Abstract
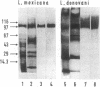
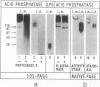
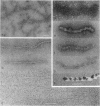
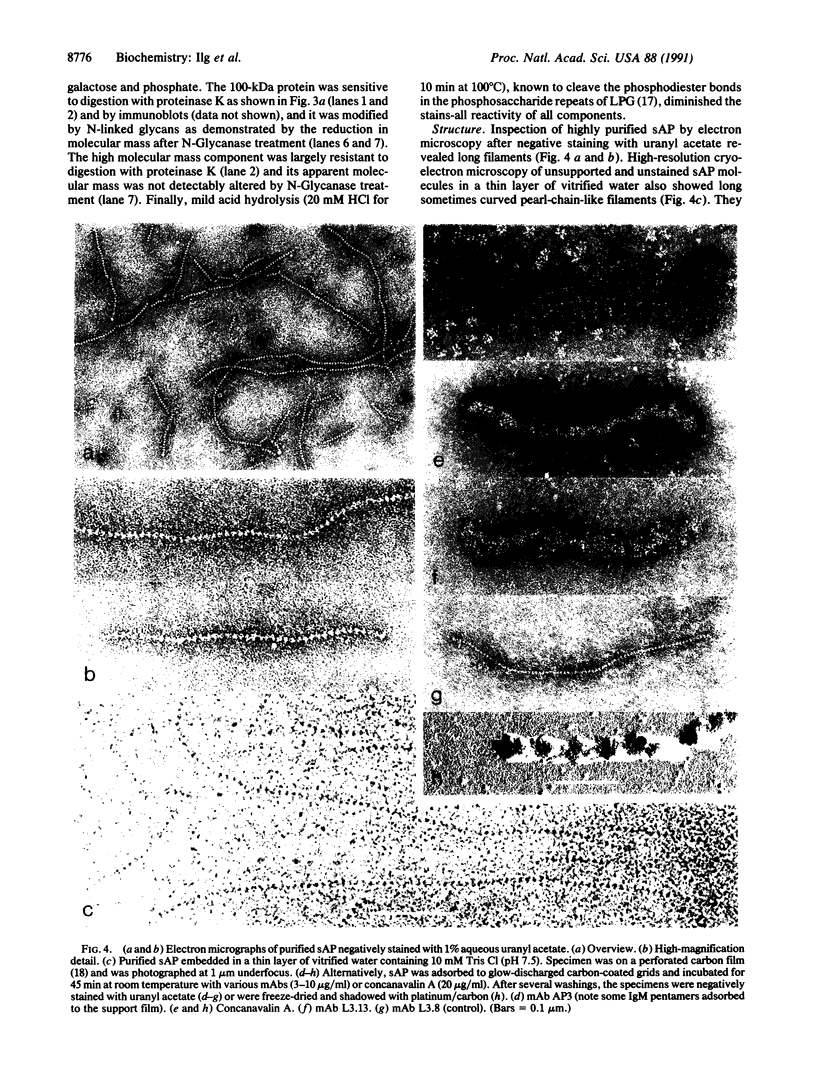
In the promastigote, or insect stage, most species of the parasitic protozoan Leishmania secrete an acid phosphatase. The enzyme purified from the culture medium of Leishmania mexicana is shown to be a complex [13.3% (wt/wt) protein, 74.4% (wt/wt) carbohydrate, and 12.3% (wt/wt) phosphate] composed of a predominant phosphorylated glycoprotein with a relative molecular mass of 100 kDa and noncovalently associated high molecular mass (proteo)phosphoglycans. Electron microscopy discloses long filaments composed of a central chain of protein subunits surrounded by a diffuse glycocalix that can be decorated by monoclonal antibodies or concanavalin A. In contrast to the polymeric structure of the L. mexicana enzyme, the acid phosphatase secreted by Leishmania donovani is mono- or oligomeric but not filamentous.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bates P. A., Dwyer D. M. Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Dec;26(3):289–296. doi: 10.1016/0166-6851(87)90081-8. [DOI] [PubMed] [Google Scholar]
- Bates P. A., Gottlieb M., Dwyer D. M. Leishmania donovani: identification of glycoproteins released by promastigotes during growth in vitro. Exp Parasitol. 1988 Dec;67(2):199–209. doi: 10.1016/0014-4894(88)90067-7. [DOI] [PubMed] [Google Scholar]
- Bates P. A., Hermes I., Dwyer D. M. Golgi-mediated post-translational processing of secretory acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1990 Mar;39(2):247–255. doi: 10.1016/0166-6851(90)90063-r. [DOI] [PubMed] [Google Scholar]
- Dibenedetto G., Cozzani I. Nonspecific acid phosphatase from Schizosaccharomyces pombe. Purification and physical chemical properties. Biochemistry. 1975 Jul;14(13):2847–2852. doi: 10.1021/bi00684a009. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Identification and partial characterization of an extracellular acid phosphatase activity of Leishmania donovani promastigotes. Mol Cell Biol. 1982 Jan;2(1):76–81. doi: 10.1128/mcb.2.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. F., Coombs G. H. Phosphomonoesterases of Leishmania mexicana mexicana and other flagellates. Mol Biochem Parasitol. 1987 Apr;23(3):285–296. doi: 10.1016/0166-6851(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Ilg T., Menz B., Winter G., Russell D. G., Etges R., Schell D., Overath P. Monoclonal antibodies to Leishmania mexicana promastigote antigens. I. Secreted acid phosphatase and other proteins share epitopes with lipophosphoglycan. J Cell Sci. 1991 May;99(Pt 1):175–180. doi: 10.1242/jcs.99.1.175. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Perez L., Schnur L. F. Lipophosphoglycan and secreted acid phosphatase of Leishmania tropica share species-specific epitopes. Mol Biochem Parasitol. 1990 Jun;41(2):233–240. doi: 10.1016/0166-6851(90)90186-p. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Polakis S. E. Acetyl coenzyme A carboxylase. Curr Top Cell Regul. 1974;8(0):139–195. [PubMed] [Google Scholar]
- Lovelace J. K., Dwyer D. M., Gottlieb M. Purification and characterization of the extracellular acid phosphatase of Leishmania donovani. Mol Biochem Parasitol. 1986 Sep;20(3):243–251. doi: 10.1016/0166-6851(86)90105-2. [DOI] [PubMed] [Google Scholar]
- Lovelace J. K., Gottlieb M. Comparison of extracellular acid phosphatases from various isolates of Leishmania. Am J Trop Med Hyg. 1986 Nov;35(6):1121–1128. doi: 10.4269/ajtmh.1986.35.1121. [DOI] [PubMed] [Google Scholar]
- Lovelace J. K., Gottlieb M. Effect of tunicamycin on the extracellular acid phosphatase of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Jan 2;22(1):19–28. doi: 10.1016/0166-6851(87)90065-x. [DOI] [PubMed] [Google Scholar]
- Medina-Acosta E., Karess R. E., Schwartz H., Russell D. G. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989 Dec;37(2):263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- Menz B., Winter G., Ilg T., Lottspeich F., Overath P. Purification and characterization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol Biochem Parasitol. 1991 Jul;47(1):101–108. doi: 10.1016/0166-6851(91)90152-v. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schweingruber A. M., Schoenholzer F., Keller L., Schwaninger R., Trachsel H., Schweingruber M. E. Glycosylation and secretion of acid phosphatase in Schizosaccharomyces pombe. Eur J Biochem. 1986 Jul 1;158(1):133–140. doi: 10.1111/j.1432-1033.1986.tb09730.x. [DOI] [PubMed] [Google Scholar]
- Stierhof Y. D., Schwarz H., Menz B., Russell D. G., Quinten M., Overath P. Monoclonal antibodies to Leishmania mexicana promastigote antigens. II. Cellular localization of antigens in promastigotes and infected macrophages. J Cell Sci. 1991 May;99(Pt 1):181–186. doi: 10.1242/jcs.99.1.181. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Orlandi P. A., Jr, Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989 Apr 25;264(12):6711–6715. [PubMed] [Google Scholar]
- Turco S. J. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990 Feb;70(2):241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., Vitters E., van den Berg W. B., Schram D., van de Putte L. B. Determination of small quantities of sulfate (0-12 nmol) in serum, urine, and cartilage of the mouse. Anal Biochem. 1987 Jun;163(2):408–417. doi: 10.1016/0003-2697(87)90242-9. [DOI] [PubMed] [Google Scholar]