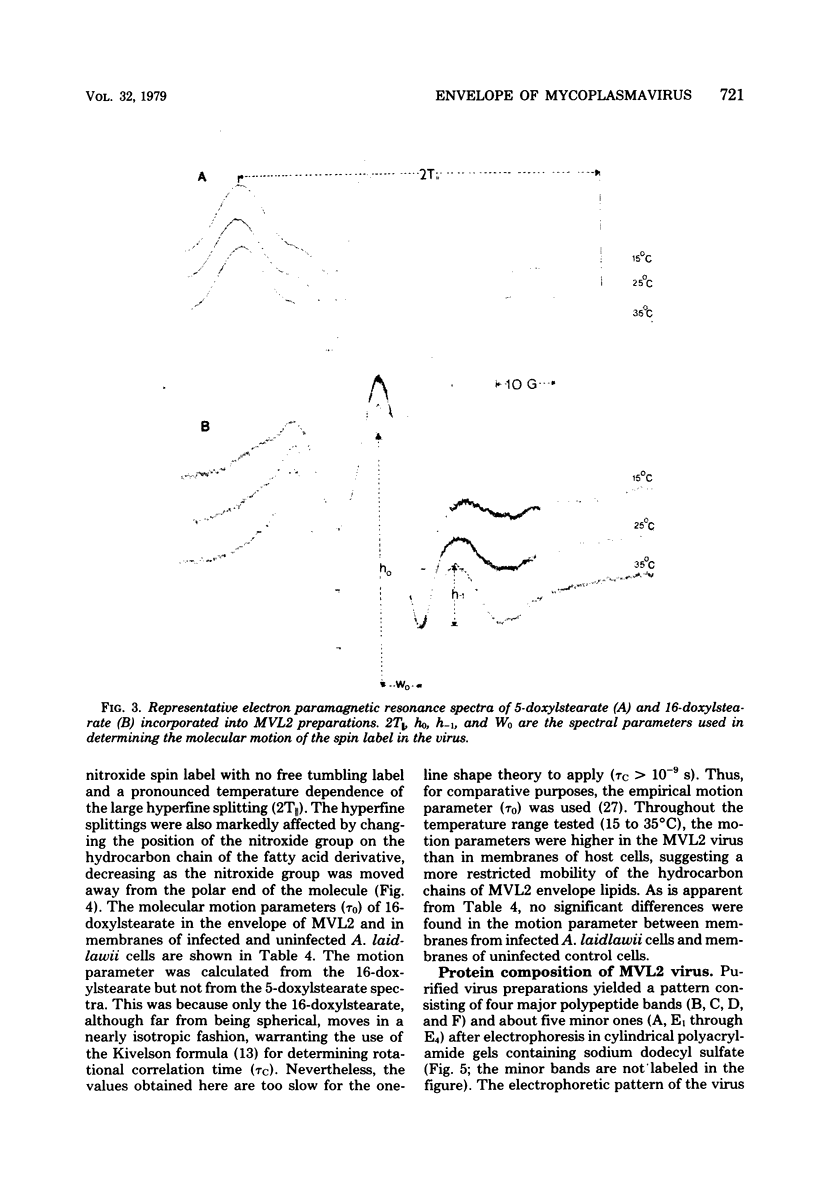
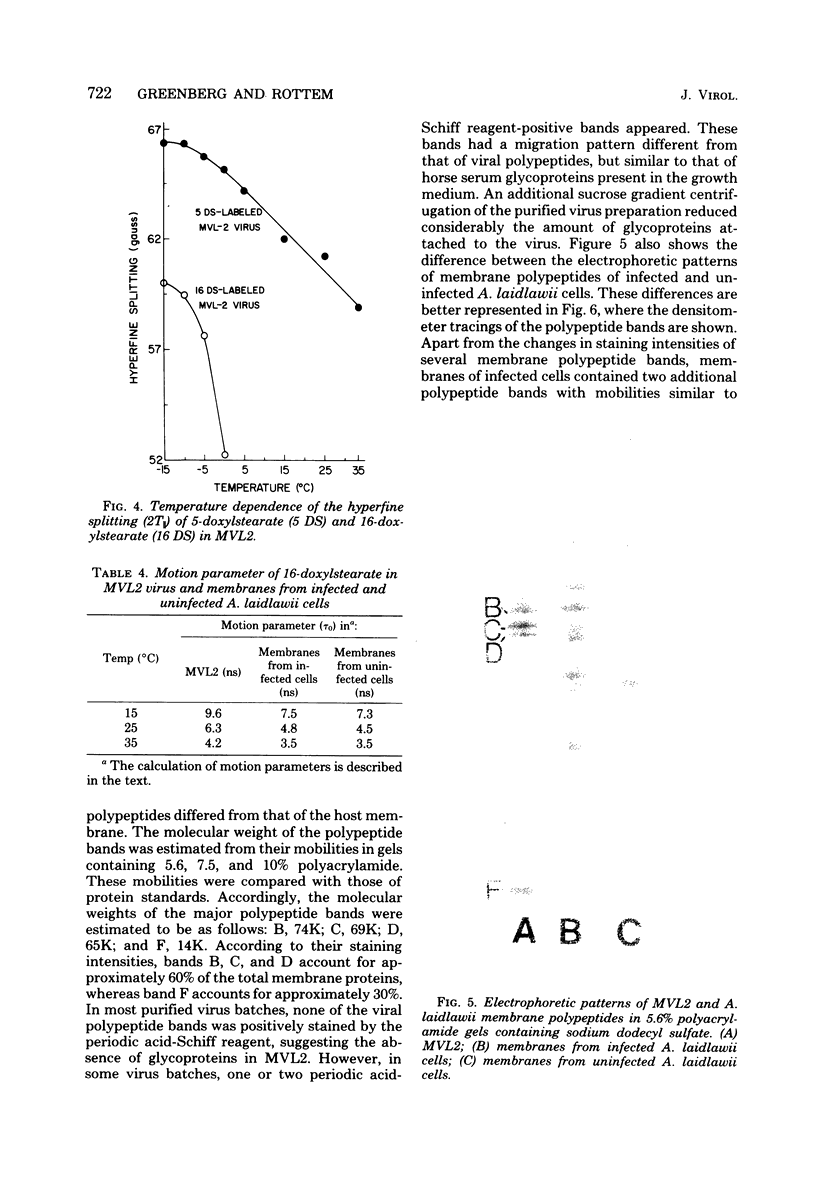
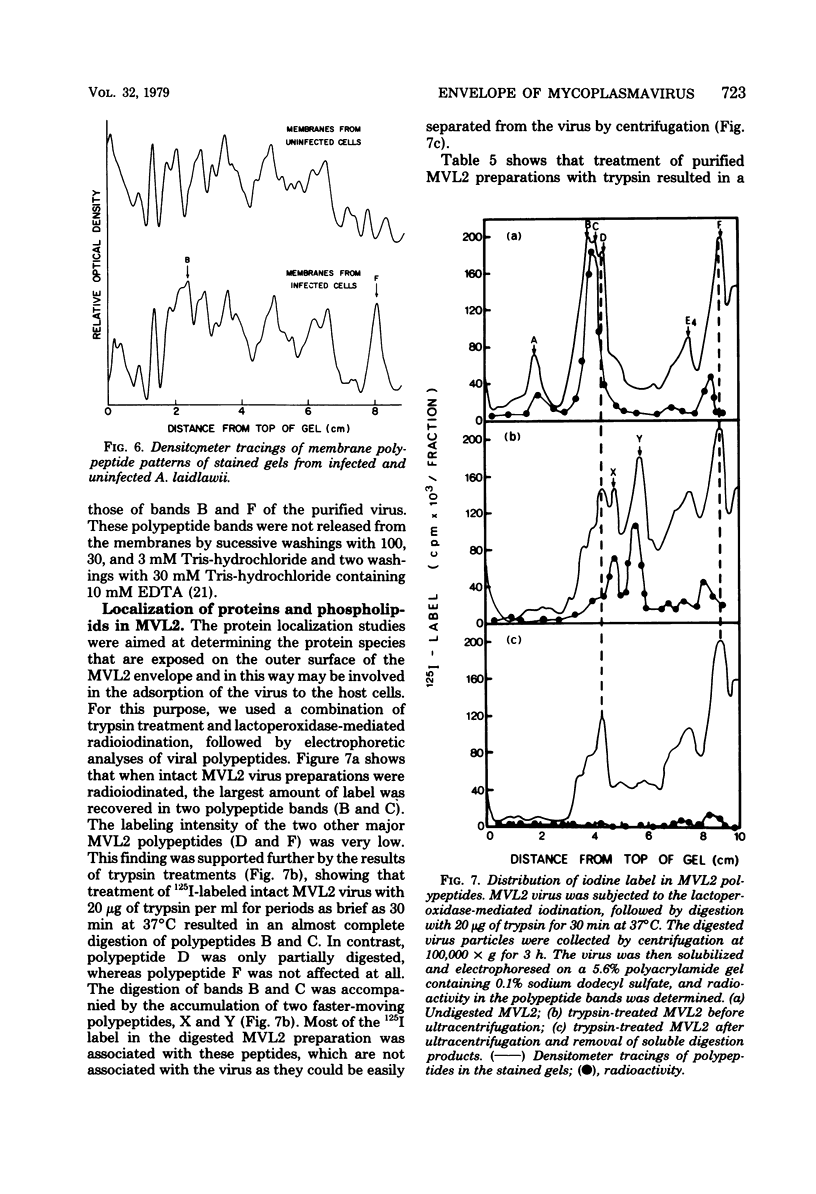
Abstract
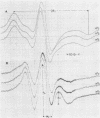
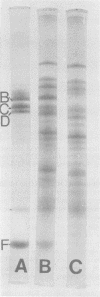
MVL2 virus was purified from culture supernatants of infected Acholeplasma laidlawii cells by differential centrifugation, followed by velocity centrifugation in sucrose gradients. The purified virus contained 0.08 to 0.1 mumol of lipid phosphorous per ml of viral protein. Thin-layer chromatography of viral lipids revealed the presence of phospho-, glyco-, and phosphoglycolipids identical with those found in the host cell membrane, but the relative amount of phosphatidylglycerol was much lower than that in the virus. The fatty acid composition of lipids incorporated into the virus included lipids synthesized before and after infection. The freedom of motion of spin-labeled fatty acids in MVL2 depended markedly on temperature and on the position of the nitroxide group on the hydrocarbon chain of the probe, suggesting that the local environment of the probe has the properties of a lipid bilayer. Nevertheless, the lipid hydrocarbon chains in MVL2 appear to be less mobile than those in membranes of the host cells. Polyacrylamide gel electrophoresis of purified MVL2 revealed four major and about five minor polypeptide bands. None of the polypeptide bands gave a positive periodic acid-Schiff reaction. Lactoperoxidase-mediated iodination, followed by proteolytic digestion of intact MVL2 particles, revealed that at least two major polypeptides are localized on the external surface of the viral envelope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar A., Rottem S., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. VI. Composition and disposition of proteins in membranes from aging Mycoplasma hominis cultures. Biochim Biophys Acta. 1976 Mar 5;426(2):258–270. doi: 10.1016/0005-2736(76)90336-9. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Singal S. A., Op den Kamp J. A., van Deenen L. L. Recognition of different pools of phosphatidylglycerol in intact cells and isolated membranes of Acholeplasma laidlawii by phospholipase A2. Biochemistry. 1977 Apr 5;16(7):1290–1295. doi: 10.1021/bi00626a008. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Snoek G. T., Op Den Kamp J. A., Van Deenen L. L. Phospholipid requirement of the membrane-bound Mg2+-dependent adenosinetriphosphatase in Acholeplasma laidlawaii. Biochim Biophys Acta. 1977 Jun 16;467(3):346–356. doi: 10.1016/0005-2736(77)90312-1. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XII. The fatty acids and lipid content of bacteriophage PM2. Virology. 1972 Aug;49(2):385–393. doi: 10.1016/0042-6822(72)90491-6. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N., Garwes D. J., Bruce J., Wyld S. G. Further studies on the morphology and composition of Mycoplasmatales virus-laidlawii 2. J Gen Virol. 1973 Feb;18(2):127–133. doi: 10.1099/0022-1317-18-2-127. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Isolation of a virus infecting a strain of Mycoplasma laidlawii. Nature. 1970 Mar 21;225(5238):1165–1165. doi: 10.1038/2251165a0. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasmatales virus-laidlawii 2, a new virus isolated from Acholeplasma laidlawii. J Gen Virol. 1971 Jul;12(1):65–67. doi: 10.1099/0022-1317-12-1-65. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Membrane properties of saturated fatty acid mutants of yeast revealed by spin labels. Chem Phys Lipids. 1971 Dec;7(4):245–265. doi: 10.1016/0009-3084(71)90004-1. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. doi: 10.1146/annurev.bb.07.060178.001035. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Lombardi P. S., Cole B. C. Induction of a pH-stable interferon in sheep lymphocytes by Mycoplasmatales virus MVL2. Infect Immun. 1978 Apr;20(1):209–214. doi: 10.1128/iai.20.1.209-214.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Das J., Christensen J. R. Viruses of mycoplasmas and spiroplasmas. Adv Virus Res. 1977;21:343–380. doi: 10.1016/s0065-3527(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Putzrath R. M., Maniloff J. Growth of an enveloped mycoplasmavirus and establishment of a carrier state. J Virol. 1977 May;22(2):308–314. doi: 10.1128/jvi.22.2.308-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzrath R. M., Maniloff J. Properties of a persistent viral infection: possible lysogeny by an enveloped nonlytic mycoplasmavirus. J Virol. 1978 Oct;28(1):254–261. doi: 10.1128/jvi.28.1.254-261.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Rottem S., Markowitz O., Razin S. Thermal regulation of the fatty acid composition of lipopolysaccharides and phospholipids of Proteus mirabilis. Eur J Biochem. 1978 Apr 17;85(2):445–450. doi: 10.1111/j.1432-1033.1978.tb12258.x. [DOI] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Gaffney B. J. Effect of the viral proteins on the fluidity of the membrane lipids in Sindbis virus. J Mol Biol. 1974 Dec 5;90(2):343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- Shaw N. The detection of lipids on thin-layer chromatograms with the periodate-Schiff reagents. Biochim Biophys Acta. 1968 Oct 22;164(2):435–436. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golde L. M., McElhaney R. N., van Deenen L. L. A membrane-bound lysophospholipase from Mycoplasma laidlawii strain B. Biochim Biophys Acta. 1971 Feb 2;231(1):245–249. doi: 10.1016/0005-2760(71)90275-x. [DOI] [PubMed] [Google Scholar]