Summary
Drought is one of the major abiotic stresses that directly implicate plant growth and crop productivity. Although many genes in response to drought stress have been identified, genetic improvement to drought resistance especially in food crops is showing relatively slow progress worldwide. Here, we reported the isolation of abscisic acid, stress and ripening (ASR) genes from upland rice variety, IRAT109 (Oryza sativa L. ssp. japonica), and demonstrated that overexpression of OsASR5 enhanced osmotic tolerance in Escherichia coli and drought tolerance in Arabidopsis and rice by regulating leaf water status under drought stress conditions. Moreover, overexpression of OsASR5 in rice increased endogenous ABA level and showed hypersensitive to exogenous ABA treatment at both germination and postgermination stages. The production of H2O2, a second messenger for the induction of stomatal closure in response to ABA, was activated in overexpression plants under drought stress conditions, consequently, increased stomatal closure and decreased stomatal conductance. In contrast, the loss‐of‐function mutant, osasr5, showed sensitivity to drought stress with lower relative water content under drought stress conditions. Further studies demonstrated that OsASR5 functioned as chaperone‐like protein and interacted with stress‐related HSP40 and 2OG‐Fe (II) oxygenase domain containing proteins in yeast and plants. Taken together, we suggest that OsASR5 plays multiple roles in response to drought stress by regulating ABA biosynthesis, promoting stomatal closure, as well as acting as chaperone‐like protein that possibly prevents drought stress‐related proteins from inactivation.
Keywords: Drought, Oryza sativa, OsASR5, water content, ABA, stomata
Introduction
Drought is a major environmental stress affecting plant growth and reducing crop productivity. Due to the water shortage and inadequate rainfall in the rice‐growing season, improving drought resistance becomes especially important for stabilizing rice productivity and production. However, drought resistance is a complex trait that involves a series of physiological, morphological, cellular and molecular adaptive pathways (Nguyen et al., 1997; Umezawa et al., 2006; Valliyodan and Nguyen, 2006), resulting in a quite slow progress in the genetic improvement of drought resistance worldwide.
Multiple strategies are adapted by plants in response to drought stress; among them, drought avoidance and drought tolerance are the two major mechanisms for improving drought resistance (Luo, 2010; Price et al., 2002). Drought avoidance assists plants maintaining tissue water potential by deep root and reducing water loss, especially through promoting stomatal closure (Hu and Xiong, 2014). Upon drought stress, abscisic acid (ABA), a key plant hormone, increases dramatically, which in turn leads to a number of molecular and cellular responses, among which the best known are inducing stress‐related genes and triggering stomatal closure (Daszkowska‐Golec and Szarejko, 2013; Lee and Luan, 2012; Ye et al., 2012). Significant research findings over the last 10 years have shown that ABA stimulates H2O2 generation mainly by NADPH oxidase in guard cells, and the generated H2O2 plays a vital role as essential signal molecules that mediate ABA‐induced stomatal closure by activating plasma membrane calcium channels (Kwak et al., 2003; Mustilli et al., 2002; Pei et al., 2000; Wang and Song, 2008; Zhang et al., 2001). Recently, H2O2‐induced stomatal closure through ABA‐independent pathway was reported in rice. A zinc finger transcription factor, DST, negatively regulates H2O2‐induced stomatal closure by the direct modulation of genes related to H2O2 scavenging (Huang et al., 2009). A rice homologue of SRO, OsSRO1c, increased stomatal closure by the regulation of H2O2 homeostasis possibly through down‐regulation of DST (You et al., 2013). So far, the genes that regulate stomatal movement through ABA‐dependent and H2O2‐mediated pathway in crops have not been identified, and the mechanism of stomata‐regulated drought tolerance in crops is largely unknown.
In most species, abscisic acid, stress and ripening (ASR) genes belong to a small gene family that is characterized by the presence of an ABA/WDS domain, and have been identified from monocot to dicot; nevertheless, they do not present in Arabidopsis (Gonzalez and Iusem, 2014). ASR genes were found to express in various organs and growth stages among different species, and responsive to ABA and various abiotic stresses, including drought, cold and salt stresses (Cakir et al., 2003; Chen et al., 2011; Henry et al., 2011; Hu et al., 2013; Huang et al., 2000; Joo et al., 2013; Kalifa et al., 2004; Maskin et al., 2001; Perez‐Diaz et al., 2014; Philippe et al., 2010; Saumonneau et al., 2012). Although these genes were discovered two decades earlier and were reported in response to diverse abiotic stresses, till date we lack the complete understanding of the exact molecular functions and physiological roles under drought stress.
Yeast one‐hybrid experiments revealed that the grape (Vitis vinifera) ASR ortholog named VvMSA binds to the promoter of a hexose transporter gene VvHT1 (Cakir et al., 2003). By the yeast two‐hybrid approach, a protein partner of VvMSA was isolated and characterized as a DREB transcription factor (Saumonneau et al., 2008). Likewise, tobacco (Nicotiana tabacum) ASR ortholog named NtTIP1 interacts with a tobacco bZIP transcription factor in vivo, and they possibly function in flower development and stress response (Hwan et al., 2012). Until recently, genome‐wide chromatin immunoprecipitation data identified that rice OsASR5 binds to the promoter of the putative targets genes, including an ABC transporter required for Al tolerance (Arenhart et al., 2014). Similarly, the targets of tomato ASR1 were reported to be genes involving in cell wall synthesis and remodelling as well as water transporter like aquaporins (Ricardi et al., 2014). Interestingly, tomato, plantain and lily ASR proteins were reported to perform a chaperone‐like activity that protects reporter enzymes from denaturation induced by freezing or heat in vitro (Dai et al., 2011; Hsu et al., 2011; Konrad and Bar‐Zvi, 2008). Moreover, several studies on the heterologous and homologous expression of ASR genes in plant species were reported for functional characterization of ASR genes. Overexpression of the ASR gene from plantain (Musa paradisiaca; MpASR) and lily (Lilium longiflorum; LLA23) in Arabidopsis enhanced osmotic, cold and freezing tolerances possibly by acting as osmoprotectant, respectively (Dai et al., 2011; Hsu et al., 2011). Transgenic tobacco plants overexpressing the ASR gene from tomato (Solanum lycopersicum; ASR1) or Salicornia brachiata (SbASR‐1) exhibited improved tolerance to osmotic stress (Jha et al., 2012; Kalifa et al., 2004) and from wheat (Triticum aestivum; TaASR1) showed enhanced tolerance to water stress (Hu et al., 2013). The ZmASR1 protein influences branched‐chain amino acid biosynthesis and transgenic maize (Zea mays) plants overexpression of ZmASR1 maintained kernel yield under water‐limited conditions (Virlouvet et al., 2011). Overexpression of OsASR1 or OsASR3 in transgenic rice plants also resulted in enhanced tolerances to cold and drought stresses in terms of photosynthetic efficiency (Joo et al., 2013; Kim et al., 2009). It appears that the exact functions of the ASR proteins are still baffling, as the possible roles of the ASR genes could not be simply deduced by sequence homology with other known proteins (Virlouvet et al., 2011).
Upland rice (UR) has been evolved as ‘drought‐resistant type’ derived from natural and artificial selection under drought stress conditions, while lowland rice (LR) is ‘drought‐sensitive type’ in rice; thus, identification and elucidating the function of drought‐responsive genes from UR will promote our understanding of drought tolerance mechanism in rice. To gain new insight into ASR functions in response to drought stress, we characterized the ASR gene family from UR variety, IRAT109 (O. sativa L. ssp. japonica). Three drought‐responsive ASR genes, OsASR3, OsASR5 and OsASR6, were identified from UR, and using OsASR5 overexpression plants and the loss‐of‐function mutant, the function and molecular mechanism of OsASR5 in drought tolerance were characterized and discussed, respectively.
Results
Expression profile of ASR genes in UR and LR
Genes preferentially expressed in UR under drought stress conditions were the probable candidate genes to improve drought tolerance. For that reason, the expression changes in the ASR genes in response to drought were analysed between UR variety, IRAT109, and LR variety, Nipponbare (O. sativa L. ssp. japonica). Rice contains six ASR paralogous genes (Philippe et al., 2010); among them, OsASR3 was up‐regulated in IRAT109, and OsASR5 and OsASR6 were induced and up‐regulated by drought in IRAT109 relative to Nipponbare (Figure S1). To further study the functions of the ASR genes in response to abiotic stress in rice, we currently focused on the characterization of OsASR5.
To investigate whether the tissue‐specific expression of OsASR5 is different between the two varieties, the expression patterns of OsASR5 in various organs during seedling and productive stages were analysed by quantitative real‐time PCR (qRT‐PCR). As shown in Figure 1a, OsASR5 was expressed in various organs at seedling and reproductive stages, interestingly, highly expressed in the sheath and stem tissues of IRAT109 as compared to Nipponbare during reproductive stage. The temporal and spatial expression pattern of OsASR5 was further investigated by transforming Nipponbare with a fusion gene of Pro OsASR5:OsASR5‐GFP. The GFP signal was observed in pistil, stamen, glume, guard cell, leaf, root, sheath and in vascular bundles (Figure S2).
Figure 1.
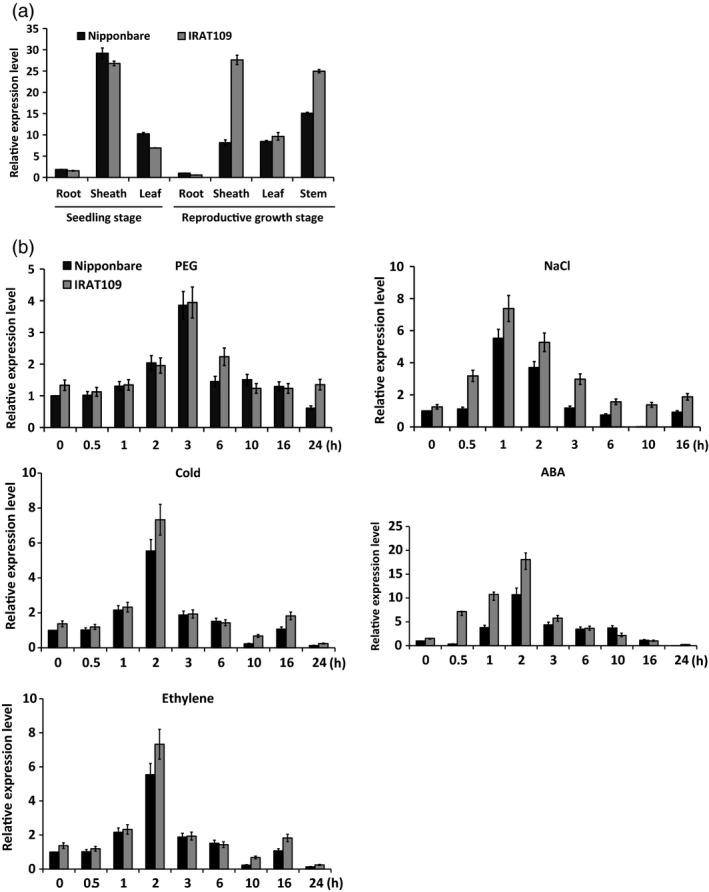
Expression analysis of the OsASR5 gene. (a) Real‐time PCR analysis of the expression level of OsASR5 in different tissues of LR variety, Nipponbare, and UR variety, IRAT109. (b) Stress‐inducible expression of OsASR5 under PEG, NaCl, cold, ABA and ethylene treatments. Error bars indicate standard error (SE) based on three replicates.
To speculate the function of OsASR5, the transcript levels of OsASR5 in response to polyethylene glycol (PEG), high salinity, cold, ABA and ethylene were analysed in the leaf tissues. The OsASR5 transcript was induced rapidly by PEG, NaCl, cold, ABA and ethylene for 1–3 h after treatments both in IRAT109 and in Nipponbare; interestingly, the expression levels of OsASR5 in IRAT109 were much higher than those in Nipponbare (Figure 1b). For instance, there was a significant increase in the OsASR5 transcripts in 1–2 h after ABA treatment in both varieties; however, the transcript levels of OsASR5 showed 1.5‐ to 2.0‐folds in IRAT109 as compared with Nipponbare. These data suggest that OsASR5 was responsive to multiple abiotic stresses preferentially in UR variety.
Expression of OsASR5 enhances osmotic and drought tolerance in E. coli and Arabidopsis
To examine the potential role of OsASR5 in protecting cells from osmotic stress, heterologous expression of OsASR5 in E. coli (BL21) was carried out. Cells transformed with the empty vector were used as a control (Figure 2a). The growth of the cells transformed either with empty vector or with recombinant plasmid showed nonsignificant differences on fresh LB media. On solid media containing 0.5 m mannitol, the transformants expressing GST‐OsASR5 fusion protein showed higher growth rate than those expressed GST protein only (Figure 2b). On liquid media with 0.5 m mannitol, the growth rate of the transformants expressing GST‐OsASR5 fusion protein was threefold higher than the control after incubation for 10 h (Figure 2c). These results clearly indicate that the heterologous expression of OsASR5 protein increased E. coli tolerance to osmotic stress.
Figure 2.
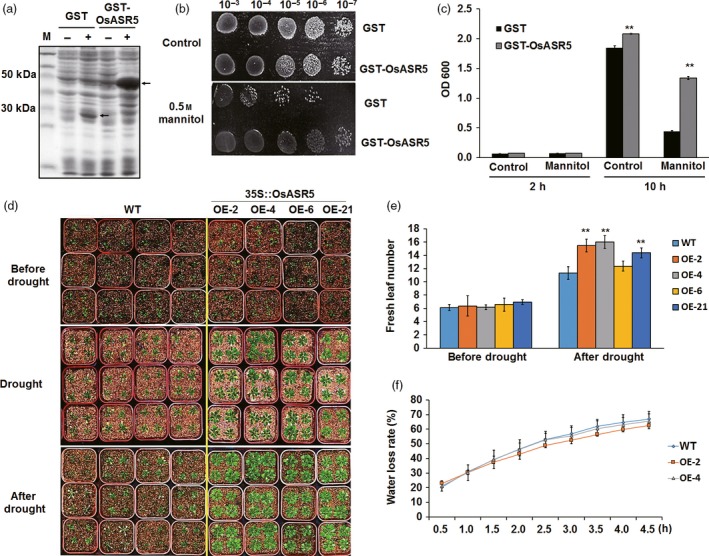
Enhanced osmotic and drought tolerance in E. coli and Arabidopsis. (a) Isopropylb‐D‐thiogalactopyranoside (IPTG)‐inducible expression of GST and GST‐OsASR5 fusion proteins. GST and GST‐OsASR5 were not (−) or were (+) induced by IPTG. Arrows indicate expression proteins. (b) Growth analysis of cells spotted on LB agar plate supplemented with 0.5 m mannitol. (c) Growth analysis of cells cultured in liquid medium supplemented with 0.5 m mannitol (n = 3). Cell growth densities were measured at 600 nm at the indicated time points. (d) Drought stress tolerance assay of OsASR5 overexpression Arabidopsis transgenic lines and WT by stopping irrigation for 3 weeks and recovery with rewatering for 4 days. (e) Fresh leaf numbers of OsASR5 overexpression Arabidopsis transgenic lines and WT before and after drought stress (n = 3, four plants in each repeat). (f) Water loss rate in the detached leaves of OsASR5 overexpression Arabidopsis transgenic lines and WT under normal conditions (n = 3, 12 leaves in each repeat). Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
To examine whether overexpression of OsASR5 in Arabidopsis would increase the tolerance of transgenic lines to drought stress, ten T3 transgenic lines were obtained and four of them with highest transcript levels of OsASR5 were used to verify the function of OsASR5 (Figure S3). There existed no developmental differences between overexpressed and wild‐type (WT) plants under the normal conditions. However, under drought stress conditions, the transgenic plants gave more green leaves with higher leaf area as compared with WT plants that showed a significant inhibited growth. Moreover, all of the transgenic plants showed the complete recovery after rewatering, while only half of the WT recovered, and water loss rates of detached leaves from transgenic plants were lower than those from WT. These results indicate that heterologous overexpression of OsASR5 in Arabidopsis enhances drought tolerance, suggesting that OsASR5 is functional in dicots.
Overexpression of OsASR5 significantly enhances osmotic and drought tolerance in rice
To directly investigate the function of OsASR5 in response to osmotic and drought stress in rice, seven transgenic lines with overexpressing OsASR5 were obtained. Of them, two lines (OE‐3 and OE‐19) with highest transcription levels of OsASR5 were selected to verify the function of OsASR5 (Figure S4). The performances of OsASR5 overexpression lines under high osmotic stress caused by adding high salinity or mannitol were examined. The growth of the OsASR5 overexpression seedlings was less inhibited (Figure 3a,b), exhibiting higher relative shoot growth and relative shoot fresh weight than those of the nontransgenic (NT) seedlings (Figure 3c,d). These results indicate that overexpressing OsASR5 in rice could enhance the tolerance of overexpression lines to osmotic stress.
Figure 3.
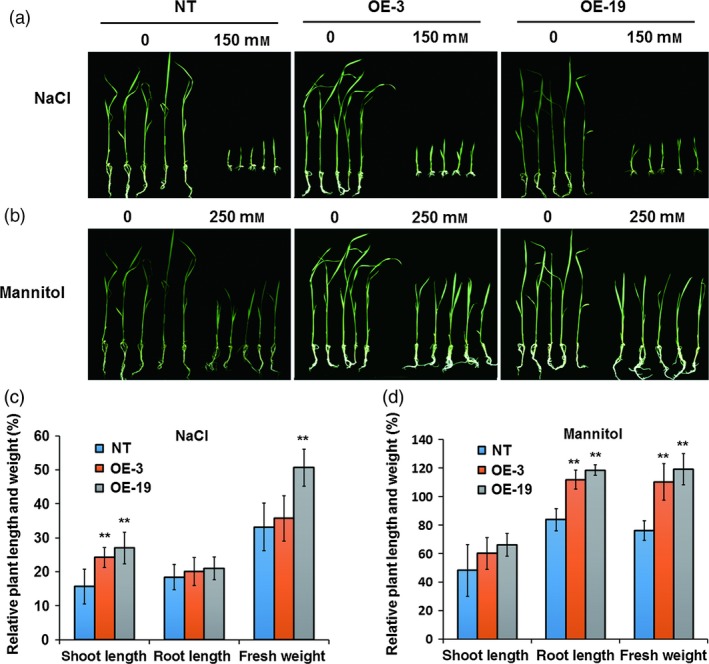
Increased osmotic tolerance of OsASR5 overexpression plants. (a, b) Growth performance of OsASR5 overexpression and NT seedlings under high salinity and mannitol treatments at the seventh d after transplanting, respectively (n = 3, five plants in each repeat). (c, d) The relative plant length and fresh weight of OsASR5 overexpression and NT seedlings corresponding to a, b, respectively. Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
Three‐week‐old seedlings grown in liquid medium were treated with PEG to create physiological dehydration stress conditions; after recovery was performed, OsASR5 overexpression lines showed a stronger growth recovery phenotype than that of the NT plants. Almost 31.6%–52.5% of OsASR5 overexpression plants survived, while only 21.6%–22.5% of the NT survived under this treatment (Figure 4a). Furthermore, the OsASR5 overexpression and NT plants were planted in the soil and well watered at the tillering stage. There were no developmental differences between OsASR5 overexpression and NT plants when normal irrigation was performed. However, after 1 week of stopping irrigation and 2 weeks of recovery, the OsASR5 overexpression lines showed a distinct recovery rate from that of the NT plants. Almost 33.3%–41.1% of OsASR5 overexpression plants survived, whereas only 5.5%–6.6% of the NT plants survived this treatment (Figure 4b). Therefore, it is evidence that overexpression of OsASR5 results in increased tolerance to drought stress in rice.
Figure 4.
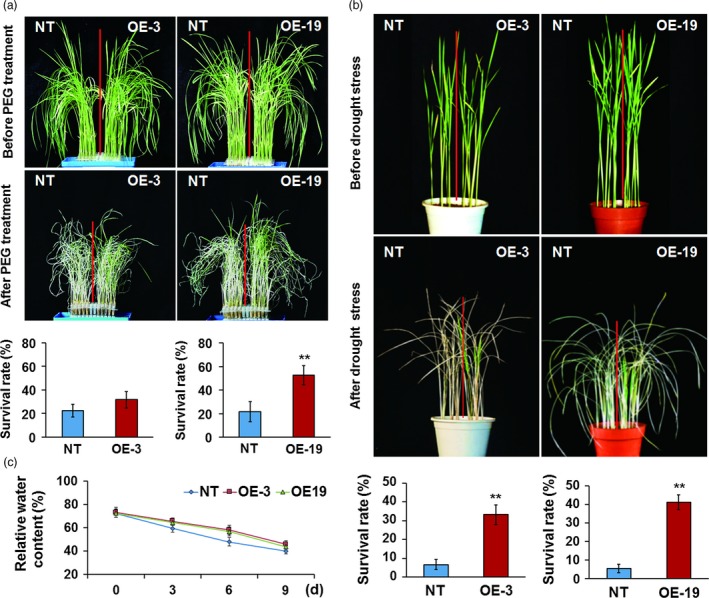
Enhanced drought tolerance of OsASR5 overexpression plants. (a) Physiological dehydration stress tolerance assay of OsASR5 overexpression and NT plants under 15% PEG6000 treatment. Survival rates of OsASR5 overexpression and NT plants after dehydration stress were examined (n = 3, 15 plants in each repeat). (b) Drought stress tolerance assay of OsASR5 overexpression and NT plants by stopping irrigation for 1 week and recovery with rewatering for 2 weeks. Survival rates of OsASR5 overexpression and NT plants after drought stress were examined (n = 3, nine plants in each repeat). (c) Relative water content of OsASR5 overexpression and NT plants under 15% PEG6000 treatment at the indicated time points (n = 3). Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
Because relative water content (RWC) is one of the most important traits to detect drought tolerance, the RWC in the leaves of OsASR5 overexpression and NT plants was measured during drought stress. High RWC in the leaves of OsASR5 overexpression plants was observed as compared with that of NT (Figure 4c), suggesting that OsASR5 possibly plays an important role in reducing water evaporation especially under drought stress conditions. To further evaluate the physiological and biochemical changes in OsASR5 overexpression plants, the contents of free proline and soluble carbohydrates in OsASR5 overexpression and NT plants were measured following drought stress. The contents of proline and sugar in both OsASR5 overexpression and NT plants rose continuously during drought treatment, whereas no significant differences were observed between OsASR5 overexpression and NT plants (Figure S5), suggesting that overexpression of OsASR5 does not regulate the accumulation of proline and sugar in transgenic plants.
Overexpression of OsASR5 increases stomatal closure
As water loss is mainly occurred through stomatal opening in plants, the reduced water loss in the OsASR5 overexpression plants prompted us to investigate stomatal aperture. The leaf stomatal apertures of OsASR5 overexpression and NT plants were observed by using scanning electron microscopy. As shown in Figure 5a,b, the percentages of completely closure, completely open and partially open stomata in the OsASR5 overexpression plants were not obviously different as compared with the NT plants under normal conditions. However, under drought stress conditions, 62.0% of stomata completely closed in the OsASR5 overexpression plants, while only 43.1% completely closed in the NT plants; in contrast, only 36.7% partially opened in the OsASR5 overexpression plants, but 54.4% partially opened in the NT plants, whereas nonsignificant differences in the percentage of completely open stomata were observed. Nonsignificant differences were observed for the stomatal density between overexpression and NT plants (Figure 5c). Moreover, the stomatal conductance was obviously decreased in OsASR5 overexpression plants as compared with the NT plants under drought stress conditions (Figure 5d). These results clearly demonstrate that overexpressing OsASR5 possibly affects the stomatal movements especially under drought stress conditions.
Figure 5.
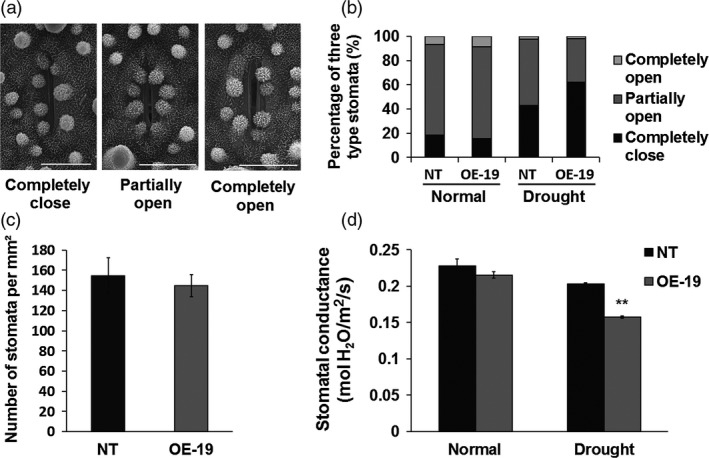
Overexpression of OsASR5 increasing stomatal closure. (a) Scanning electron microscopy images of three levels of stomatal apertures. Bar, 5 μm. (b) The percentage of three levels of stomatal apertures in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions (n = 300 stomata for NT under normal conditions; n = 248 stomata for OE‐19 under normal conditions; n = 322 stomata for NT under drought stress; n = 272 stomata for OE‐19 under drought stress). (c) Stomatal density of the middle leaves of OsASR5 overexpression and NT plants (n = 3). Three random scopes were used in each repeat. (d) Stomatal conductance of OsASR5 overexpression and NT plants (n = 3). Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
Overexpression of OsASR5 increased endogenous ABA level and sensitivity to exogenous ABA
Because ABA can induce stomatal closure and consequently decrease water loss, the endogenous ABA levels were measured in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions. The result showed that the endogenous ABA levels in both OsASR5 overexpression and NT seedlings were clearly increased by drought stress; interestingly, the level of ABA was much higher in OsASR5 overexpression seedlings (1513 ng/g fresh weight) than that in NT seedlings (1120 ng/g fresh weight), whereas no obvious difference was observed under normal conditions (Figure 6a). We hypothesized that OsASR5 may be involved in regulating ABA biosynthesis in drought stress. To confirm this, the transcript levels of ABA biosynthesis and responsive genes were analysed. As shown in Figure 6b, the expression levels of OsNCED4 and OsNCED5, RAB16A and RAB16C were highly up‐regulated by drought stress in OsASR5 overexpression seedlings as compared with NT seedlings, whereas the expression levels of these genes showed nonsignificant differences between OsASR5 overexpression and NT seedlings under normal conditions. These results indicate that OsASR5 may play an important role in drought‐induced ABA biosynthesis through up‐regulation of ABA biosynthesis genes, such as OsNCED4 and OsNCED5, and regulating ABA‐responsive genes, such as RAB16A and RAB16C.
Figure 6.
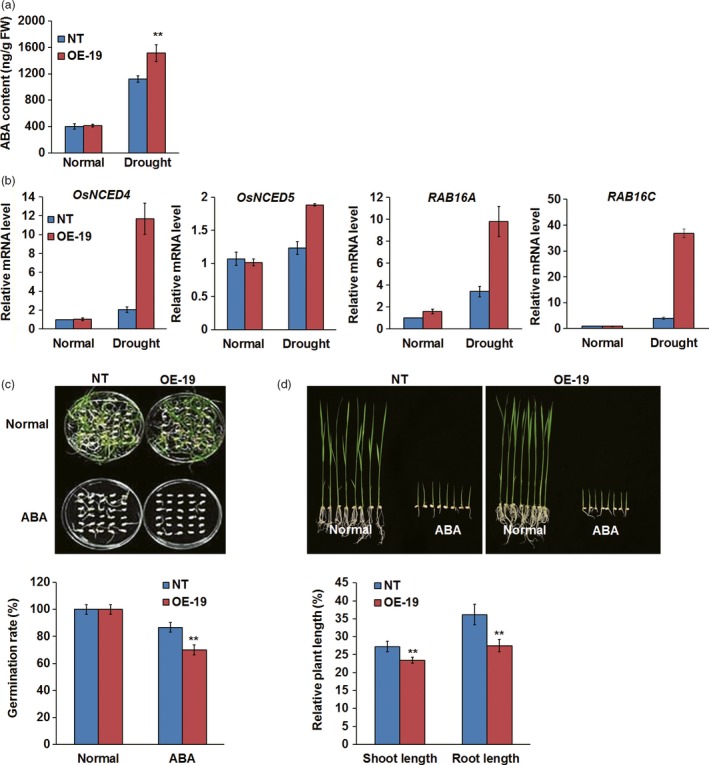
ABA accumulation and sensitivity of OsASR5 overexpression plants. (a) ABA contents of OsASR5 overexpression and NT plants under normal and drought stress conditions (n = 3). (b) Real‐time PCR analysis of the expression of ABA biosynthesis and responsive genes under normal and drought stress conditions (n = 3). (c) Germination rates of OsASR5 overexpression and NT seeds under ABA treatment (n = 3, 30 seeds in each repeat). (d) Growth performance and relative plant length of OsASR5 overexpression and NT seedlings under ABA treatment (n = 3, five plants in each repeat). Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
As the expression of OsASR5 was induced by ABA, we speculate that OsASR5 may play a positive role in ABA signalling in rice. To confirm this hypothesis, the exogenous ABA sensitivities of OsASR5 overexpression lines were investigated at germination and postgermination stages. As shown in Figure 6c, no obvious difference in germination rate was observed between overexpression and NT plants in the normal medium, whereas the germination rates of overexpression lines were significantly lower than those of the NT plants in the medium with 2.5 μm ABA at the end of treatment. Similarly, the relative shoot length and root length of the OsASR5 overexpression plants were significantly shorter than those of the NT plants, while nonsignificant differences were observed for the growth rate between OsASR5 overexpression and NT seedlings in the normal medium at the postgermination stage (Figure 6d). These results demonstrate that overexpressing OsASR5 increased exogenous ABA sensitivity at both germination and postgermination stages, indicating that OsASR5 may be a positive regulator of ABA signalling in rice.
OsASR5 modulates H2O2 homeostasis in drought stress
As ABA induced H2O2 generation in Arabidopsis (Pei et al., 2000; Zhang et al., 2001), and the accumulation of H2O2 resulting in stomatal closure was reported in rice (Huang et al., 2009; You et al., 2013), the H2O2 levels in the leaves of OsASR5 overexpression and NT plants were necessarily to be examined. A higher production of H2O2 was detected in the leaves of OsASR5 overexpression plants under drought stress conditions (Figure 7a,b), suggesting that the accumulation of H2O2 may increase stomatal closure in OsASR5 overexpression plants.
Figure 7.
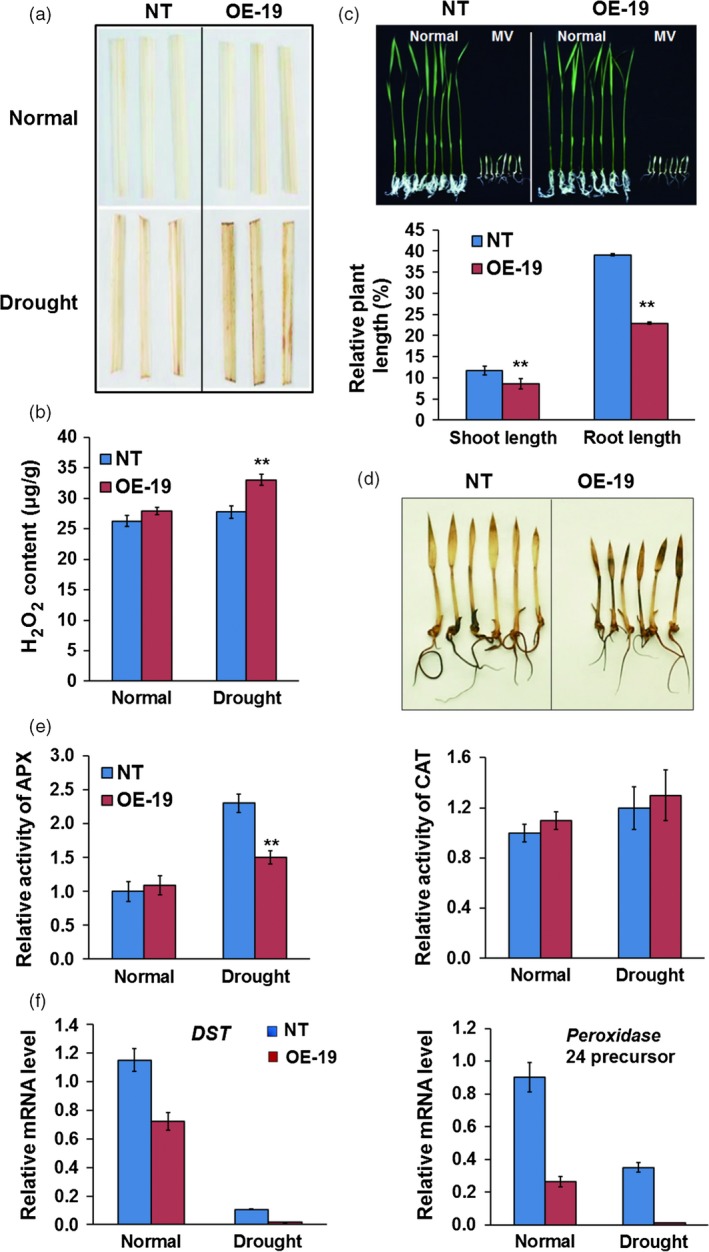
H2O2 accumulation in OsASR5 overexpression plants. (a) 3,3φ‐Diaminobenzidine (DAB) staining for H2O2 in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions. (b) Quantitative measurement of H2O2 in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions (n = 3, three plants in each repeat). (c) Growth performance and relative plant length of OsASR5 overexpression and NT plants after MV treatment (n = 3, five plants in each repeat). (d) DAB staining for H2O2 in the leaves of OsASR5 overexpression and NT plants after MV treatment corresponding to C. (e) Activity of APX and CAT in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions (n = 3). (f) Expression of DST and peroxidase 24 precursor in the leaves of OsASR5 overexpression and NT plants under normal and drought stress conditions. Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
Overexpression of OsASR5 inducing H2O2 accumulation also prompted us to determine whether OsASR5 is involved in oxidative stress response. Germinated seedlings of the OsASR5 overexpression and NT plants were sown on 1/2 Murashige and Skoog (MS) and 1/2 MS medium containing 2 μm methyl viologen (MV). The growth rate was markedly reduced, and more H2O2 was accumulated in OsASR5 overexpression seedlings as compared with NT seedlings after oxidative stress treatment, while no obvious changes were observed under normal conditions (Figure 7c,d). These results suggest that overexpression of OsASR5 is sensitive to oxidative stress.
To understand the mechanism of OsASR5 in modulating H2O2 homeostasis, the activities of H2O2‐scavenging enzymes were measured in OsASR5 overexpression and NT plants. The results showed that the activity of APX was reduced in OsASR5 overexpression plants as compared with NT plants under drought stress conditions (Figure 7e). Because peroxidase 24 precursor that encodes a peroxidase to scavenge H2O2 was regulated by DST, a negative regulator of H2O2 accumulation (Huang et al., 2009), expression levels of DST and peroxidase 24 precursor were analysed in OsASR5 overexpression plants. The results revealed the expression of DST and peroxidase 24 precursor was significantly repressed in OsASR5 overexpression plants as compared with NT plants (Figure 7f). These results demonstrate that OsASR5 could regulate H2O2 homeostasis by affecting the activity of H2O2‐scavenging enzyme, APX, and suppressing DST and its downstream gene, peroxidase 24 precursor.
The osasr5 mutant is sensitive to drought stress
To confirm the function of OsASR5 in response to drought stress, T‐DNA insertion line, osasr5, was obtained. The T‐DNA was inserted in the promoter region, in 310 bp upstream of ATG (Figure S6A). Real‐time PCR analysis showed that almost no OsASR5 transcript was detected in the insertion line, indicating that osasr5 was true loss‐of‐function mutant line (Figure S6B). The growth of osasr5 mutant (Dongjing background) is similar to that of Dongjing (DJ); however, osasr5 mutant was hypersensitive to drought stress by 15% PEG6000 treatment (Figure 8a). The survival rate of osasr5 mutant was only 39%–44%, while 80%–83% of the DJ was recovered (Figure 8b). Low relative water content was observed in the leaves of osasr5 mutant under 15% PEG6000 treatment (Figure 8c). Moreover, osasr5 mutant showed reduced ABA sensitivity as compared with DJ at germination stage (Figure 8d,e); osasr5 mutant also showed the increased growth rate and reduced H2O2 accumulation after oxidative stress treatment (Figure 8f). Together, these results reconfirm the function of OsASR5 in drought stress tolerance.
Figure 8.
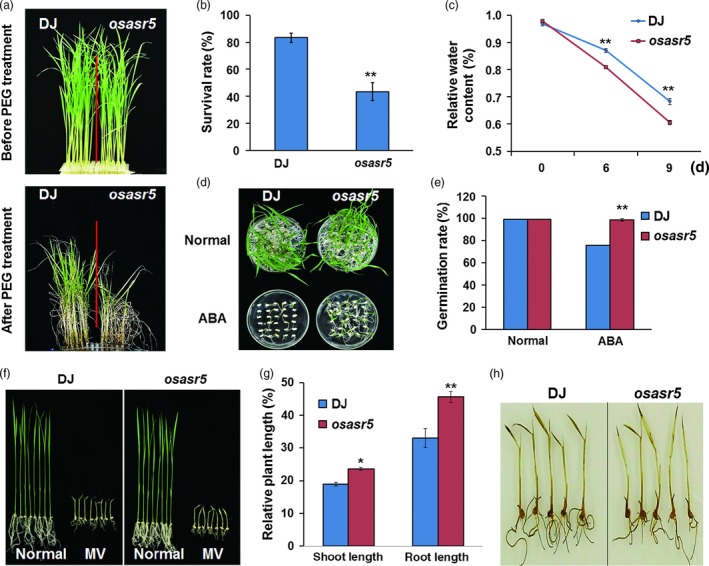
Increased drought and reduced ABA and oxidative sensitivities of the loss‐of‐function osasr5 mutant. (a) Physiological dehydration stress assay of osasr5 mutant and DJ with 15% PEG6000 treatment. (b) Survival rates of osasr5 mutant and DJ after dehydration stress (n = 3). (c) Relative water content of osasr5 mutant and DJ with 15% PEG6000 treatment observed at three different time intervals (0, 3 and 9d). (d) Germination performance of osasr5 mutant and DJ under ABA treatment (n = 3, 30 seeds in each repeat). (e) Germination rates of osasr5 mutant and DJ corresponding to d. (f) Growth performance of osasr5 mutant and DJ after MV treatment (n = 3, five plants in each repeat). (g) Relative plant length of osasr5 mutant and DJ corresponding to f. (h) DAB staining for H2O2 in the leaves of osasr5 mutant and DJ corresponding to f. Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
OsASR5 interacts with stress‐related proteins in chloroplast
In our homologous in vivo system with transgenic rice protoplast expressing OsASR5‐GFP fusion protein, we verified that the OsASR5 protein was localized in chloroplast and nucleus (Figure S7). As ASR proteins were reported to bind with DNA motif (Arenhart et al., 2014; Ricardi et al., 2014), it is necessary to analyse the transcription activity of OsASR5. However, the expression of BD (GAL4‐binding domain)‐OsASR5 fusion protein in yeast did not lead to reporter gene expression, and did not form homodimers to function (Figure S8), which indicated that OsASR5 has no transcriptional activity in yeast. To further elucidate the function of OsASR5, a cDNA library of IRAT109 treated with drought stress was constructed for screening OsASR5‐interacting proteins by yeast two‐hybrid (Y2H) system. Using the full‐length OsASR5 as bait, 24 positive clones were identified, and seven of them were confirmed to be unique interacting proteins (Figure 9a, Table S1). The interactions of OsASR5 with a heat‐shock protein, HSP40, and with a 2OG‐Fe (II) oxygenase family protein in the chloroplasts of tobacco epidermal cells and rice protoplast were confirmed by bimolecular fluorescence complementation (BiFC) assay (Figure 9b).
Figure 9.
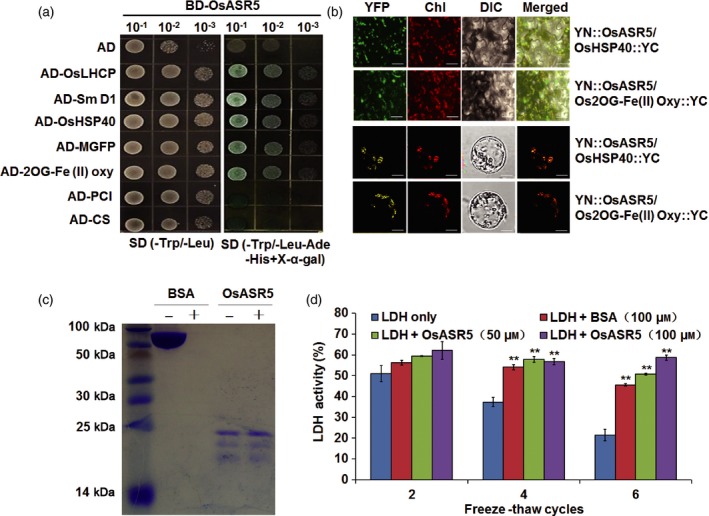
Interaction proteins and molecular chaperone activity of OsASR5. (a) Y2H assay of OsASR5 interacting proteins. BD‐OsASR5 co‐transformed with AD empty vector is used as negative control. Three different concentrations of yeast cells were grown on control plate (‐Trp/‐Leu) and selective plate (‐Trp/‐Leu/‐Ade/‐His/X‐α‐gal). (b) BiFC assay for the in vivo interaction of OsASR5 with OsHSP40 and with OsFe(II) Oxy in tobacco epidermal cells (upper) and rice protoplast (lower). Bar, 10 μm. (c) OsASR5 and BSA (control) were not (−) or were (+) boiled at 100 °C for 30 min (n = 3). (d) LDH activity in the presence or absence of OsASR5 during cycles of freeze–thaw treatments (n = 3). Data are mean ± SE. ** indicates significant difference at P < 0.01 probability.
OsASR5 functions as chaperone‐like protein
ASR proteins are low molecular weight charged and hydrophilic proteins (Goldgur et al., 2007; Gonzalez and Iusem, 2014), while hydrophilic proteins were shown to possess chaperone‐like activity (Garay‐Arroyo et al., 2000). OsASR5 was predicted to be a hydrophilic protein (data not shown) and was heat stable (Figure 9c), which indicates that OsASR5 is not likely to aggregate during high temperature treatment or boiling. We also examined whether OsASR5 exhibits chaperone activity to protect protein from inactivation. The activity of LDH in the presence or absence of OsASR5 in vitro was detected during cycles of freeze–thaw treatment. The activity of LDH was significantly reduced after four cycles of freeze–thaw, while the enzyme activity was markedly retained in the presence of OsASR5 (Figure 9d). It is worth to note that the effect of enzyme protection by OsASR5 is superior to BSA, a cryoprotectant, after six cycles of freeze–thaw. Thus, these results indicate that OsASR5 can function as chaperone‐like protein and stabilize proteins against inactivation.
Discussion
OsASR genes preferentially expressed in UR are probably drought‐responsive genes in rice
Previous studies reported comparative expression profiles of UR and LR under drought stress conditions using cDNA microarray technology (Ding et al., 2013; Lenka et al., 2011; Wang et al., 2007). In addition, differently expressed genes in the two genotypes were identified, and several of them were currently proved to be involved in drought response. For instance, SNAC1, OsLEA3‐1 and OsMIOX were strongly induced in UR variety by drought stress as compared with LR variety, and overexpression of these genes separately in LR variety showed significantly improved drought tolerance (Duan et al., 2012; Hu et al., 2006; Xiao et al., 2007). In recent years, the expression patterns of ASR genes both in tissue‐specific and in abiotic stresses were characterized by several groups (Joo et al., 2013; Philippe et al., 2010); however, we firstly analysed the expression changes of the rice ASR gene family between UR variety and LR variety. As described in Figure S1, OsASR3 was up‐regulated in UR variety, OsASR5 and OsASR6 were strongly induced by drought stress in UR variety, and the expression levels of these genes were 4.2‐ to 89.6‐fold higher in UR variety than those in LR variety during drought stress. Based on our findings and the knowledge gathered, we could deduce that OsASR3, OsASR5 and OsASR6 up‐regulated in UR variety were possibly drought stress‐responsive genes in rice. In order to confirm the hypothesis, these ASR genes were overexpressed into the japonica rice, Nipponbare, separately. And the role of OsASR5 in response to drought stress was identified firstly.
OsASR5 plays a positive role in drought stress response
It is widely accepted that the genes induced by abiotic stresses may play positive roles in abiotic stress tolerances. The transcription of OsASR5 was strongly induced by dehydration, high salinity, cold, ABA and ethylene treatments. OsASR5 overexpression lines showed improved growth performance under simulated osmotic stress conditions brought by NaCl or mannitol treatment, and enhanced the survival rate under dehydration conditions created by PEG treatment or dry soil conditions brought by restricting irrigation. Expression of OsASR5 also enhanced osmotic and drought stress tolerances in E. coli and Arabidopsis, respectively. Furthermore, overexpression of OsASR5 showed no obvious changes in morphological phenotype in rice and Arabidopsis transgenic lines under normal conditions. These results suggest that OsASR5 is a positive regulator of the responses to drought, osmotic and dehydration stresses, implying the usefulness of OsASR5 in genetic improvement of abiotic stress tolerance in several crop species.
OsASR5 confers tolerance to drought stress through a stomatal closure pathway associated with ABA and H2O2 signalling
Stomata control uptake of CO2 for photosynthesis and restrict water loss by modulating transpiration, thereby playing crucial roles in abiotic stress tolerance (Hetherington and Woodward, 2003; Schroeder et al., 2001). Due to the essential roles of the stomata for plants, the molecular mechanisms of stomatal movement integrated by phytohormone, environmental signalling and many ion channels have been frequently studied in Arabidopsis (Daszkowska‐Golec and Szarejko, 2013). So far, a total of seven drought‐responsive genes that regulating stomatal movement have been identified in rice (Gao et al., 2011; Hu et al., 2006; Huang et al., 2009; Manavalan et al., 2012; Wei et al., 2014; You et al., 2013; Zhang et al., 2011). Among which, SNAC1, OsSDIR1, hrf1, SQS and OsCPK9 were characterized to be sensitive to ABA, modulating stomatal movement possibly through an ABA‐dependent pathway (Gao et al., 2011; Hu et al., 2006; Manavalan et al., 2012; Wei et al., 2014; Zhang et al., 2011a), while DST and OsSRO1 regulated stomatal movement due to the accumulation of H2O2 through an ABA‐independent pathway (Huang et al., 2009; You et al., 2013). Therefore, knowledge on control of stomatal closure and opening remains fragmented in rice. In this study, OsASR5 was strongly induced by exogenous ABA treatment, and the endogenous ABA level of OsASR5 overexpression plants under drought stress conditions was much higher than that of NT. Furthermore, OsASR5 overexpression plants were more sensitive, and osasr5 mutant was more insensitive to exogenous ABA treatment than that of their WT, respectively. These results indicated that the OsASR5 was involved in an ABA‐dependent pathway.
H2O2 generation was dependent on ABA concentration and was essential for ABA‐induced stomatal closure in plants (Kwak et al., 2003; Wang and Song, 2008; Zhang et al., 2001). We found a higher accumulation of H2O2 along with the increased ABA level, and the coincidence of reduced rate of water loss with increased stomatal closure in OsASR5 overexpression plants under drought stress conditions. To our knowledge, we suggest that OsASR5 modulates stomatal closure probably due to the H2O2 accumulation through ABA‐dependent pathway under drought stress conditions.
Possible functions of the OsASR5 protein in chloroplast
ASR proteins were previously reported to be localized in both the cytosol and nucleus (Chen et al., 2011; Ricardi et al., 2012; Takasaki et al., 2008), only in the nucleus (Hu et al., 2013; Hwan et al., 2012) or in multiple cellular compartments such as nucleus, cytoplasm and chloroplasts (Arenhart et al., 2014). However, the precise function for the localization of ASR proteins in these subcellular compartments is not clear. We identified HSP40 and a 2OG‐Fe (II) oxygenase family protein that interacted with OsASR5 in the chloroplasts of tobacco epidermal cells and rice protoplasts, separately. This is the first report on the interaction of ASR proteins in the chloroplast. HSPs are stimulated in response to abiotic stress and play an important role in protecting plants against many stresses (Alvim et al., 2001; Cho and Hong, 2006; Sato and Yokoya, 2008; Timperio et al., 2008; Wang et al., 2014). A 2OG‐Fe (II) oxygenase family protein in rice affects water transport in leaves by affecting the composition and structure of leaf secondary cell walls (Fang et al., 2012). These evidences imply that OsASR5‐interacting proteins, HSP40 and 2OG‐Fe (II) oxygenase family protein may be playing important roles in response to water stress.
Abiotic stress may result in protein aggregation and degradation. Plants use a number of mechanisms to protect protein from inactivation, including chaperones and chaperone‐like proteins, and low molecular weight organic molecules (Konrad and Bar‐Zvi, 2008). In vitro assay with purified OsASR5 protein, we confirmed that OsASR5 could protect LDH from cold‐induced inactivation, function as chaperone‐like protein. Therefore, we could conclude that OsASR5 may function as molecular chaperone for the HSP40 and 2OG‐Fe (II) oxygenase family protein in chloroplast, and possibly prevent HSP40 and 2OG‐Fe (II) oxygenase family protein from inactivation under drought stress conditions.
Based on our knowledge, we try to summarize a model to explain the role of OsASR5 in improving drought stress tolerance in plant (Figure 10). In conclusion, OsASR5 has multiple roles in the regulation of drought stress tolerance by increasing ABA and H2O2 accumulation, thus leading to stomatal closure and reduce water loss, besides by acting as chaperone‐like protein that possibly protects some drought stress‐related proteins from inactivation under drought stress conditions. Furthermore, overexpression of OsASR5 did not alter the morphological phenotype of the transgenic lines. Therefore, through this study, we could successfully identify a gene, OsASR5, which may be potentially useful for engineering drought tolerance in plant.
Figure 10.
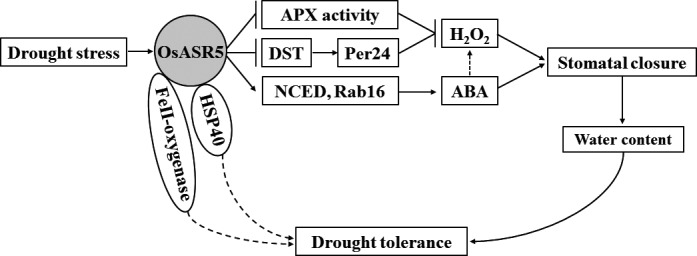
A proposed model explaining the function of OsASR5 in the regulation of stomatal status and drought stress tolerance. Under drought stress, the expression of OsASR5 was up‐regulated,resulting in up‐regulation of ABA biosynthesis and responsive genes,such as OsNCED4 and OsNCED5,RAB16A and RAB16C, leading to ABA accumulation and increased sensitivity to exogenous ABA. Up‐regulation of OsASR5 also affected the activity of H2O2‐scavenging enzyme, APX, and suppressed DST and its downstream gene, peroxidase 24 precursor, leading to H2O2 accumulation. ABA and H2O2 accumulation promotes stomatal closure, resulting in increased water content and finally enhancing drought tolerance. Furthermore, OsASR5 functioned as molecular chaperone and interacted with HSP40 and 2OG‐Fe (II) oxygenase family protein, may prevent those drought stress‐related proteins from inactivation under drought stress conditions. However, the function of those interacted proteins for drought stress tolerance remains to be elucidated in future studies.
Experimental procedures
Plant materials and growth conditions
The UR variety, IRAT109, and LR variety, Nipponbare, were used in this study. The japonica rice variety, DJ and mutant osasr5 seeds were obtained from the POSTECH RISD (http://www.postech.ac.kr/life/pfg/risd/). Seeds of IRAT109 and Nipponbare were germinated at 32 °C for 2 days and then grown in Hoagland nutrient solution under controlled conditions with 28 ± 2 °C temperature, 200 μmol/m2s2 light intensity with 14‐h light/10‐h dark photoperiod and 80% relative humidity. Four‐week‐old seedlings were subjected to different treatments with 15% PEG6000 (w/v), 200 mm NaCl, low temperature (4 °C), 100 μm ABA, 2 mm ethylene and drought by stopping irrigation. The leaf tissues were harvested at 0‐, 0.5‐, 1‐, 2‐, 3‐, 6‐, 10‐, 16‐ and 24‐h time points for PEG, low temperature, ABA and ethylene treatments. Likewise, leaf tissues were harvested at 0‐, 0.5‐, 1‐, 2‐, 3‐, 6‐, 10‐ and 16‐h time points for NaCl treatment and at 0‐, 4‐, 8‐, 11‐, 14‐, 17‐, 21‐, 25‐ and 28‐d time points for drought treatment. All these harvested leaf samples were then rapidly freezed in liquid nitrogen and stored at −80 °C for further expression analysis of OsASR5 between UR and LR.
Osmotic, drought and oxidative stress treatments
For osmotic stress treatment, the seeds of T3 transgenic and NT lines were germinated on 1/2 MS medium under 14‐h light (28 °C)/10‐h dark (25 °C) photoperiod conditions for 5 days and transplanted to 1/2 MS medium containing 150 mm NaCl and 250 mm mannitol, respectively. The shoot length, root length and fresh weight of transgenic lines and NT plants were measured after 7 days. For dehydration treatment, 3‐week‐old seedlings of OsASR5 overexpression and NT plants, mutant osasr5 and DJ plants grown in normal Hoagland solution were treated with 15% PEG6000 solution for 14 days and then recovered in normal Hoagland solution for 7 days. The survival rates of each line were examined. For drought treatment, 2‐week‐old seedlings of OsASR5 overexpression Arabidopsis transgenic lines and WT grown at 10‐h light (22 °C)/14‐h dark (18 °C) photoperiod in flowerpots with soil and vermiculite (1 : 2) were not irrigated. After 3 weeks of stopping irrigation, the seedlings were observed for recovery by rewatering for 4 days. Fresh leaf numbers of OsASR5 overexpression Arabidopsis transgenic lines and WT before and after drought stress were examined. One‐month‐old seedlings of the OsASR5 overexpression rice transgenic lines and NT plants grown in flowerpots with soil and vermiculite (1 : 1) were not irrigated. After 1 week of stopping irrigation, the seedlings were observed for recovery by rewatering for 2 weeks. Seedlings were regarded as survivals if the fresh and green young leaves emerged after water supply. The survival ratio was calculated according to the number of survival plants over the treated plants in each flowerpot. For oxidative treatment, the seeds of OsASR5 overexpression and NT plants germinated on normal 1/2 MS medium were transplanted to 1/2 MS medium containing 2 μm MV, and the plant length was measured at 5 days after transplanting.
Imaging of stomatal opening and measurement of stomatal conductance
Leaves of 1‐month‐old OsASR5 overexpression and NT plants with drought treatment (without irrigation for 3 days) or normal growth were detached and directly fixed by 2.5% glutaraldehyde. The stomatal pictures were obtained using a scanning electron microscopy (JSM‐6390lv, JEOL, Japan), and the percentages of stomatal completely open, partially open and completely close were calculated. The second fully expanded leaves, counting from the top of the same plants used for imaging stomata, were applied to measure stomatal conductance with a portable gas analysis system (LI‐COR 6400, LI‐COR, Inc.).
Endogenous ABA level and exogenous ABA sensitivity assay
Endogenous ABA levels were determined according to the method as described previously (Xiong et al., 2014). To test the ABA sensitivity at germination stage, seeds of OsASR5 overexpression and NT, osasr5 and DJ lines were germinated on 1/2 MS medium containing 2.5 μm ABA and the germination rates were calculated at the fifth day after initiation. To test the sensitivity at postgermination stage, the seeds of overexpression and NT plants germinated on normal 1/2 MS medium were transplanted to 1/2 MS medium containing 2.5 μm ABA. The shoot length and root length of each seedlings were measured after 7 days of the ABA treatment at 14‐h light (28 °C)/10‐h dark (25 °C) photoperiod.
Other methods
Details of the methods for RNA isolation and qRT‐PCR analysis, plasmid construction and plant transformation, subcellular localization, physiological and biochemical indexes assay, transactivation, yeast two‐hybrid and BiFC assays are available in supplementary methods at PBJ online.
Supporting information
Figure S1 Drought inducible expression of ASR genes in UR and LR varieties.
Figure S2 The temporal and spatial expression pattern of OsASR5 in the transgenic lines harbouring a fusion gene of Pro OsASR5:OsASR5‐GFP.
Figure S3 RT‐PCR analysis of OsASR5 transcript levels in different Arabidopsis transgenic lines.
Figure S4 Transcription levels of OsASR5 in OsASR5 overexpression rice transgenic lines.
Figure S5 Free proline and soluble sugar contents of OsASR5 overexpression and NT plants under 15% PEG6000 treatment.
Figure S6 Identification of osasr5 T‐DNA insertion mutant.
Figure S7 Subcellular localization of OsASR5‐GFP fusion protein.
Figure S8 OsASR5 transcriptional activation and homodimerization analysis.
Table S1 OsASR5 interacting proteins identified in yeast two‐hybrid screening.
Appendix S1 Supplementary methods.
Acknowledgements
This work was supported by grants from Ministry of Agriculture of the People's Republic of China (Grant Nos. 2014ZX08009‐003‐002 and 2014ZX08001‐003‐002. http://english.agri.gov.cn/np/); Ministry of Science and Technology of the People's Republic of China (Grant No.2015DFG31900.www.istcp.org.cn) National Natural Science Foundation of the People's Republic of China (Grant No. 31061140458. http://www.nsfc.gov.cn/Portal0/default166.htm). The authors declare no conflicts of interest.
References
- Alvim, F.C. , Carolino, S.M. , Cascardo, J.C. , Nunes, C.C. , Martinez, C.A. , Otoni, W.C. and Fontes, E.P. (2001) Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol. 126, 1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenhart, R.A. , Bai, Y. , de Oliveira, L.F. , Neto, L.B. , Schunemann, M. , Maraschin Fdos, S. , Mariath, J. et al (2014) New insights into aluminum tolerance in rice: the ASR5 protein binds the STAR1 promoter and other aluminum‐responsive genes. Mol. Plant, 7, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir, B. , Agasse, A. , Gaillard, C. , Saumonneau, A. , Delrot, S. and Atanassova, R. (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell, 15, 2165–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.Y. , Liu, D.J. , Jiang, Y.M. , Zhao, M.L. , Shan, W. , Kuang, J.F. and Lu, W.J. (2011) Molecular characterization of a strawberry FaASR gene in relation to fruit ripening. PLoS ONE, 6, e24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, E.K. and Hong, C.B. (2006) Over‐expression of tobacco NtHSP70‐1 contributes to drought‐stress tolerance in plants. Plant Cell Rep. 25, 349–358. [DOI] [PubMed] [Google Scholar]
- Dai, J.R. , Liu, B. , Feng, D.R. , Liu, H.Y. , He, Y.M. , Qi, K.B. , Wang, H.B. et al (2011) MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep. 30, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Daszkowska‐Golec, A. and Szarejko, I. (2013) Open or close the gate – stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X. , Li, X. and Xiong, L. (2013) Insight into differential responses of upland and paddy rice to drought stress by comparative expression profiling analysis. Int. J. Mol. Sci. 14, 5214–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J. , Zhang, M. , Zhang, H. , Xiong, H. , Liu, P. , Ali, J. , Li, J. et al (2012) OsMIOX, a myo‐inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci. 196, 143–151. [DOI] [PubMed] [Google Scholar]
- Fang, L. , Zhao, F. , Cong, Y. , Sang, X. , Du, Q. , Wang, D. , Li, Y. et al (2012) Rolling‐leaf14 is a 2OG‐Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves. Plant Biotechnol. J. 10, 524–532. [DOI] [PubMed] [Google Scholar]
- Gao, T. , Wu, Y. , Zhang, Y. , Liu, L. , Ning, Y. , Wang, D. , Tong, H. et al (2011) OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 76, 145–156. [DOI] [PubMed] [Google Scholar]
- Garay‐Arroyo, A. , Colmenero‐Flores, J.M. , Garciarrubio, A. and Covarrubias, A.A. (2000) Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 275, 5668–5674. [DOI] [PubMed] [Google Scholar]
- Goldgur, Y. , Rom, S. , Ghirlando, R. , Shkolnik, D. , Shadrin, N. , Konrad, Z. and Bar‐Zvi, D. (2007) Desiccation and zinc binding induce transition of tomato abscisic acid stress ripening 1, a water stress‐ and salt stress‐regulated plant‐specific protein, from unfolded to folded state. Plant Physiol. 143, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, R.M. and Iusem, N.D. (2014) Twenty years of research on Asr (ABA‐stress‐ripening) genes and proteins. Planta, 239, 941–949. [DOI] [PubMed] [Google Scholar]
- Henry, I.M. , Carpentier, S.C. , Pampurova, S. , Van Hoylandt, A. , Panis, B. , Swennen, R. and Remy, S. (2011) Structure and regulation of the Asr gene family in banana. Planta 234, 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington, A.M. and Woodward, F.I. (2003) The role of stomata in sensing and driving environmental change. Nature, 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Hsu, Y.F. , Yu, S.C. , Yang, C.Y. and Wang, C.S. (2011) Lily ASR protein‐conferred cold and freezing resistance in Arabidopsis. Plant Physiol. Biochem. 49, 937–945. [DOI] [PubMed] [Google Scholar]
- Hu, H. and Xiong, L. (2014) Genetic engineering and breeding of drought‐resistant crops. Annu. Rev. Plant Biol. 65, 715–741. [DOI] [PubMed] [Google Scholar]
- Hu, H. , Dai, M. , Yao, J. , Xiao, B. , Li, X. , Zhang, Q. and Xiong, L. (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl Acad. Sci. USA, 103, 12987–12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W. , Huang, C. , Deng, X. , Zhou, S. , Chen, L. , Li, Y. , Wang, C. et al (2013) TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 36, 1449–1464. [DOI] [PubMed] [Google Scholar]
- Huang, J.C. , Lin, S.M. and Wang, C.S. (2000) A pollen‐specific and desiccation‐associated transcript in Lilium longiflorum during development and stress. Plant Cell Physiol. 41, 477–485. [DOI] [PubMed] [Google Scholar]
- Huang, X.Y. , Chao, D.Y. , Gao, J.P. , Zhu, M.Z. , Shi, M. and Lin, H.X. (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 23, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwan, Y.S. , Hyon, K.I.M.S. , Berberich, T. and Kusano, T. (2012) Identification and properties of a small protein that interacts with a tobacco bZIP‐type transcription factor TBZF. Plant Biotechnol. 29, 395–399. [Google Scholar]
- Jha, B. , Lal, S. , Tiwari, V. , Yadav, S.K. and Agarwal, P.K. (2012) The SbASR‐1 gene cloned from an extreme halophyte Salicornia brachiata enhances salt tolerance in transgenic tobacco. Mar. Biotechnol. (NY) 14, 782–792. [DOI] [PubMed] [Google Scholar]
- Joo, J. , Lee, Y.H. , Kim, Y.K. , Nahm, B.H. and Song, S.I. (2013) Abiotic stress responsive rice ASR1 and ASR3 exhibit different tissue‐dependent sugar and hormone‐sensitivities. Mol. Cells, 35, 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalifa, Y. , Perlson, E. , Gilad, A. , Konrad, Z. , Scolnik, P.A. and Bar‐Zvi, D. (2004) Over‐expression of the water and salt stress‐regulated Asr1 gene confers an increased salt tolerance. Plant Cell Environ. 27, 1459–1468. [Google Scholar]
- Kim, S.J. , Lee, S.C. , Hong, S.K. , An, K. , An, G. and Kim, S.R. (2009) Ectopic expression of a cold‐responsive OsAsr1 cDNA gives enhanced cold tolerance in transgenic rice plants. Mol. Cells 27, 449–458. [DOI] [PubMed] [Google Scholar]
- Konrad, Z. and Bar‐Zvi, D. (2008) Synergism between the chaperone‐like activity of the stress regulated ASR1 protein and the osmolyte glycine‐betaine. Planta 227, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M. , Mori, I.C. , Pei, Z.M. , Leonhardt, N. , Torres, M.A. , Dangl, J.L. , Bloom, R.E. et al (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS‐dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.C. and Luan, S. (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. [DOI] [PubMed] [Google Scholar]
- Lenka, S.K. , Katiyar, A. , Chinnusamy, V. and Bansal, K.C. (2011) Comparative analysis of drought‐responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 9, 315–327. [DOI] [PubMed] [Google Scholar]
- Luo, L.J. (2010) Breeding for water‐saving and drought‐resistance rice (WDR) in China. J. Exp. Bot. 61, 3509–3517. [DOI] [PubMed] [Google Scholar]
- Manavalan, L.P. , Chen, X. , Clarke, J. , Salmeron, J. and Nguyen, H.T. (2012) RNAi‐mediated disruption of squalene synthase improves drought tolerance and yield in rice. J. Exp. Bot. 63, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskin, L. , Gudesblat, G.E. , Moreno, J.E. , Carrari, F.O. , Frankel, N. , Sambade, A. , Rossi, M. et al (2001) Differential expression of the members of the Asr gene family in tomato (Lycopersicon esculentum). Plant Sci. 161, 739–746. [Google Scholar]
- Mustilli, A.C. , Merlot, S. , Vavasseur, A. , Fenzi, F. and Giraudat, J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell, 14, 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, H.T. , Babu, R.C. and Blum, A. (1997) Breeding for drought resistance in rice: physiology and molecular genetics considerations. Crop Sci. 37, 1426–1434. [Google Scholar]
- Pei, Z.M. , Murata, Y. , Benning, G. , Thomine, S. , Klusener, B. , Allen, G.J. , Grill, E. et al (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature, 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Perez‐Diaz, J. , Wu, T.M. , Perez‐Diaz, R. , Ruiz‐Lara, S. , Hong, C.Y. and Casaretto, J.A. (2014) Organ‐ and stress‐specific expression of the ASR genes in rice. Plant Cell Rep. 33, 61–73. [DOI] [PubMed] [Google Scholar]
- Philippe, R. , Courtois, B. , McNally, K.L. , Mournet, P. , El‐Malki, R. , Le Paslier, M.C. , Fabre, D. et al (2010) Structure, allelic diversity and selection of Asr genes, candidate for drought tolerance, in Oryza sativa L. and wild relatives. TAG 121, 769–787. [DOI] [PubMed] [Google Scholar]
- Price, A.H. , Cairns, J.E. , Horton, P. , Jones, H.G. and Griffiths, H. (2002) Linking drought‐resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J. Exp. Bot. 53, 989–1004. [DOI] [PubMed] [Google Scholar]
- Ricardi, M.M. , Guaimas, F.F. , Gonzalez, R.M. , Burrieza, H.P. , Lopez‐Fernandez, M.P. , Jares‐Erijman, E.A. , Estevez, J.M. et al (2012) Nuclear import and dimerization of tomato ASR1, a water stress‐inducible protein exclusive to plants. PLoS ONE, 7, e41008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardi, M.M. , Gonzalez, R.M. , Zhong, S. , Dominguez, P.G. , Duffy, T. , Turjanski, P.G. , Salgado Salter, J.D. et al (2014) Genome‐wide data (ChIP‐seq) enabled identification of cell wall‐related and aquaporin genes as targets of tomato ASR1, a drought stress‐responsive transcription factor. BMC Plant Biol. 14, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y. and Yokoya, S. (2008) Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat‐shock protein, sHSP17.7. Plant Cell Rep. 27, 329–334. [DOI] [PubMed] [Google Scholar]
- Saumonneau, A. , Agasse, A. , Bidoyen, M.T. , Lallemand, M. , Cantereau, A. , Medici, A. , Laloi, M. et al (2008) Interaction of grape ASR proteins with a DREB transcription factor in the nucleus. FEBS Lett. 582, 3281–3287. [DOI] [PubMed] [Google Scholar]
- Saumonneau, A. , Laloi, M. , Lallemand, M. , Rabot, A. and Atanassova, R. (2012) Dissection of the transcriptional regulation of grape ASR and response to glucose and abscisic acid. J. Exp. Bot. 63, 1495–1510. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I. , Allen, G.J. , Hugouvieux, V. , Kwak, J.M. and Waner, D. (2001) Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Takasaki, H. , Mahmood, T. , Matsuoka, M. , Matsumoto, H. and Komatsu, S. (2008) Identification and characterization of a gibberellin‐regulated protein, which is ASR5, in the basal region of rice leaf sheaths. Mol. Genet. Genomics, 279, 359–370. [DOI] [PubMed] [Google Scholar]
- Timperio, A.M. , Egidi, M.G. and Zolla, L. (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J. Proteomics. 71, 391–411. [DOI] [PubMed] [Google Scholar]
- Umezawa, T. , Fujita, M. , Fujita, Y. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr. Opin. Biotechnol. 17, 113–122. [DOI] [PubMed] [Google Scholar]
- Valliyodan, B. and Nguyen, H.T. (2006) Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 9, 189–195. [DOI] [PubMed] [Google Scholar]
- Virlouvet, L. , Jacquemot, M.P. , Gerentes, D. , Corti, H. , Bouton, S. , Gilard, F. , Valot, B. et al (2011) The ZmASR1 protein influences branched‐chain amino acid biosynthesis and maintains kernel yield in maize under water‐limited conditions. Plant Physiol. 157, 917–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. and Song, C.P. (2008) Guard‐cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 178, 703–718. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Zhang, H. , Gao, F. , Li, J. and Li, Z. (2007) Comparison of gene expression between upland and lowland rice cultivars under water stress using cDNA microarray. TAG 115, 1109–1126. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Lin, S. , Song, Q. , Li, K. , Tao, H. , Huang, J. , Chen, X. et al (2014) Genome‐wide identification of heat shock proteins (Hsps) and Hsp interactors in rice: Hsp70s as a case study. BMC Genom. 15, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S. , Hu, W. , Deng, X. , Zhang, Y. , Liu, X. , Zhao, X. , Luo, Q. et al (2014) A rice calcium‐dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 14, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, B. , Huang, Y. , Tang, N. and Xiong, L. (2007) Over‐expression of a LEA gene in rice improves drought resistance under the field conditions. TAG 115, 35–46. [DOI] [PubMed] [Google Scholar]
- Xiong, H. , Li, J. , Liu, P. , Duan, J. , Zhao, Y. , Guo, X. , Li, Y. et al (2014) Overexpression of OsMYB48‐1, a novel MYB‐related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE, 9, e92913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, N. , Jia, L. and Zhang, J. (2012) ABA signal in rice under stress conditions. Rice (NY) 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, J. , Zong, W. , Li, X. , Ning, J. , Hu, H. , Li, X. , Xiao, J. et al (2013) The SNAC1‐targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J. Exp. Bot. 64, 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, L. , Dong, F. , Gao, J. , Galbraith, D.W. and Song, C.P. (2001) Hydrogen peroxide is involved in abscisic acid‐induced stomatal closure in Vicia faba . Plant Physiol. 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Xiao, S. , Li, W. , Feng, W. , Li, J. , Wu, Z. , Gao, X. et al (2011) Overexpression of a Harpin‐encoding gene hrf1 in rice enhances drought tolerance. J. Exp. Bot. 62, 4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Drought inducible expression of ASR genes in UR and LR varieties.
Figure S2 The temporal and spatial expression pattern of OsASR5 in the transgenic lines harbouring a fusion gene of Pro OsASR5:OsASR5‐GFP.
Figure S3 RT‐PCR analysis of OsASR5 transcript levels in different Arabidopsis transgenic lines.
Figure S4 Transcription levels of OsASR5 in OsASR5 overexpression rice transgenic lines.
Figure S5 Free proline and soluble sugar contents of OsASR5 overexpression and NT plants under 15% PEG6000 treatment.
Figure S6 Identification of osasr5 T‐DNA insertion mutant.
Figure S7 Subcellular localization of OsASR5‐GFP fusion protein.
Figure S8 OsASR5 transcriptional activation and homodimerization analysis.
Table S1 OsASR5 interacting proteins identified in yeast two‐hybrid screening.
Appendix S1 Supplementary methods.


