Abstract
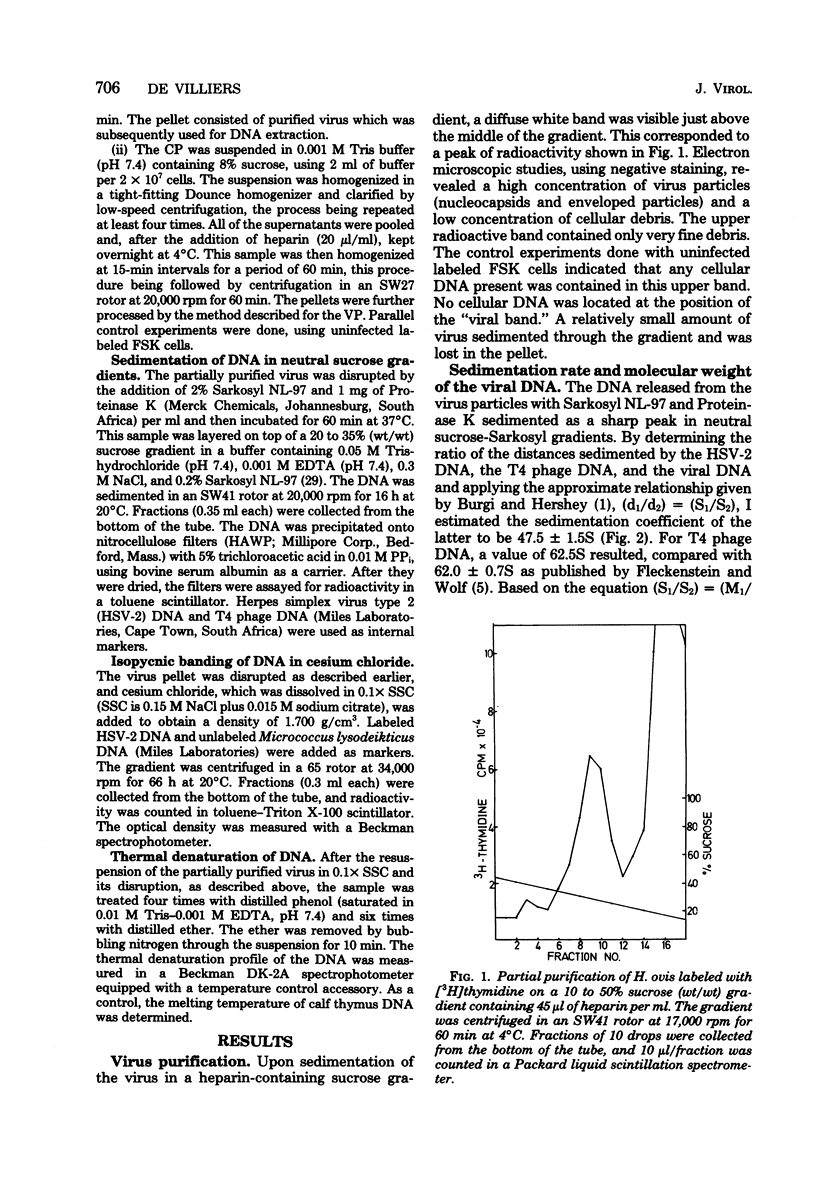
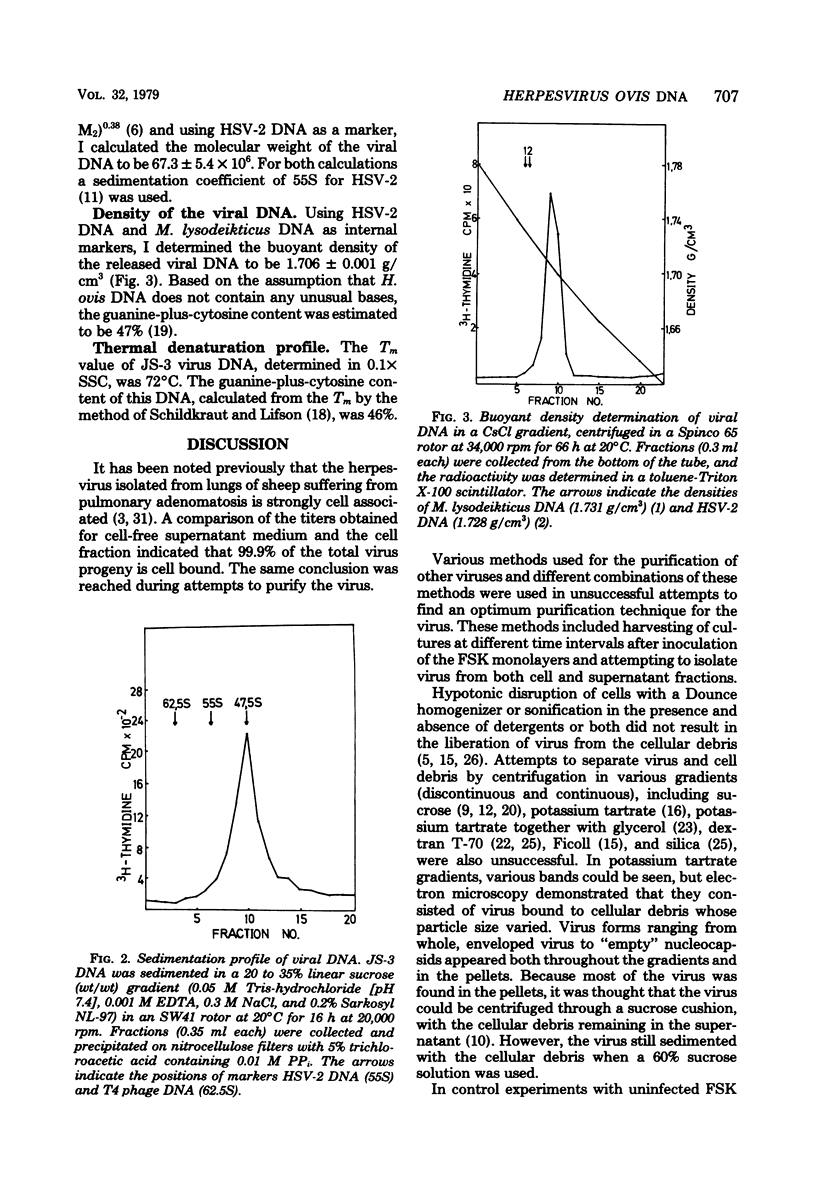
A procedure incorporating the use of heparin was developed to purify Herpesvirus ovis. The viral DNA has a buoyant density of 1.706 +/- 0.001 g/cm3, and the sedimentation constant was estimated to be 47.5 +/- 1.5S; from the latter, the molecular weight was calculated as 67.3 +/- 5.4 X 10(6). Estimates of the guanine-plus-cytosine content made from the buoyant density and melting point (72 degrees C) gave levels of 47 and 46%, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Haines H. G., Biswal N., Benyesh-Melnick M. The characterization of Varicella-zoster virus DNA. J Gen Virol. 1972 Jan;14(1):111–114. doi: 10.1099/0022-1317-14-1-111. [DOI] [PubMed] [Google Scholar]
- Mackay J. M. Tissue culture studies of sheep pulmonary adenomatosis (jaagsiekte). II. Transmission of cytopathic effects to normal cultures. J Comp Pathol. 1969 Jan;79(1):147–154. doi: 10.1016/0021-9975(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Krauss H. H., Moulton J. E., Wandera J. G. Morphologic study of virus-infected lung cell cultures from sheep pulmonary adenomatosis (Jaagsiekte). Lab Invest. 1972 May;26(5):528–533. [PubMed] [Google Scholar]
- Matis J., Lesso J., Mucha V., Matisová E. Purification and separation of enveloped and unenveloped herpes simplex virus particles. Acta Virol. 1975 Jul;19(4):273–280. [PubMed] [Google Scholar]
- Miller G. The oncogenicity of Epstein-Barr virus. J Infect Dis. 1974 Aug;130(2):187–205. doi: 10.1093/infdis/130.2.187. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Hyman R. W., Rapp F. Isolation of herpes simplex virus DNA from the "hirt supernatant". Virology. 1976 Dec;75(2):481–483. doi: 10.1016/0042-6822(76)90046-5. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Smith W., Mackay J. M. Morphological observations on a virus associated with sheep pulmonary adenomatosis (jaagsiekte). J Comp Pathol. 1969 Oct;79(4):421–424. doi: 10.1016/0021-9975(69)90061-9. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., Almeida J. D. Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol. 1977 Aug;36(2):345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- VAHERI A., CANTELL K. THE EFFECT OF HEPARIN ON HERPES SIMPLEX VIRUS. Virology. 1963 Dec;21:661–662. doi: 10.1016/0042-6822(63)90242-3. [DOI] [PubMed] [Google Scholar]
- Vahlne A. G., Blomberg J. Purification of herpes simplex virus. J Gen Virol. 1974 Feb;22(2):297–302. doi: 10.1099/0022-1317-22-2-297. [DOI] [PubMed] [Google Scholar]
- Verwoerd D. W., Els H. J., De Villiers E. M., Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972 Oct;10(4):783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D. W., Meyer-Scharrer E., Broekman J., De Villiers E. The serological relationship of herpesvirus ovis to other herpesviruses and its possible involvement in the aetiology of jaagsiekte. Onderstepoort J Vet Res. 1979 Mar;46(1):61–63. [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M., Els H. J., Verwoerd D. W. Characteristics of an ovine herpesvirus associated with pulmonary adenomatosis (jaagsiekte) in sheep. S Afr J Med Sci. 1975;40(4):165–175. [PubMed] [Google Scholar]



