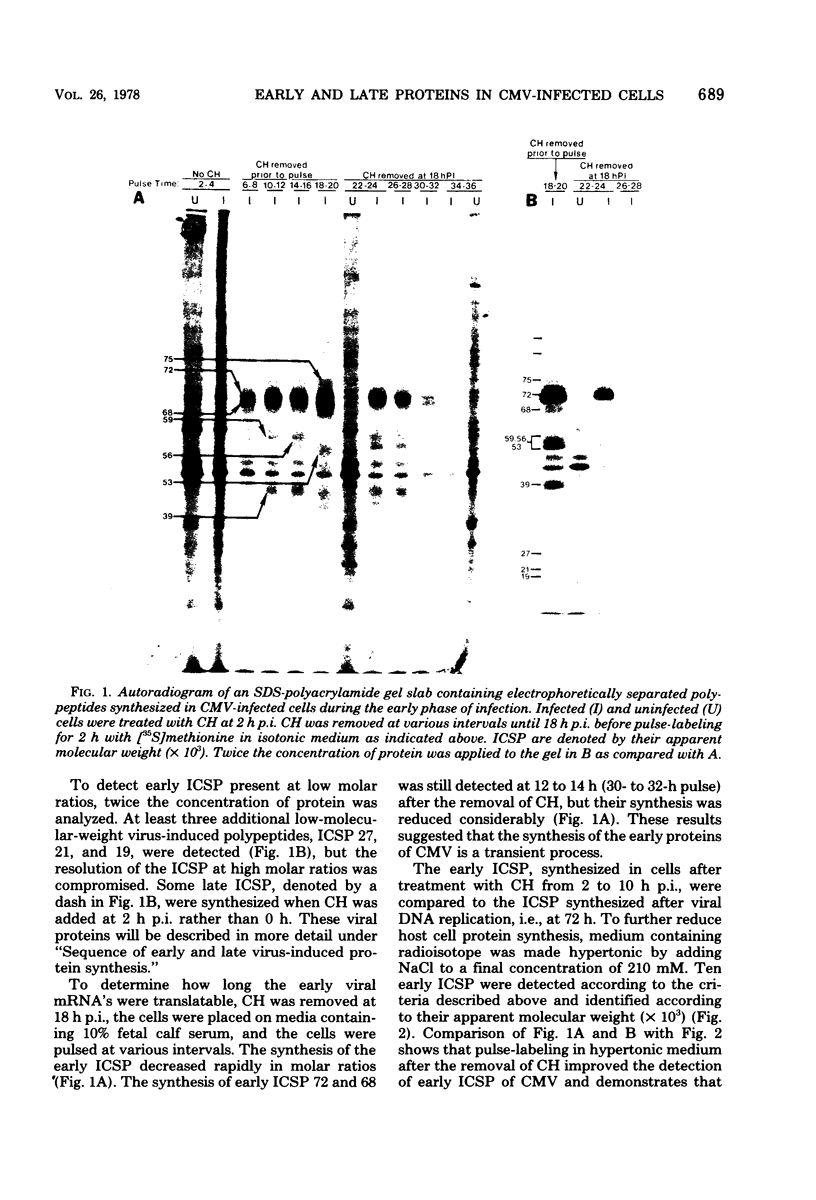
Abstract
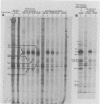
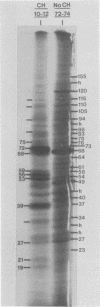
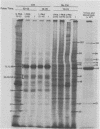
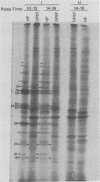
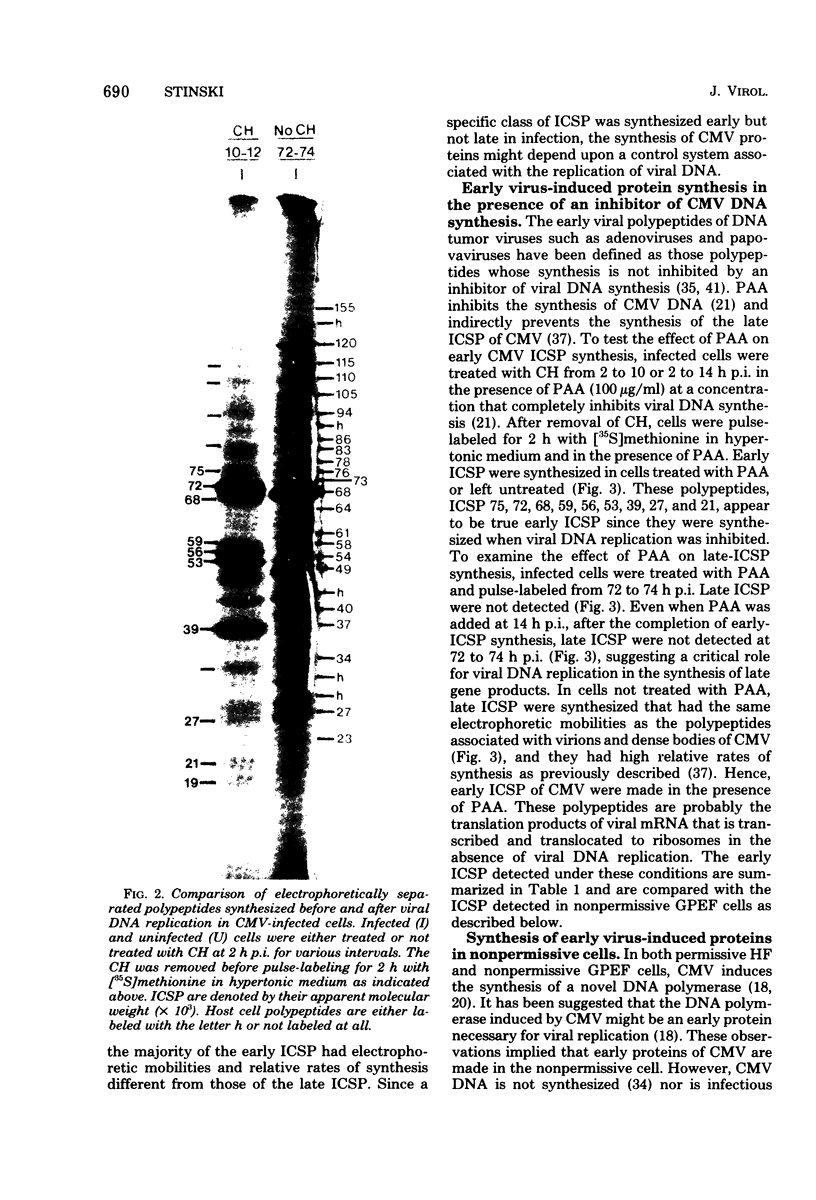
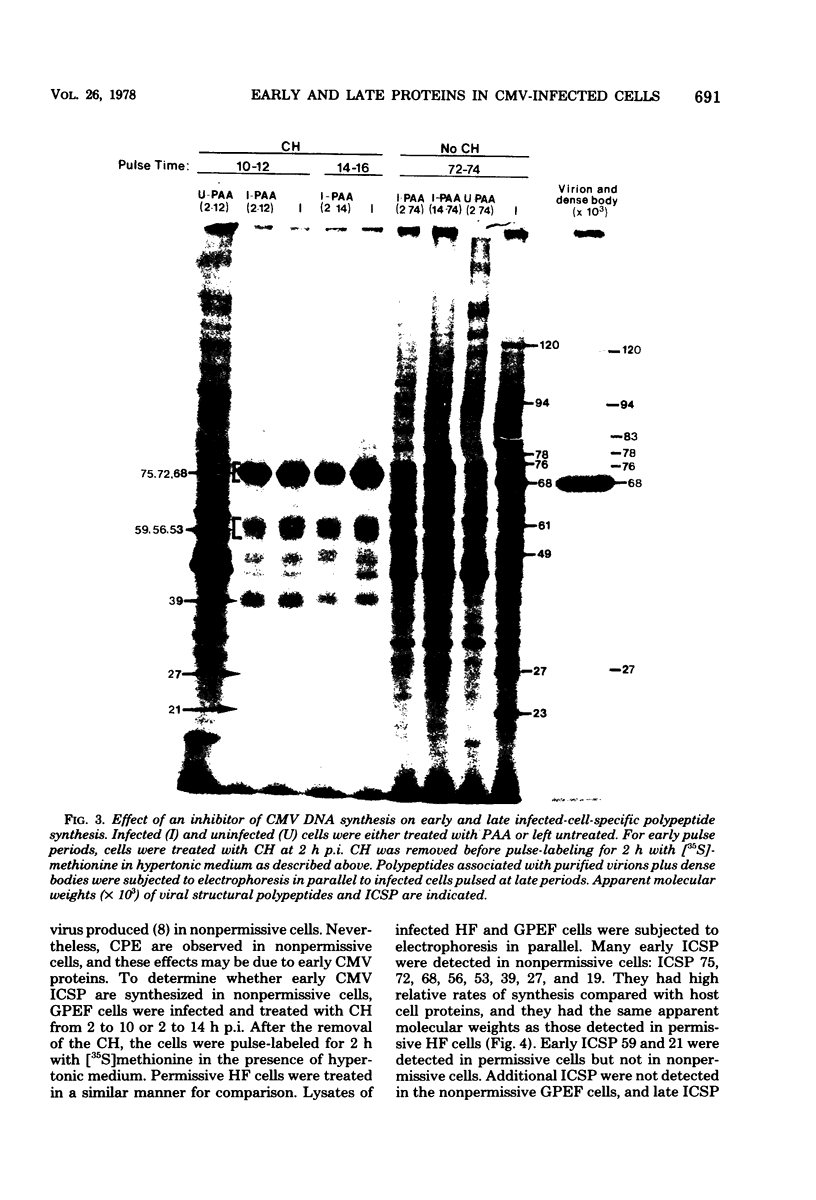
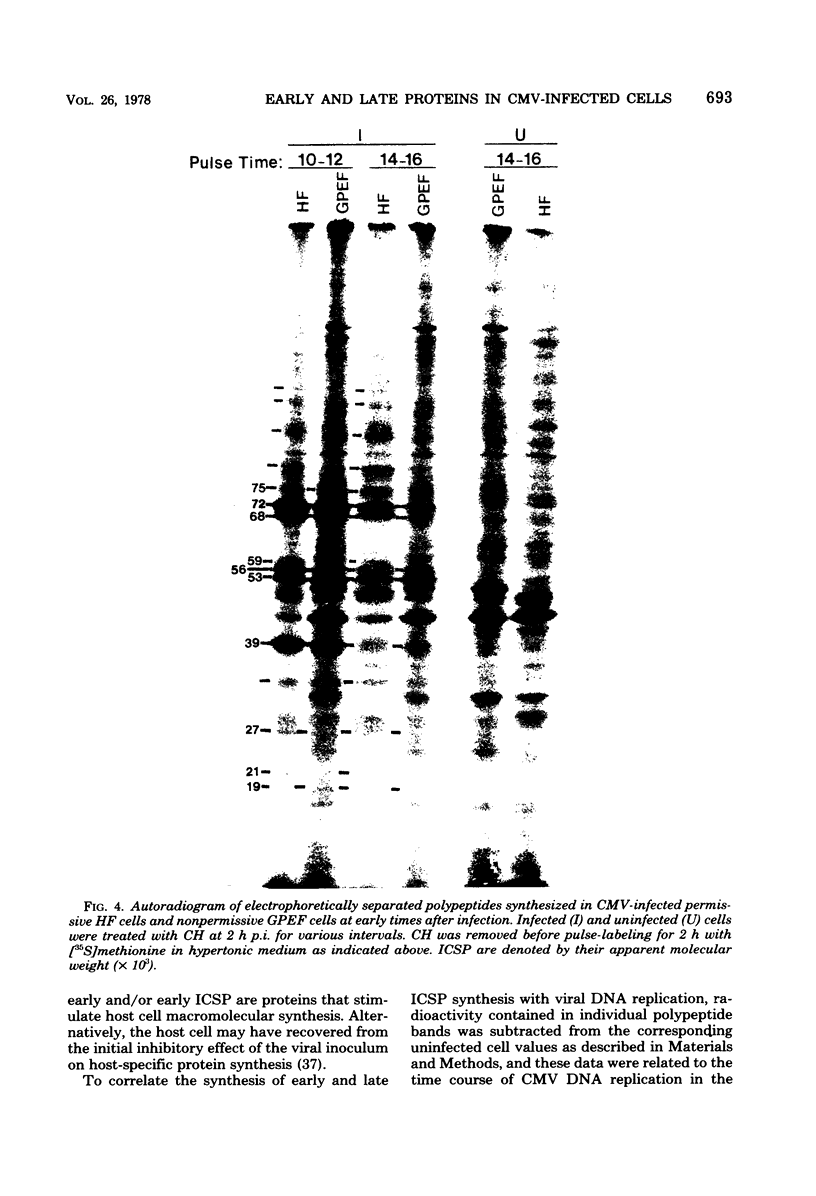
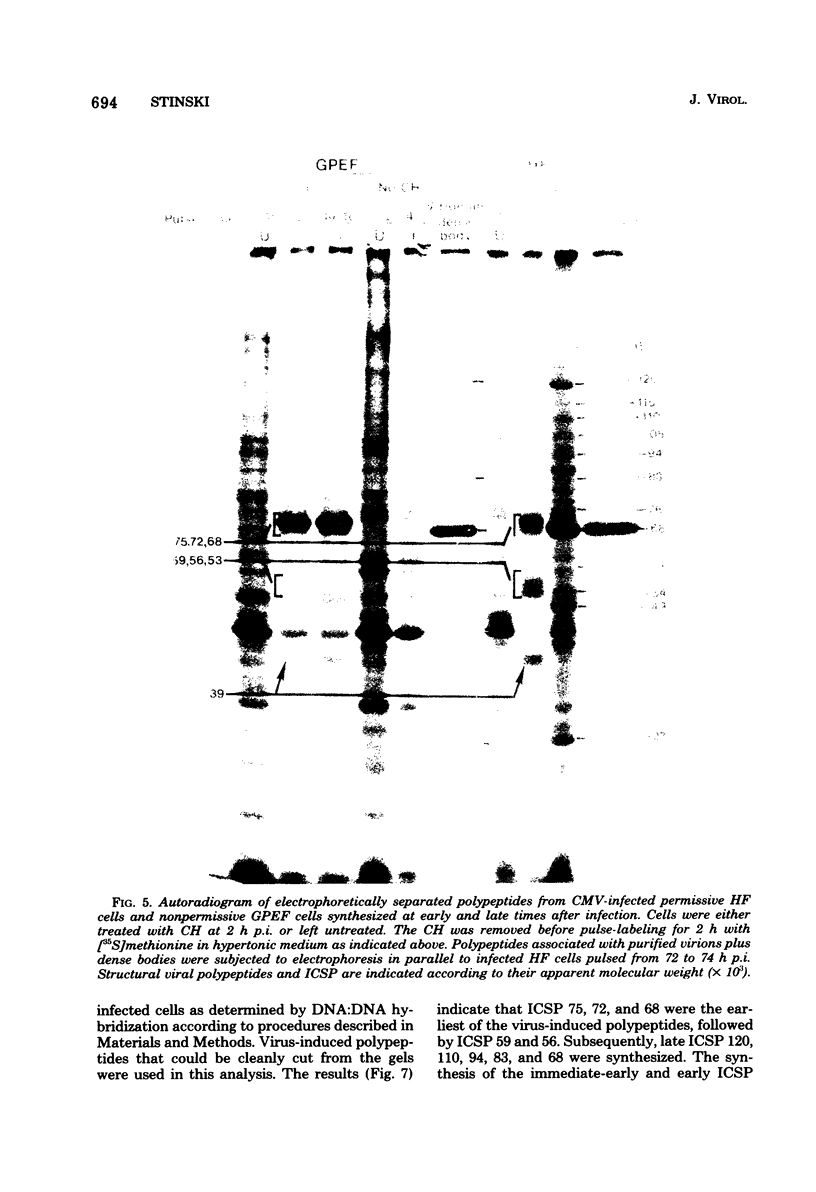
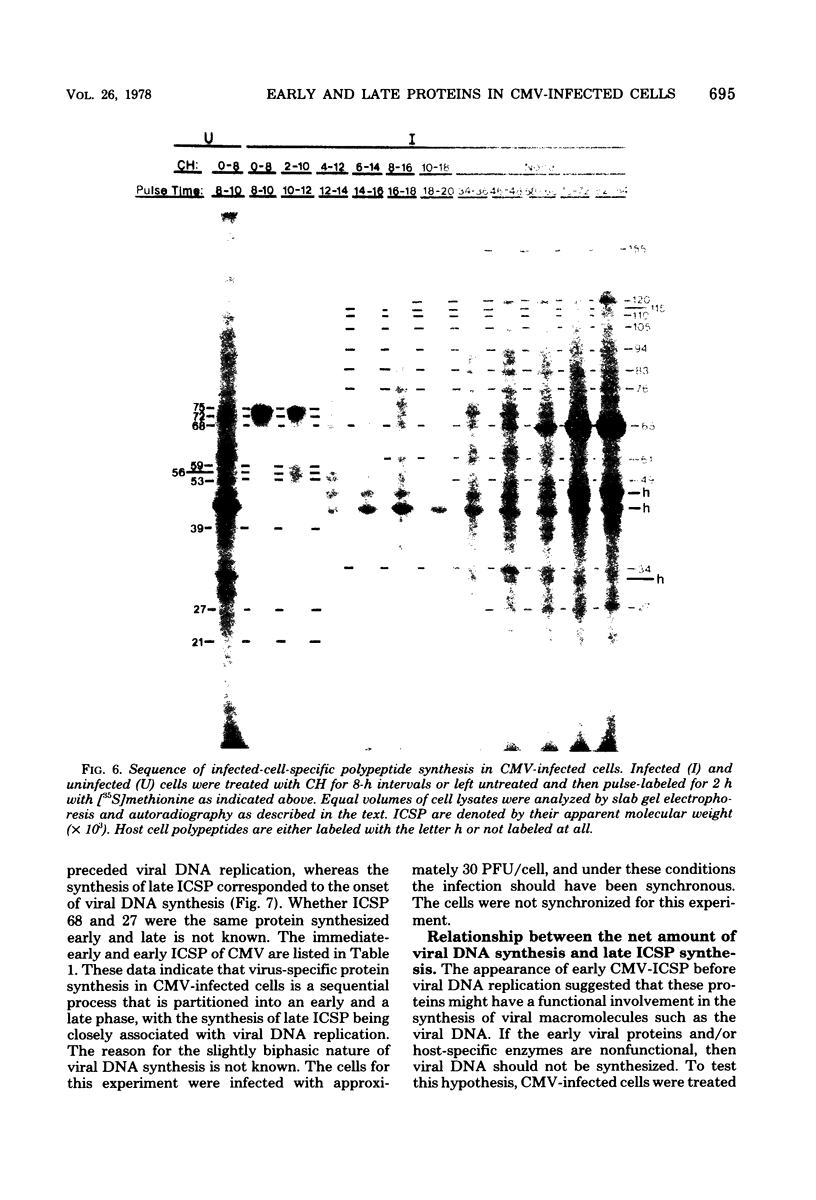
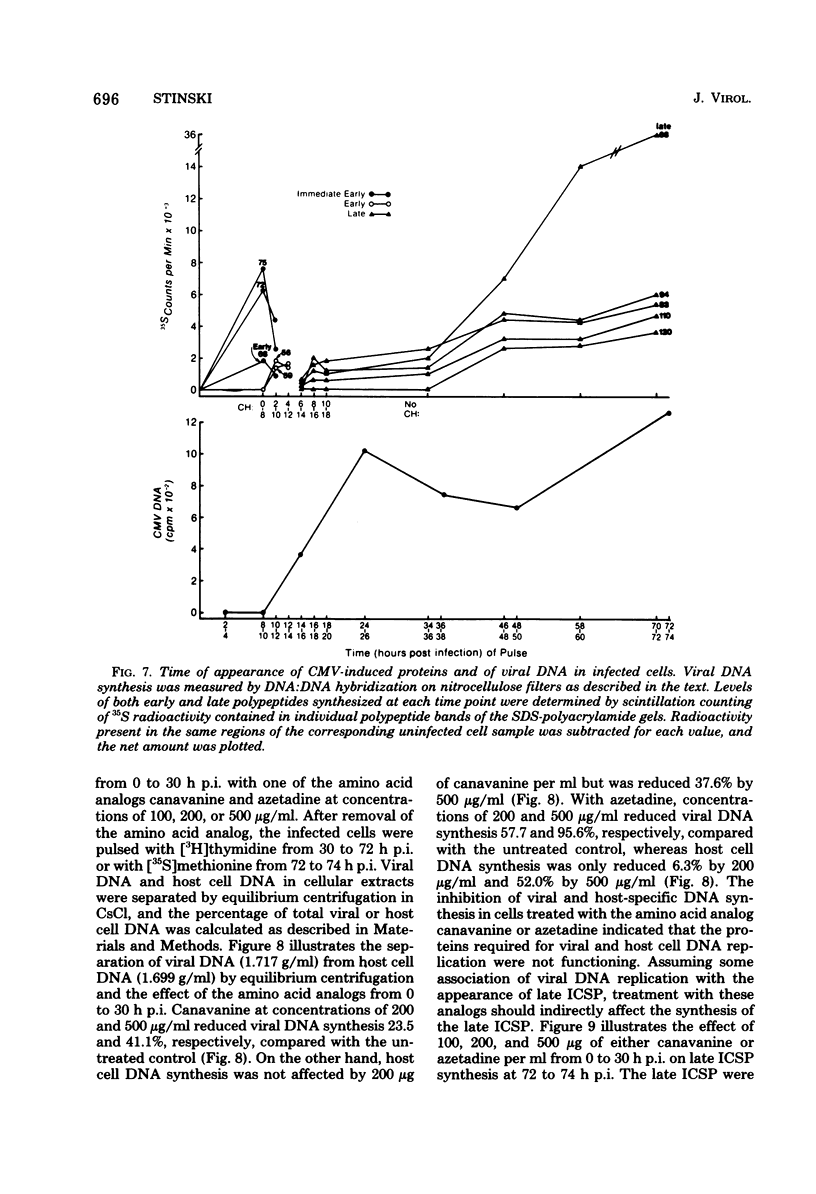
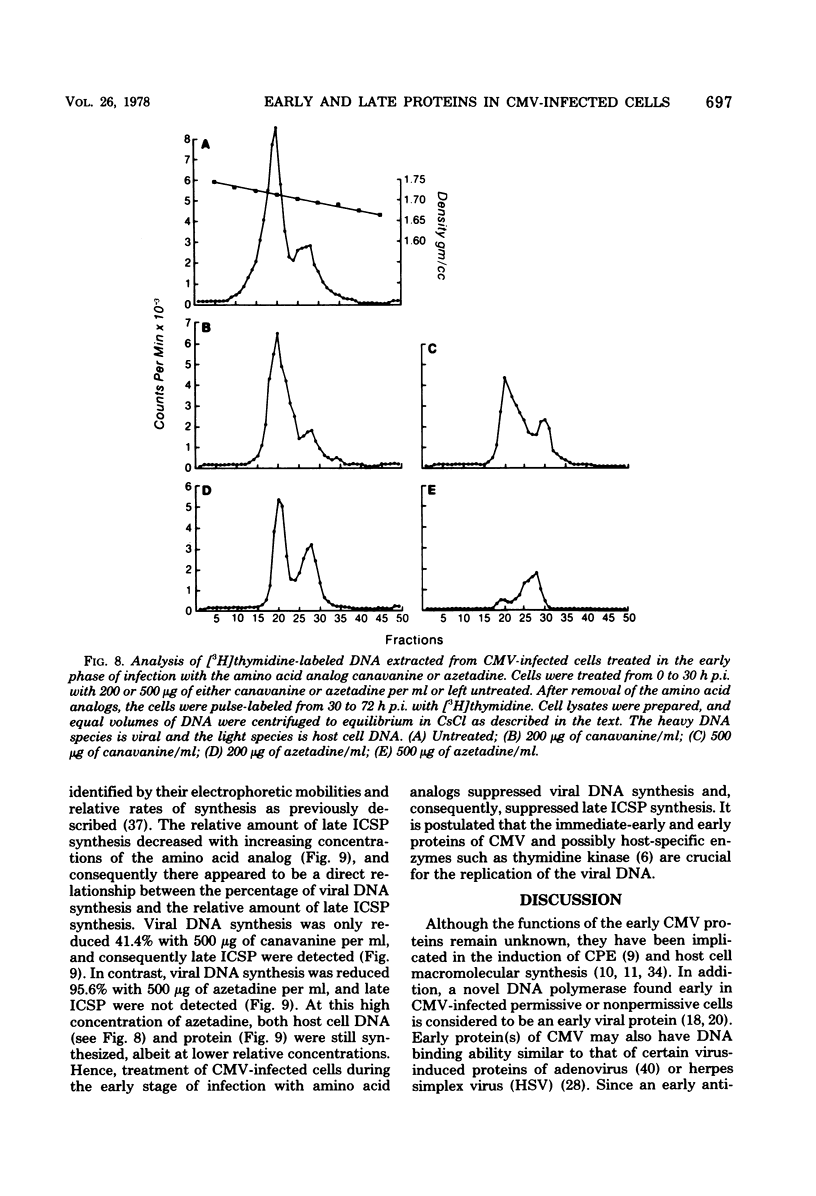
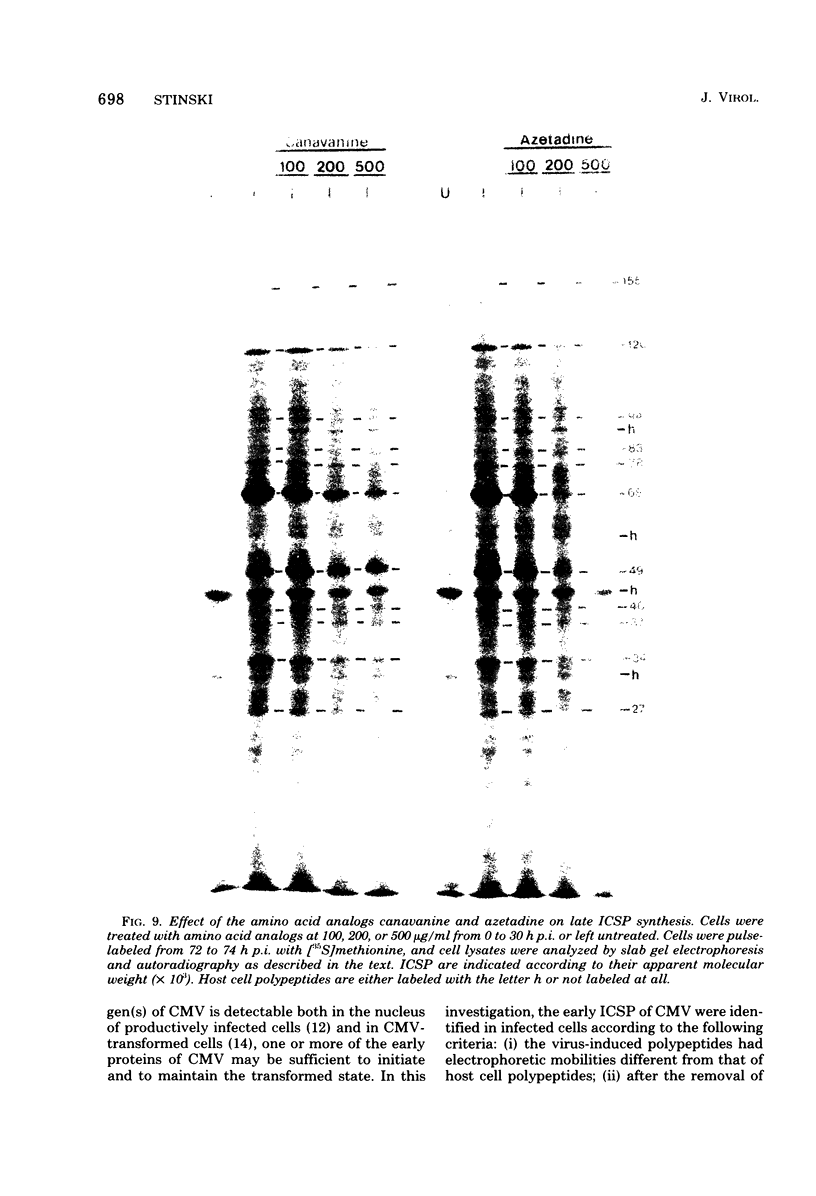
At least 10 distinct early virus-induced polypeptides were synthesized within 0 to 6 h after infection of permissive cells with cytomegalovirus. These virus-induced polypeptides were synthesized before and independently of viral DNA replication. A majority of these early virus-induced polypeptides were also synthesized in nonpermissive cells, which do not permit viral DNA replication. The virus-induced polypeptides synthesized before viral DNA replication were hypothesized to be nonstructural proteins coded for by the cytomegalovirus genome. Their synthesis was found to be a sequential process, since three proteins preceded the synthesis of the others. Synthesis of all early cytomegalovirus-induced proteins was a transient process; the proteins reached their highest molar ratios before the onset of viral DNA replication. Late viral proteins were synthesized at the time of the onset of viral DNA replication, which was approximately 15 h after infection. Their synthesis was continuous and increased in molar ratios with the accumulation of newly synthesized viral DNA in the cells. The presence of the amino acid analog canavanine or azetadine during the early stage of infection suppressed viral DNA replication. The amount of viral DNA synthesis was directly correlated to the relative amount of late viral protein synthesis. Because synthesis of late viral proteins depended upon viral DNA replication, the proteins were not detected in permissive cells treated with an inhibitor of viral DNA synthesis or in nonpermissive cells that are restrictive for cytomegalovirus DNA replication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Physiological state of human embryonic lung cells affects their response to human cytomegalovirus. J Virol. 1977 Jul;23(1):126–132. doi: 10.1128/jvi.23.1.126-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- England J. M., Howett M. K., Tan K. B. Effect of hypertonic conditions on protein synthesis in cells productively infected with simian virus 40. J Virol. 1975 Nov;16(5):1101–1107. doi: 10.1128/jvi.16.5.1101-1107.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fioretti A., Furukawa T., Santoli D., Plotkin S. A. Nonproductive infection of guinea pig cells with human cytomegalovirus. J Virol. 1973 Jun;11(6):998–1003. doi: 10.1128/jvi.11.6.998-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Tanaka S., Plotkin S. A. Stimulation of macromolecular synethesis in guinea pig cells by human CMV. Proc Soc Exp Biol Med. 1975 Jan;148(1):211–214. doi: 10.3181/00379727-148-38508. [DOI] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Geder L. Evidence for early nuclear antigens in cytomegalovirus-infected cells. J Gen Virol. 1976 Aug;32(2):315–319. doi: 10.1099/0022-1317-32-2-315. [DOI] [PubMed] [Google Scholar]
- Geder L., Rapp F. Evidence for nuclear antigens in cytomegalovirus-transformed human cells. Nature. 1977 Jan 13;265(5590):184–186. doi: 10.1038/265184a0. [DOI] [PubMed] [Google Scholar]
- Gubbins E. J., Newlon C. S., Kann M. D., Donelson J. E. Sequence organization and expression of a yeast plasmid DNA. Gene. 1977 May;1(3-4):185–207. doi: 10.1016/0378-1119(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Shanmugam G., Wold W. S., Green M. Detection of adenovirus type 2-induced early polypeptides using cycloheximide pretreatment to enhance viral protein synthesis. J Virol. 1976 Jul;19(1):232–242. doi: 10.1128/jvi.19.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Furukawa T., Plotkin S. A. Induction of DNA Polymerase in WI-38 and guinea pig cells infected with human cytomegalovirus (HCMV). Virology. 1976 Mar;70(1):251–255. doi: 10.1016/0042-6822(76)90266-x. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A., Levine A. J., Anderson S., Osborn M., Rosenwirth B., Weber K. The relationship between group C adenovirus tumor antigen and the adenovirus single-strand DNA-binding protein. Cell. 1976 Apr;7(4):575–584. doi: 10.1016/0092-8674(76)90208-7. [DOI] [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Rapp F., Geder L., Murasko D., Lausch R., Ladda R., Huang E. S., Webber M. M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975 Oct;16(4):982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S. C., Hutt R. Cell DNA replication as a function in the synthesis of human cytomegalovirus. J Gen Virol. 1977 Oct;37(1):65–73. doi: 10.1099/0022-1317-37-1-65. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Furukawa T., Plotkin S. A. Human cytomegalovirus stimulates host cell RNA synthesis. J Virol. 1975 Feb;15(2):297–304. doi: 10.1128/jvi.15.2.297-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Maizel J. V., Jr The polypeptides of adenovirus. IV. Detection of early and late virus-induced polypeptides and their distribution in subcellular fractions. Virology. 1974 Feb;57(2):402–408. doi: 10.1016/0042-6822(74)90180-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]