suggests that while peripheral changes are occurring, the dominant factor in this model appears to be increased central excitability. In addition, it may be that the peripheral resistance to wind-down stems from a few sensitized efferent neurons running through the dorsal nerve causing the persistent peripheral activity over multiple stimulations after a pinch. Still, it is unclear as to whether these results can be extended to behavioral habituation, which was not observed by Walters et al. (2001). It will be interesting to determine whether these results with non-noxious force hold true over a wider range of forces.
Possible Role for NMDARs and HCN Channels
Based on genetic analysis of the recently sequenced M. sexta genome (M. Kanost, unpublished; available at https://i5k.nal.usda.gov/Manduca_sexta; Poelchau et al., 2015) and NCBI Protien (http://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al., 1990; RRID:SCR_003257) we found conserved sequences for NMDAR and HCN channels. Msex2.09307, hereafter referred to as MsNR1 showed 67% identity with Drosophila DmNR1 (NP_730940.1) and 46% identity with human NMDAR1 (NP_015566.1). Likewise, Msex2.0523, hereafter referred to as MsIH, showed 83.7% identity with Drosophila DmIH (NP_001246320.1) and 51.6% with human HCN2 (NP_001185.3). The conserved nature of NMDAR and HCN channel sequences in the M. sexta genome, including functional regions such as membrane segments, selectivity filters, and drug binding sites, voltage sensing, and cyclic nucleotide binding domain (Moriyoshi et al., 1991; Ferrer-Montiel et al., 1995; Marx et al., 1999; Bucchi et al., 2013) supports the hypothesis that these M. sexta sequences are most likely NMDA receptors and HCN channels.
While to our knowledge this is the first instance of ivabradine used in invertebrates, the ability of compounds such as MK-801, AP5, and ZD7288 to work in a number of other insect and invertebrate species including other Lepidoptera (Cattaert and Birman, 2001; Chiang et al., 2002a, 2002b; Pirtle and Satterlie, 2004; Bhatt and Cooper, 2005; Zhong and Zucker, 2005; Cheung et al., 2006; Geister et al., 2008; Xia and Chiang, 2009; Huang et al., 2015) suggest that the drug actions are likely conserved in M. sexta as well. Futhermore, all of the inhibitors seem to selectively affect central sensitization and not mechanosensation, based on our in vitro and in vivo studies where the firing or strike threshold is not desensitized beyond control levels before or after a pinch despite doses 100–1000 fold higher than the IC50’s. This suggests a central mechanism for the drug actions as opposed to peripheral inhibition or nonspecific effects.
The prevention of sensitization with MK-801 and AP5 when administered before the pinch procedure (Fig. 6AB, 7E), but not after (Fig. 6C), suggests that induction of sensitization is NMDAR-dependent, potentially in a manner consistent with previously described LTP-based nociceptive sensitization in models such as Aplysia (Lin and Glanzman 1994a, 1994b, for a review see Ji et al., 2003). It is unclear whether this is the only mechanism for sensitization in our model, as most studied sensory systems in insects are cholinergic rather than glutamatergic (Salvcaterra and Kitamoto, 2001). Moreover, mAChR have been shown to play a role in sensitization of motor neurons and the proleg withdrawal (Trimmer and Weeks, 1989, 1991, 1993; Trimmer, 1994; see also Trimmer, 1995), putatively via NO and cGMP (Qazi and Trimmer, 1999; Zayas et al., 2000, 2002; Zayas and Trimmer, 2007; see also Trimmer, 1995). Thus, given (i) the global and non-site specific nature of sensitization of the strike, (ii) the fact that sensory-induced muscarinic receptor mediated effects in M. sexta are not seen during stimulation of single afferents (Trimmer and Weeks, 1993), and (iii) the expression of NMDAR – albeit less abundant – at sensory postsynaptic membranes (Daniels et al., 2008), suggests that a role for NMDARs in central sensitization is not impossible. Clearly we will want to assess the roles of muscarinic receptors in the future. However, given the robust response to NMDAR inhibitors, it will be very important to determine the neuroanatomy of our nociceptive network. For instance, it will be important to determine whether NMDAR are located within the brain or other second order neurons as suggested by work by Daniels et al. (2008), and whether these neurons function in a modulatory role on the cholinergic sensory neurons via interneurons within the ganglia. For example, work by Xia et al. (2005) demonstrated that memory in Drosophila can be NMDAR dependent, and that the receptors are highly expressed in the mushroom body suggesting that in insects, sensitization may require certain brain regions expressing NMDAR. This question may be resolved with further development of our in vitro preparation.
Block of sensitization by ivabradine and ZD7288 suggest that HCN channels also play a central role in sensitization. In particular, ivabradine was very effective in suppressing sensitization of the defensive strike with an IC50 of 0.7ng/g, or approximately 4 nM (Cymborowski et al., 1982). This value is strikingly smaller than reported patch clamp IC50’s in the low μM range for heterologously expressed channels (Thollon et al., 2007; Biel et al., 2009), suggesting a powerful role for Ih in this learned pain paradigm. Most likely only partial block is necessary to disrupt sensitization or there may be a significant effect due to rate- or voltage-dependent block (Thollon et al., 2007). However, it is interesting to note that ivabradine showed no effect when injected prior to dissection (Fig. 7C,D) and omitted from the bath solution (washout of the hemocoel). This result, along with the in vivo intervention results (Fig. 6F), suggests that HCN channels are involved more in the maintenance than induction of sensitization. It will be interesting to see if longer exposure or chronic dosing can attenuate sensitization in a manner similar to our in vivo results.
It remains unclear how HCN channels are being activated in this model. It has been shown that the multi-dendritic complex responds to nitric-oxide by producing cGMP, and that cGMP is also increased after tissue damage in the multi-dendritic complex (Grueber and Truman, 1999; Grueber et al., 2001). Sensitization of motor neurons and of proleg withdrawal appears to arise via NO and cGMP (Qazi and Trimmer, 1999; Zayas et al., 2000, 2002; Zayas and Trimmer, 2007; see also Trimmer, 1995). Interestingly, cGMP may also have an antinociceptive action in vivo in M. sexta (Merchasin, 2009; Fuse et al., 2013; Arreola, unpublished observations). This is not necessarily incongruent since cGMP is reported to have both sensitizing and antinociceptive roles in mammals, depending upon the conditions (larger cGMP responses are more likely to be sensitizing) and the locus (central cGMP signaling is mainly antinociceptive, intense peripheral cGMP signaling is sensitizing; Kawabata et al., 1994; Aley et al., 1998; Sousa and Prado, 2001; Tegeder et al., 2002; Vivancos et al., 2003; Patil et al., 2005; Hucho and Levine, 2007). Under certain conditions cGMP even has a role alongside cAMP in nociceptive sensitization in Aplysia, rodents, and primates (Lin et al., 1997; Lewin and Walters, 1999; Levy and Strassman, 2004; Heine et al., 2011; Luo et al., 2014). It will be important to determine what the activation factor(s) for Ih is in this model.
Several vertebrate models examining Ih inhibition in pain are based on the hypothesis that sensitization occurs via hyperexcitability in peripheral sensory neurons (Chaplan et al., 2003; Lee et al., 2005; Sun et al., 2005; Luo et al., 2007; Jiang et al., 2008b; Cho et al., 2009; Takasu et al., 2010; Yeon et al., 2011; Weng et al., 2012; Acosta et al., 2012), however others also suggest that the major effect may be synaptic transmission (Jiang et al., 2008b; Papp et al., 2010; Takasu et al., 2010). Due to the primarily central sensitization, M. sexta may provide a useful setting to differentiate between the varied central and peripheral aspects of Ih and other site-specific players such as cGMP. Moreover, despite the evolutionary distance between these models, M. sexta may yield interesting evolutionary insights into the conserved nature of sensitization mechanisms like Ih and NMDAR function. This may be particularly significant given the very different sensory transmission mechanisms (cholinergic vs. glutamatergic) between insects and mammals.
Supplementary Material
Connective activity during the defensive strike. Video recording of the animal responding to noxious pinch with forceps. Audio clicks represent individual spikes recorded from the connective. Firing activity is greatly increased just prior to and during movement of the animal, and is relatively silent before and after (see also Fig. 2).
Acknowledgments
We would like to thank John T. Birmingham and Robyn J. Crook for their editorial comments on the manuscript, Scott W. Roy for input and guidance with the bioinformatics analysis, as well as Stephen Richards, Michael Kanost, and Gary Blissard for making the M. sexta genome public.
Dennis Tabuena was funded by the National Institutes of Health MBRS-RISE Fellowship (R25-GM059298) and the Genentech Foundation MS Dissertation Scholarship. Allan Solis was funded by the National Institutes of Health Bridges to the Baccalaureate fellowship (2R25-GM050078). Megumi Fuse was funded by National Institutes of Health Minority Biomedical Research Support grant (SC2 GM095428-01A1) and by the SFSU center for Computing for Life Sciences.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D.R.T. Acquisition of data: D. R. T., A. S. and K. G. Analysis and interpretation of data: D.R.T. and A. S. Drafting of the manuscript: D. R. T. and M. F. Critical revision of the manuscript for important intellectual content: D. R. T., C. A. M. and M. F. Statistical analysis: D. R. T. and C. A. M. Obtained funding: M. F. and C. A. M. Administrative, technical, and material support: M. F. and C. A. M. Study supervision: M. F.
Literature Cited
- Acosta C, McMullan S, Djouhri L, Gao L, Watkins R, Berry C, Dempsey K, Lawson SN, Zhang Z. HCN1 and HCN2 in Rat DRG Neurons: Levels in Nociceptors and Non-Nociceptors, NT3-Dependence and Influence of CFA-Induced Skin Inflammation on HCN2 and NT3 Expression. PLoS One. 2012;7:e50442. doi: 10.1371/journal.pone.0050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Babcock DT, Galko MJ. Two sides of the same coin no longer: genetic separation of nociceptive sensitization responses. Commun Integr Biol. 2009;2:517–9. doi: 10.4161/cib.2.6.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Landry C, Galko MJ. Cytokine Signaling Mediates UV-Induced Nociceptive Sensitization in Drosophila Larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21:1525–33. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Bhatt D, Cooper RL. The pharmacological and physiological profile of glutamate receptors at the Drosophila larval neuromuscular junction. Physiol Entomol. 2005;30:205–210. [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchi A, Baruscotti M, Nardini M, Barbuti A, Micheloni S, Bolognesi M, DiFrancesco D. Identification of the molecular site of ivabradine binding to HCN4 channels. PLoS One. 2013;8:e53132. doi: 10.1371/journal.pone.0053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaert D, Birman S. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J Neurobiol. 2001;48:58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo H-Q, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung U, Atwood HL, Zucker RS. Presynaptic effectors contributing to cAMP-induced synaptic potentiation in Drosophila. J Neurobiol. 2006;66:273–80. doi: 10.1002/neu.20218. [DOI] [PubMed] [Google Scholar]
- Chiang A-S, Lin W-Y, Liu H-P, Pszczolkowski MA, Fu T-F, Chiu S-L, Holbrook GL. Insect NMDA receptors mediate juvenile hormone biosynthesis. Proc Natl Acad Sci U S A. 2002a;99:37–42. doi: 10.1073/pnas.012318899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang A-S, Pszczolkowski MA, Liu H, Lin S. Ionotropic glutamate receptors mediate juvenile hormone synthesis in the cockroach, Diploptera punctata. Insect Biochem Mol Biol. 2002b;32:669–78. doi: 10.1016/s0965-1748(01)00146-1. [DOI] [PubMed] [Google Scholar]
- Cho H-J, Staikopoulos V, Furness JB, Jennings EA. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience. 2009;162:453–461. doi: 10.1016/j.neuroscience.2009.04.063. [DOI] [PubMed] [Google Scholar]
- Clatworthy AL, Walters ET. Rapid amplification and facilitation of mechanosensory discharge in Aplysia by noxious stimulation. J Neurophysiol. 1993;70:1181–94. doi: 10.1152/jn.1993.70.3.1181. [DOI] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- Crook RJ, Hanlon RT, Walters ET. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J Neurosci. 2013;33:10021–6. doi: 10.1523/JNEUROSCI.0646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymborowski B, Bogus M, Beckage NE, Williams CM, Riddiford LM. Juvenile hormone titres and metabolism during starvation-induced supernumerary larval moulting of the tobacco hornworm, Manduca sexta L. J Insect Physiol. 1982;28:129–135. [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 2008;508:131–52. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- Dixon W, Mood A. A method for obtaining and analyzing sensitivity data. J Am Stat …. 1948;43:109–126. [Google Scholar]
- Dixon WJ. The Up-and-Down Method for Small Samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- Ehrlich JS, Boulis NM, Karrer T, Sahley CL. Differential effects of serotonin depletion on sensitization and dishabituation in the leech, Hirudo medicinalis. J Neurobiol. 1992;23:270–9. doi: 10.1002/neu.480230306. [DOI] [PubMed] [Google Scholar]
- Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–6. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel AV, Sun W, Montal M. Molecular design of the N-methyl-D-aspartate receptor binding site for phencyclidine and dizolcipine. Proc Natl Acad Sci U S A. 1995;92:8021–5. doi: 10.1073/pnas.92.17.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse M, Merchasin E, McMackin M, Iwasaki K, Ramos L, Moffatt C. Soc Neurosci Abstract (SFN) San Diego, CA: 2013. Characterization of nociceptive sensitization in larval Manduca sexta. [Google Scholar]
- Fuse M, Truman JW. Modulation of ecdysis in the moth Manduca sexta: the roles of the suboesophageal and thoracic ganglia. J Exp Biol. 2002;205:1047–58. doi: 10.1242/jeb.205.8.1047. [DOI] [PubMed] [Google Scholar]
- Geister TL, Lorenz MW, Hoffmann KH, Fischer K. Effects of the NMDA receptor antagonist MK-801 on female reproduction and juvenile hormone biosynthesis in the cricket Gryllus bimaculatus and the butterfly Bicyclus anynana. J Exp Biol. 2008;211:1587–93. doi: 10.1242/jeb.016725. [DOI] [PubMed] [Google Scholar]
- van Griethuijsen LI, Banks KM, Trimmer BA. Spatial accuracy of a rapid defense behavior in caterpillars. J Exp Biol. 2013;216:379–87. doi: 10.1242/jeb.070896. [DOI] [PubMed] [Google Scholar]
- van Griethuijsen LI, Trimmer BA. Locomotion in caterpillars. Biol Rev. 2014;89:656–670. doi: 10.1111/brv.12073. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Graubard K, Truman JW. Tiling of the body wall by multidendritic sensory neurons in Manduca sexta. J Comp Neurol. 2001;440:271–283. doi: 10.1002/cne.1385. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–78. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Truman JW. Development and organization of a nitric-oxide-sensitive peripheral neural plexus in larvae of the moth, Manduca sexta. J Comp Neurol. 1999;404:127–141. doi: 10.1002/(sici)1096-9861(19990201)404:1<127::aid-cne10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Heine S, Michalakis S, Kallenborn-Gerhardt W, Lu R, Lim H-Y, Weiland J, Del Turco D, Deller T, Tegeder I, Biel M, Geisslinger G, Schmidtko A. CNGA3: a target of spinal nitric oxide/cGMP signaling and modulator of inflammatory pain hypersensitivity. J Neurosci. 2011;31:11184–11192. doi: 10.1523/JNEUROSCI.6159-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hult EF, Marchal E, Tobe SS. Identification and characterization of the NMDA receptor and its role in regulating reproduction in the cockroach Diploptera punctata. J Exp Biol. 2015;218:983–90. doi: 10.1242/jeb.115154. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive Neurons Protect Drosophila Larvae from Parasitoid Wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Galko MJ. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn. 2012;241:16–26. doi: 10.1002/dvdy.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Takle K, Jo J, Babcock DT, Ma Z, Xiang Y, Galko MJ. Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. Elife. 2015;4:e10735. doi: 10.7554/eLife.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Sun Q, Tu HY, Wan Y. Characteristics of HCN channels and their participation in neuropathic pain. Neurochem Res. 2008a;33:1979–1989. doi: 10.1007/s11064-008-9717-6. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain. 2008b;137:495–506. doi: 10.1016/j.pain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata A, Manabe S, Manabe Y, Takagi H. Effect of topical administration of L-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br J Pharmacol. 1994;112:547–50. doi: 10.1111/j.1476-5381.1994.tb13108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Chang L, Sorkin LS, Chaplan SR, Lee Doo H, Chang Leon, Linda S, Sorkin SC. Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. J Pain. 2005;6:417–424. doi: 10.1016/j.jpain.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0043597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RB, Pak C, Linn D. The structure, function and metamorphic reorganization of somatotopically projecting sensory neurons in Manduca sexta larvae. J Comp Physiol A. 1985;157:1–13. [Google Scholar]
- Levine RB, Truman JW. Dendritic reorganization of abdominal motoneurons during metamorphosis of the moth, Manduca sexta. J Neurosci. 1985;5:2424–31. doi: 10.1523/JNEUROSCI.05-09-02424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2:18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Wu J, Willis WD. Involvement of cGMP in nociceptive processing by and sensitization of spinothalamic neurons in primates. J Neurosci. 1997;17:3293–3302. doi: 10.1523/JNEUROSCI.17-09-03293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc Biol Sci. 1994a;255:113–8. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc Biol Sci. 1994b;255:215–21. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014;37:343–355. doi: 10.1016/j.tins.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Marx T, Gisselmann G, Störtkuhl KF, Hovemann BT, Hatt H. Molecular cloning of a putative voltage- and cyclic nucleotide-gated ion channel present in the antennae and eyes of Drosophila melanogaster. Invertebr Neurosci. 1999;4:55–63. doi: 10.1007/pl00022368. [DOI] [PubMed] [Google Scholar]
- McMackin MZ, Lewin MR, Tabuena DR, Arreola FE, Moffatt C, Fuse M. Use of von Frey filaments to assess nociceptive sensitization in the hornworm, Manduca sexta. J Neurosci Methods. 2016;257:139–146. doi: 10.1016/j.jneumeth.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchasin E. Unpublished master’s thesis. San Francisco State University; San Francisco, California: 2009. Characterization of Nociceptive Sensitization in Larval. [Google Scholar]
- Miyazaki S. The ionic mechanism of action potentials in neurosecretory cells and non-neurosecretory cells of the silkworm. J Comp Physiol A. 1980;140:43–52. [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–7. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Neely GG, Keene AC, Duchek P, Chang EC, Wang Q-P, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, Penninger JM. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh S, Kumar N, Bukhanova N, Chen Y, Stemkowsi PL, Smith PA. The heart-rate-reducing agent, ivabradine, reduces mechanical allodynia in a rodent model of neuropathic pain. Eur J Pain. 2014;18:1139–1147. doi: 10.1002/j.1532-2149.2014.00460.x. [DOI] [PubMed] [Google Scholar]
- Papp I, Holló K, Antal M. Plasticity of hyperpolarization-activated and cyclic nucleotid-gated cation channel subunit 2 expression in the spinal dorsal horn in inflammatory pain. Eur J Neurosci. 2010;32:1193–1201. doi: 10.1111/j.1460-9568.2010.07370.x. [DOI] [PubMed] [Google Scholar]
- Patil CS, Singh VP, Kulkarni SK. Peripheral and central activation of nitric oxide-cyclic GMP pathway by sildenafil. Inflammopharmacology. 2005;13:467–78. doi: 10.1163/156856005774649359. [DOI] [PubMed] [Google Scholar]
- Pirtle TJ, Satterlie RA. Cellular Mechanisms Underlying Swim Acceleration in the Pteropod Mollusk Clione limacina. Integr Comp Biol. 2004;44:37–46. doi: 10.1093/icb/44.1.37. [DOI] [PubMed] [Google Scholar]
- Poelchau M, Childers C, Moore G, Tsavatapalli V, Evans J, Lee C-Y, Lin H, Lin J-W, Hackett K. The i5k Workspace@NAL--enabling genomic data access, visualization and curation of arthropod genomes. Nucleic Acids Res. 2015;43:D714–9. doi: 10.1093/nar/gku983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi S, Trimmer BA. The role of nitric oxide in motoneuron spike activity and muscarinic-evoked changes in cGMP in the CNS of larval Manduca sexta. J Comp Physiol A. 1999;185:539–50. doi: 10.1007/s003590050414. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Sahley CL, Modney BK, Boulis NM, Muller KJ. The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J Neurosci. 1994;14:6715–21. doi: 10.1523/JNEUROSCI.14-11-06715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- Shimono K, Fujimoto A, Tsuyama T, Yamamoto-Kochi M, Sato M, Hattori Y, Sugimura K, Usui T, Kimura K, Uemura T. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 2009;4:37. doi: 10.1186/1749-8104-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001;897:9–19. doi: 10.1016/s0006-8993(01)01995-3. [DOI] [PubMed] [Google Scholar]
- Sun Q, Xing GG, Tu HY, Han JS, Wan Y. Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. Brain Res. 2005;1032:63–69. doi: 10.1016/j.brainres.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain. 2010;151:87–96. doi: 10.1016/j.pain.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Schmidtko A, Niederberger E, Ruth P, Geisslinger G. Dual effects of spinally delivered 8-bromo-cyclic guanosine mono-phosphate (8-bromo-cGMP) in formalin-induced nociception in rats. Neurosci Lett. 2002;332:146–50. doi: 10.1016/s0304-3940(02)00938-2. [DOI] [PubMed] [Google Scholar]
- Thollon C, Bedut S, Villeneuve N, Cogé F, Piffard L, Guillaumin J-P, Brunel-Jacquemin C, Chomarat P, Boutin J, Peglion J-L, Vilaine J-P. Use-dependent inhibition of hHCN4 by ivabradine and relationship with reduction in pacemaker activity. Br J Pharmacol. 2007;150:37–46. doi: 10.1038/sj.bjp.0706940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Bargmann CI. Invertebrate nociception: behaviors, neurons and molecules. J Neurobiol. 2004;61:161–74. doi: 10.1002/neu.20082. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Trimmer BA, Weeks JC. Effects of Nicotinic and Muscarinic Agents on an Identified Motoneurone and its Direct Afferent Inputs in Larval Manduca Sexta. J Exp Biol. 1989;337:303–337. [Google Scholar]
- Trimmer BA, Weeks JC. Activity-dependent induction of facilitation, depression, and post-tetanic potentiation at an insect central synapse. J Comp Physiol A. 1991;168:27–43. doi: 10.1007/BF00217101. [DOI] [PubMed] [Google Scholar]
- Trimmer BA, Weeks JC. Muscarinic acetylcholine receptors modulate the excitability of an identified insect motoneuron. J Neurophysiol. 1993;69:1821–36. doi: 10.1152/jn.1993.69.6.1821. [DOI] [PubMed] [Google Scholar]
- Trimmer BA. Characterization of a muscarinic current that regulates excitability of an identified insect motoneuron. J Neurophysiol. 1994;72:1862–73. doi: 10.1152/jn.1994.72.4.1862. [DOI] [PubMed] [Google Scholar]
- Trimmer BA. Current excitement from insect muscarinic receptors. Trends Neurosci. 1995;18:104–11. [PubMed] [Google Scholar]
- Vivancos GG, Parada CA, Ferreira SH. Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br J Pharmacol. 2003;138:1351–1357. doi: 10.1038/sj.bjp.0705181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop B, Levine RB. Development of the gin trap reflex in Manduca sexta: a comparison of larval and pupal motor responses. J Comp Physiol A. 1989;165:743–753. doi: 10.1007/BF00610873. [DOI] [PubMed] [Google Scholar]
- Waldrop B, Levine RB. Intersegmental interneurons serving larval and pupal mechanosensory reflexes in the moth Manduca sexta. J Comp Physiol A. 1992;171:195–205. doi: 10.1007/BF00188927. [DOI] [PubMed] [Google Scholar]
- Walters ET, Illich PA, Weeks JC, Lewin MR. Defensive responses of larval Manduca sexta and their sensitization by noxious stimuli in the laboratory and field. J Exp Biol. 2001;204:457–469. doi: 10.1242/jeb.204.3.457. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983;50:1522–42. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Walters ET. Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol. 1994;36:325–427. doi: 10.1016/s0074-7742(08)60307-4. [DOI] [PubMed] [Google Scholar]
- Wells C, Aparicio K, Salmon A, Zadel A, Fuse M. Structure–activity relationship of ETH during ecdysis in the tobacco hornworm, Manduca sexta. Peptides. 2006;27:698–709. doi: 10.1016/j.peptides.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors. Pain. 2012;153:900–914. doi: 10.1016/j.pain.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Chiang A. NMDA Receptors in Drosophila. 2009. [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu T-F, Lin W-Y, Wu C-L, Pyzocha L, Lin I-R, Saitoe M, Tully T, Chiang A-S. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–15. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon KY, Chung G, Kim YH, Hwang JH, Davies AJ, Park MK, Ahn DK, Kim JS, Jung SJ, Oh SB. Eugenol reverses mechanical allodynia after peripheral nerve injury by inhibiting hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Pain. 2011;152:2108–2116. doi: 10.1016/j.pain.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain. 2014;155:1708–1719. doi: 10.1016/j.pain.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Zayas RM, Qazi S, Morton DB, Trimmer BA. Neurons involved in nitric oxide-mediated cGMP signaling in the tobacco hornworm, Manduca sexta. J Comp Neurol. 2000;419:422–38. [PubMed] [Google Scholar]
- Zayas RM, Qazi S, Morton DB, Trimmer BA. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathway in insect neurons. J Neurochem. 2002;83:421–431. doi: 10.1046/j.1471-4159.2002.01147.x. [DOI] [PubMed] [Google Scholar]
- Zayas RM, Trimmer BA. Characterization of NO/cGMP-mediated responses in identified motoneurons. Cell Mol Neurobiol. 2007;27:191–209. doi: 10.1007/s10571-006-9091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–14. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond M, Bloom F, Landis S, Roberts J, Squire L. Fundamental Neuroscience. 1. San Diego, CA: Academic Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Connective activity during the defensive strike. Video recording of the animal responding to noxious pinch with forceps. Audio clicks represent individual spikes recorded from the connective. Firing activity is greatly increased just prior to and during movement of the animal, and is relatively silent before and after (see also Fig. 2).