Abstract
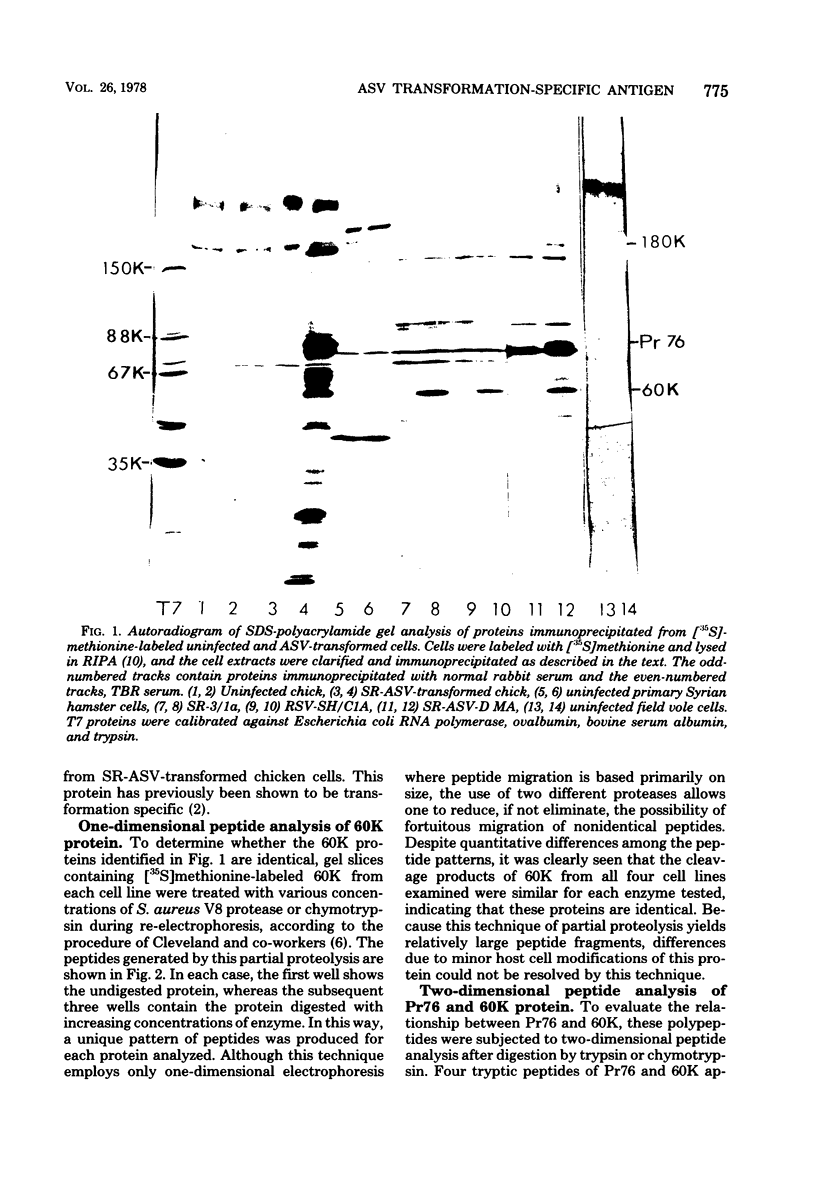
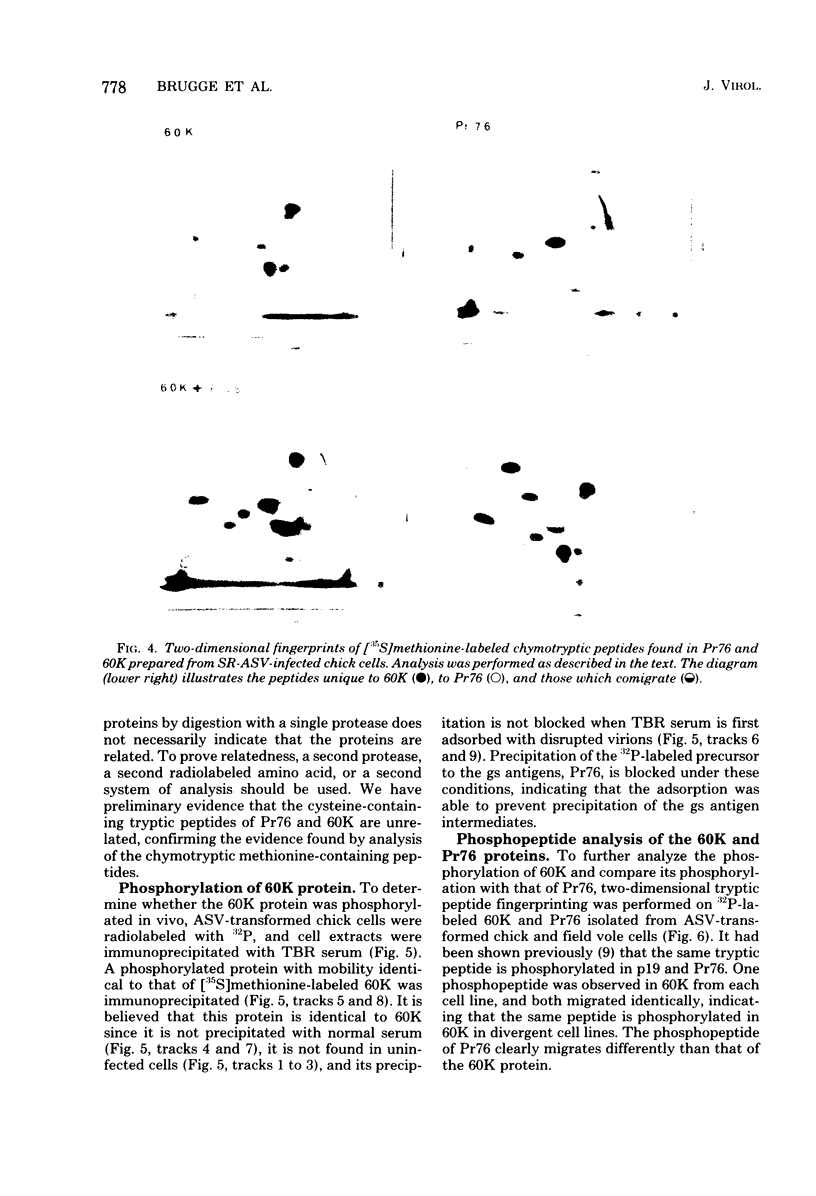
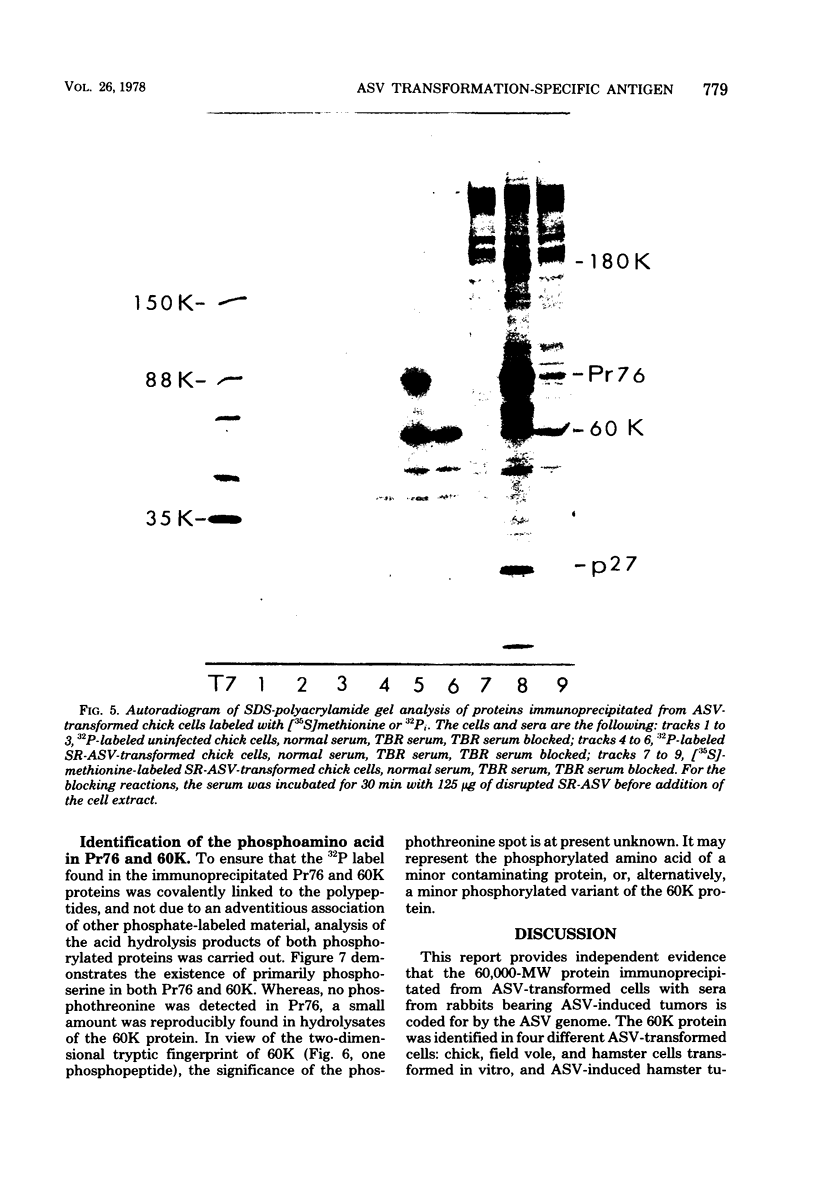
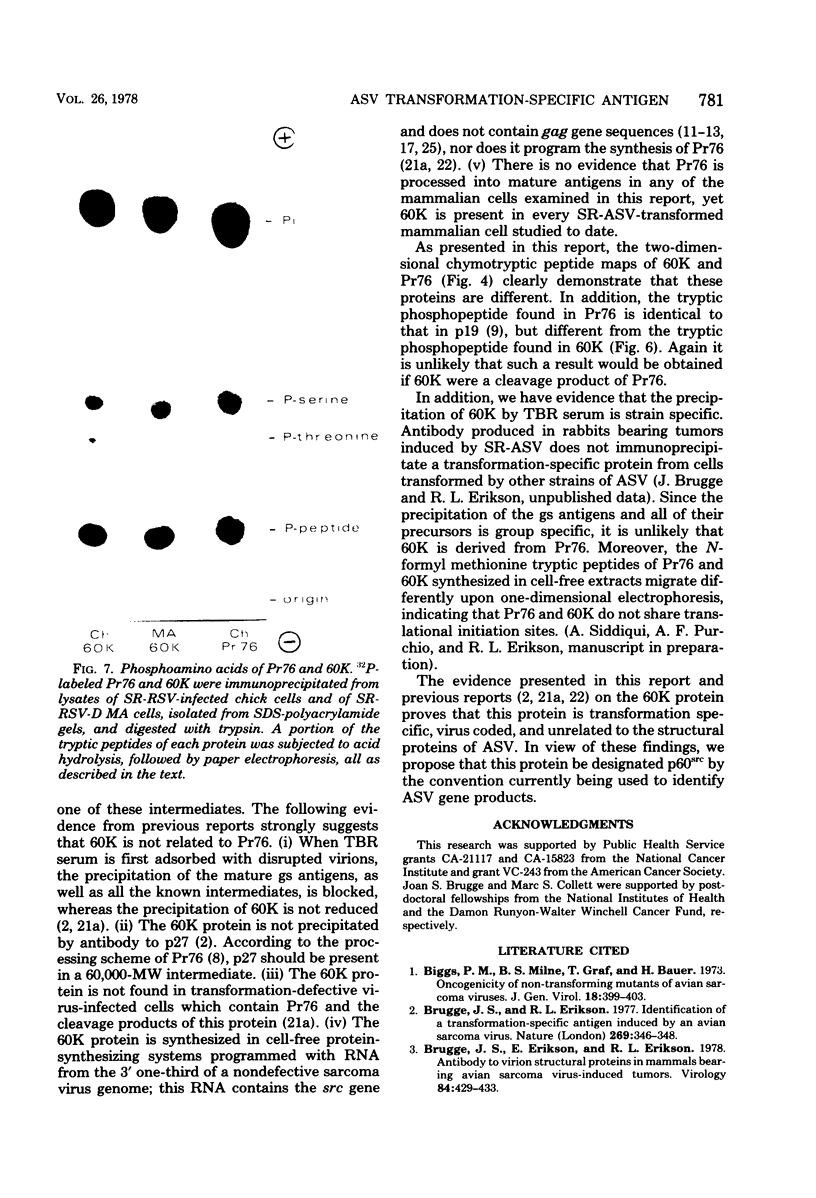
Sera from rabbits bearing tumors induced by avian sarcoma virus (ASV) were ussed to immunopecipitate virus-specific proteins from extracts of chicken, hamster, and field vole cells transformed by ASV. Two virus-specific proteins having molecular weights of 76,000 and 60,000 were found in all cell lines examined. The 76,000-molecular-weight protein, Pr76, is the precursor to the internal core proteins of ASV. The 60,000-molecular-weight (60K) transformation-specific antigen from each cell line was subjected to peptide analysis, using chymotrypsin and Staphylococcus aureus V8 protease. The resulting peptide maps of the 60K protein from the different ASV-infected cell types were similar for each enzyme, strongly suggesting that the 60K protein is virus coded. Two-dimensional analysis of chymotryptic peptides from Pr76 and 60K reveals that 60K is not related to the gs antigen precursor. Radiolabeling of ASV-transformed cells with inorganic phosphate revealed that 60K is phosphorylated in vivo. The 60K proteins isolated from both ASV-transformed chicken and field vole cells were found to contain one tryptic phosphopeptide. The tryptic phosphopeptides of 60K from both cell lines migrated identically upon two-dimensional peptide analyses, and their migration differed from that of the principal phosphopeptide of Pr76.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggs P. M., Milne B. S., Graf T., Bauer H. Oncogenicity of non-transforming mutants of avian sarcoma viruses. J Gen Virol. 1973 Mar;18(3):399–403. doi: 10.1099/0022-1317-18-3-399. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. Antibody to virion structural proteins in mammals bearing avian sarcoma virus-induced tumors. Virology. 1978 Feb;84(2):429–433. doi: 10.1016/0042-6822(78)90259-3. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Macpherson I. Reversion in Hamster Cells Transformed by Rous Sarcoma Virus. Science. 1965 Jun 25;148(3678):1731–1733. doi: 10.1126/science.148.3678.1731. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]










