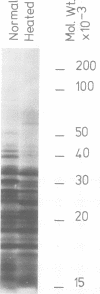
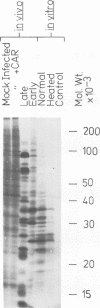
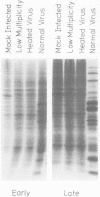
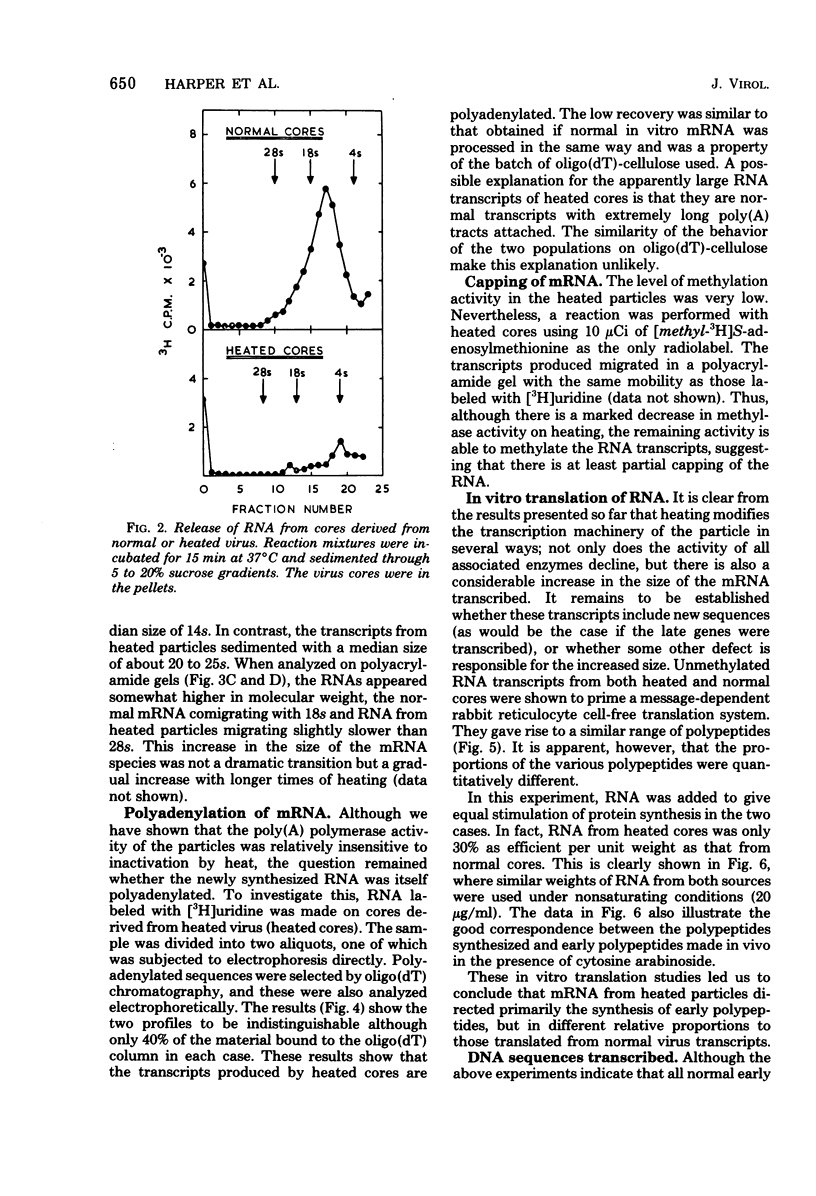
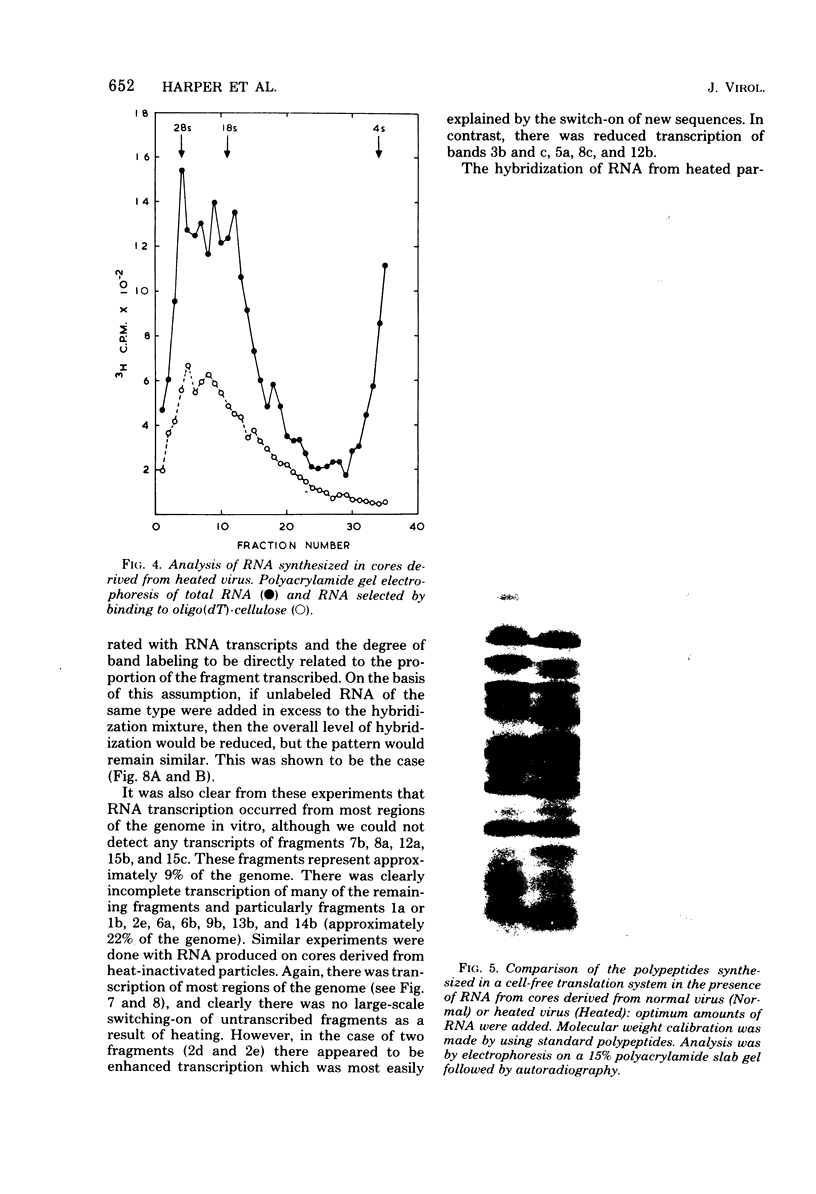
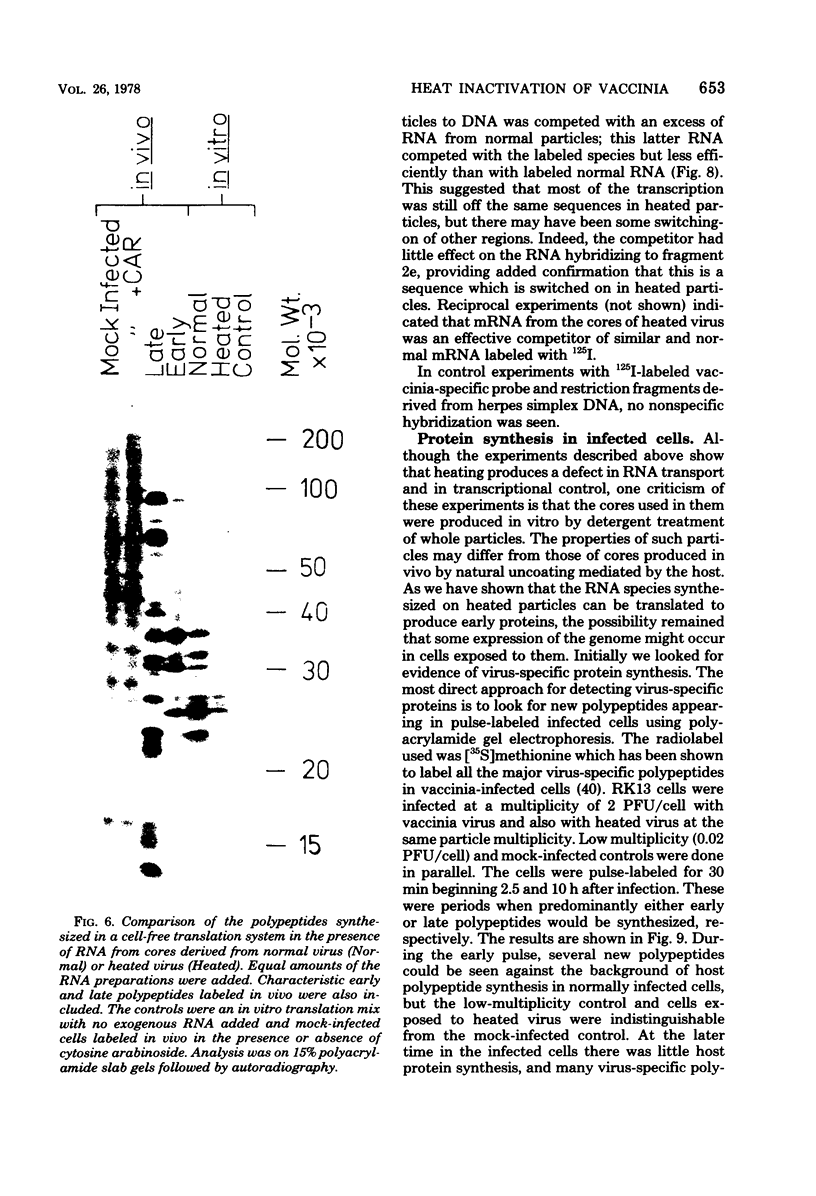
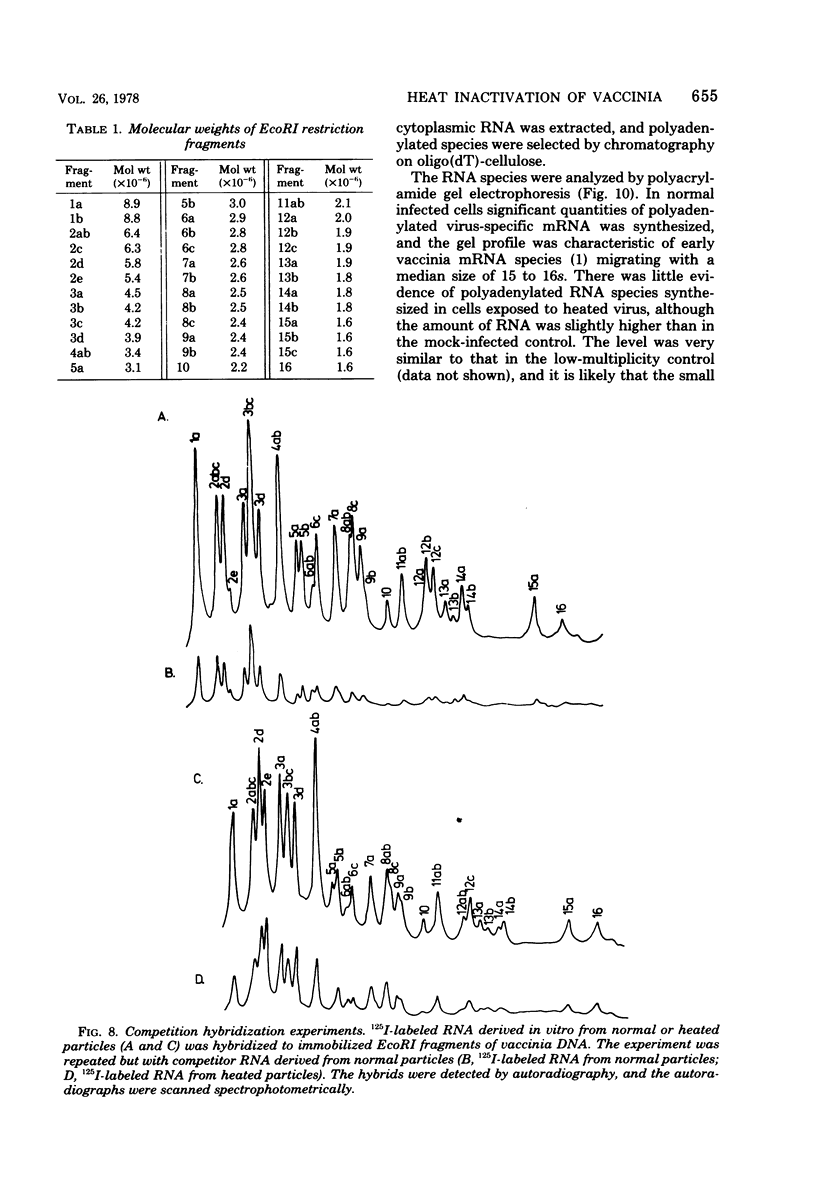
Abstract
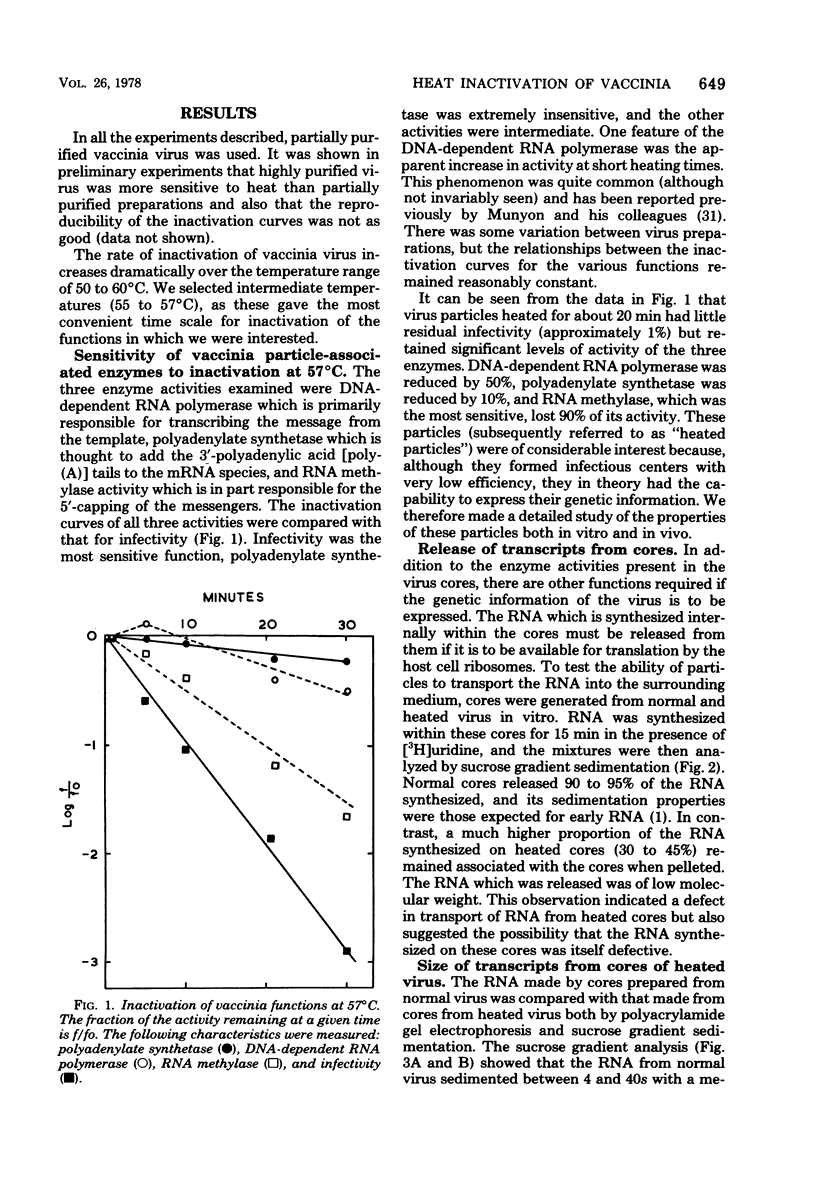
The heat inactivation characteristics of several vaccinia virus particle-associated functions known to be involved in the transcription of the genome were examined. All functions were more resistant to heat than infectivity. Noninfectious particles were generated which exhibited significant levels of activity of all enzymes examined, and their properties were investigaed both in vitro and in vivo. RNA was synthesized in vitro by such particles, although transport of the RNA into the surrounding medium was defective. This RNA was larger than that made in normal particles but it was polyadenylated and functioned in vitro as a message coding for normal early proteins. The sequences transcribed were similar to those transcribed in normal particles, and we suggest that the production of abnormally large RNA is due to a defect in transcriptional termination. We could not detect any virus-specific protein or RNA synthesis in cells exposed to these inactivated particles and conclude that the loss of infectivity caused by heating is due to a general decline in the activities of a number of particle functions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton K. T., Darby G. Patterns of transcription of messengers containing poly A in vaccinia virus-infected cells. J Gen Virol. 1974 Feb;22(2):215–224. doi: 10.1099/0022-1317-22-2-215. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. I. In vivo DNA synthesis. Virology. 1977 May 1;78(1):57–75. doi: 10.1016/0042-6822(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Fournier F., Tovell D. R., Esteban M., Metz D. H., Ball L. A., Kerr I. M. The translation of vaccinia virus messenger RNA in animal cell-free systems. FEBS Lett. 1973 Mar 15;30(3):268–272. doi: 10.1016/0014-5793(73)80667-2. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Paoletti E. Molecular complexity of vaccinia DNA and the presence of reiterated sequences in the genome. Virology. 1977 Jun 15;79(2):337–341. doi: 10.1016/0042-6822(77)90361-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR FATE OF RABBITPOX VIRUS RENDERED NONINFECTIOUS BY VARIOUS REAGENTS. Virology. 1964 Apr;22:620–633. doi: 10.1016/0042-6822(64)90084-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Jaureguiberry G., Ben-Hamida F., Chapeville F., Beaud G. Messenger activity of RNA transcribed in vitro by DNA-RNA polymerase associated to vaccinia virus cores. J Virol. 1975 Jun;15(6):1467–1474. doi: 10.1128/jvi.15.6.1467-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Munyon W., Mann J., Grace J. T., Jr Protection of vaccinia from heat inactivation by nucleotide triphosphates. J Virol. 1970 Jan;5(1):32–38. doi: 10.1128/jvi.5.1.32-38.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E. In vitro synthesis of a high molecular weight virion-associated RNA by vaccinia. J Biol Chem. 1977 Feb 10;252(3):866–871. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Use of coupled transcription and translation to study mRNA production by vaccinia cores. Nature. 1977 Oct 6;269(5628):532–534. doi: 10.1038/269532a0. [DOI] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Pogo B. G. Elimination of naturally occurring crosslinks in vaccinia virus DNA after viral penetration into cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1739–1742. doi: 10.1073/pnas.74.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL W. C. A sensitive and precise plaque assay for herpes virus. Nature. 1962 Sep 8;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- VANTSIS J. T., WILDY P. Interaction of herpes virus and HeLa cells: comparison of cell killing and infective center formation. Virology. 1962 Jun;17:225–232. doi: 10.1016/0042-6822(62)90112-5. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylation of newly synthesized viral messenger RNA by an enzyme in vaccinia virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3014–3018. doi: 10.1073/pnas.71.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]