Abstract
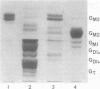
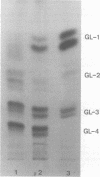
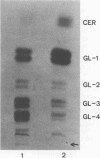
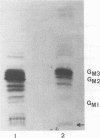
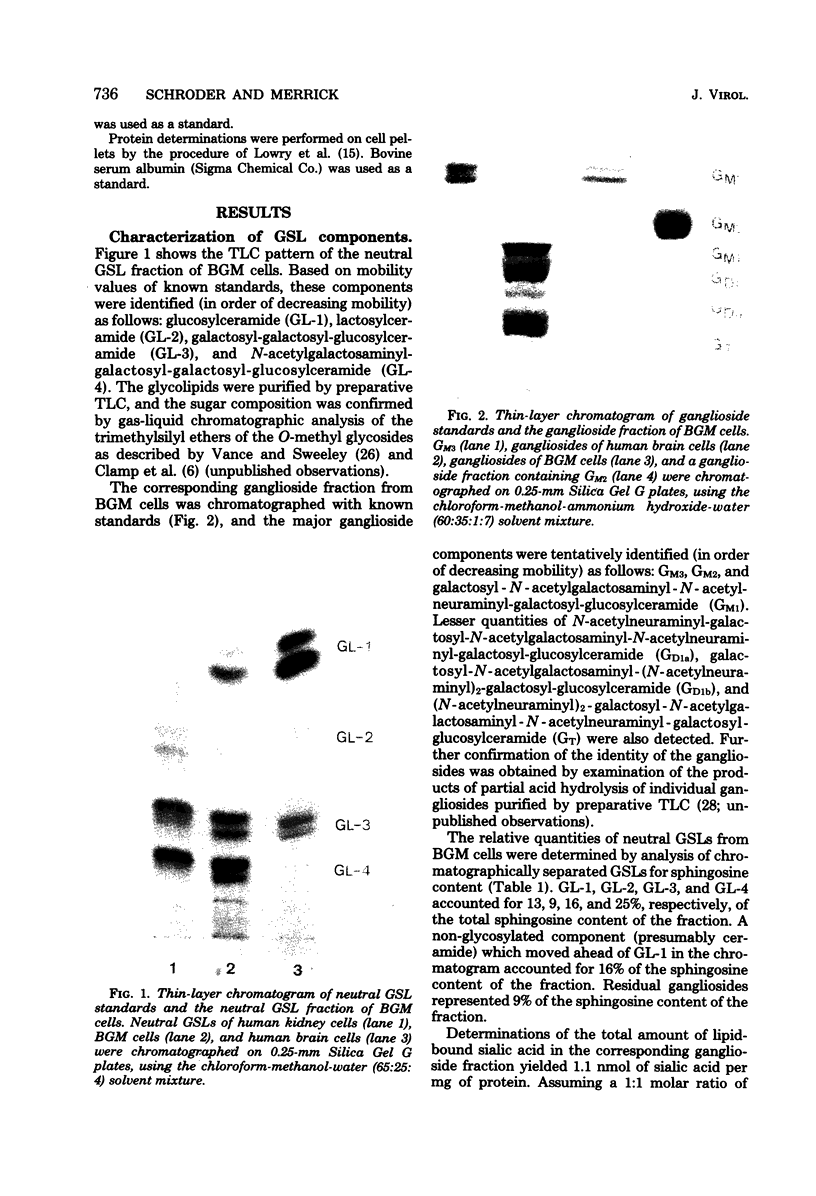
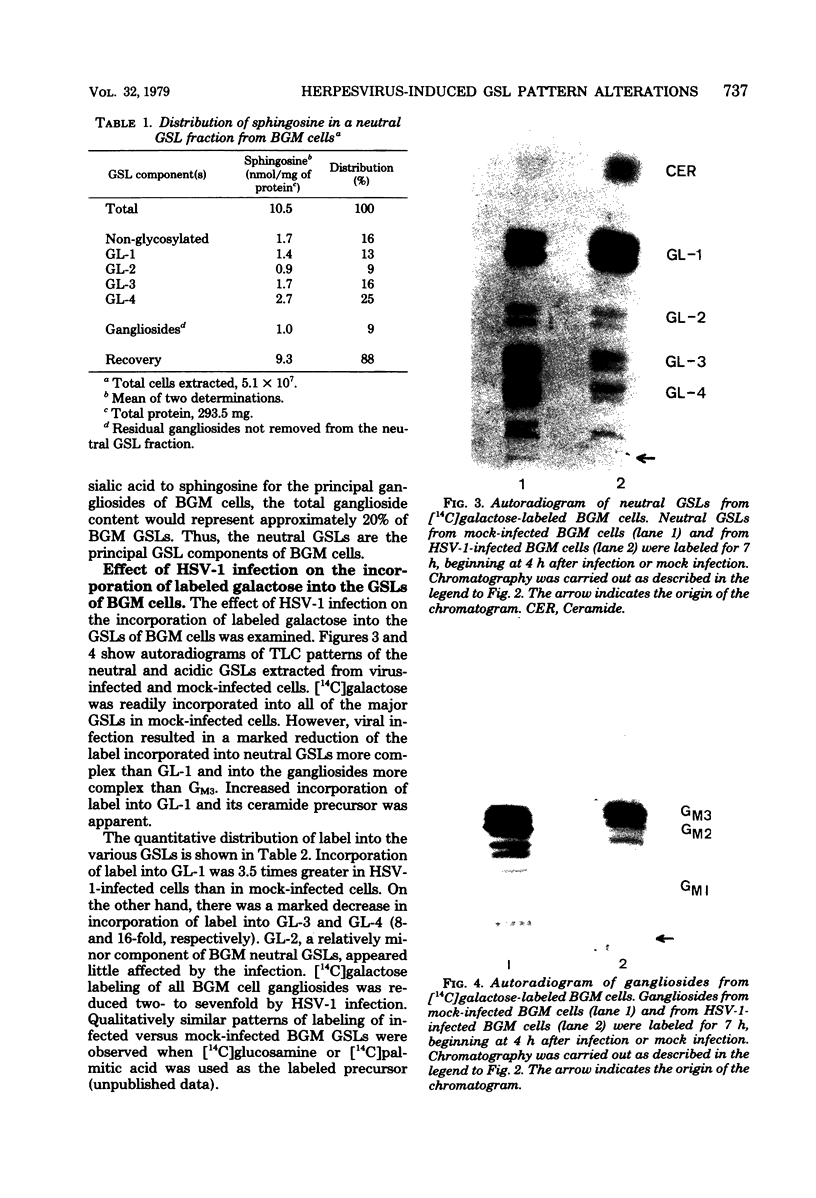
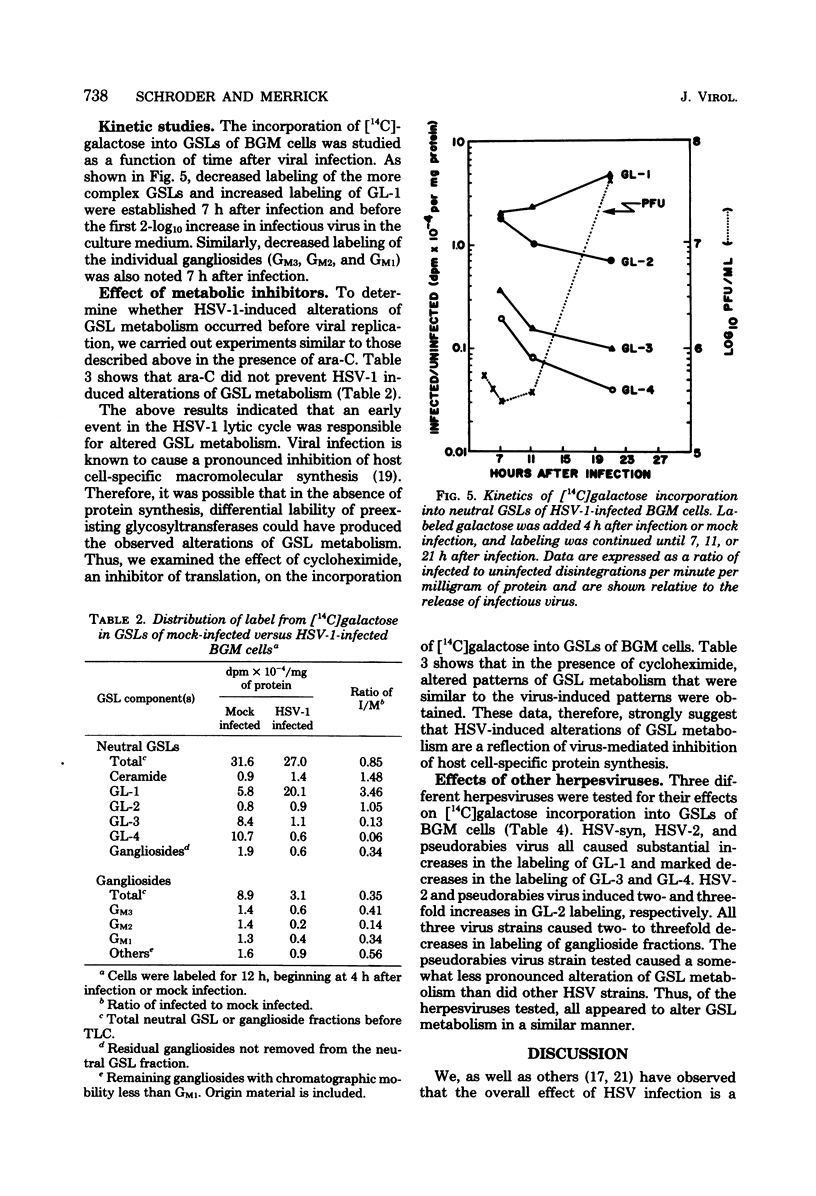
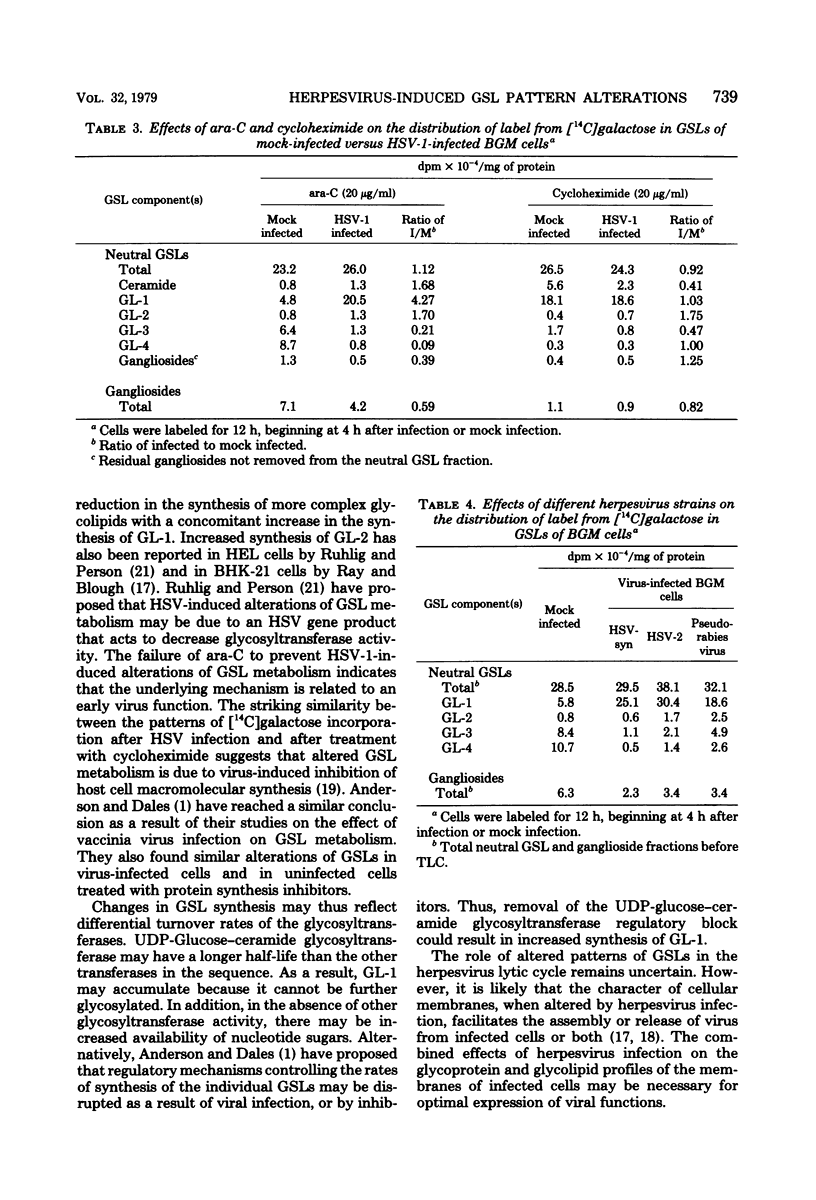
The major glycosphingolipids (GSLs) of a line of African green monkey kidney cells (BGM) were characterized as glucosylceramide, lactosylceramide, galactosyl-galactosyl-glucosylceramide, and N-acetylgalactosaminyl-galactosyl-galactosyl-glucosylceramide. Neutral GSLs accounted for approximately 80% of the total GSLs isolated. The predominant gangliosides were N-acetylneuraminyl-galactosyl-glucosylceramide, N-acetylgalactosaminyl-N-acetylneuraminyl-galactosyl- glucosylceramide, and galactosyl-N-acetylgalactosaminyl-N-acetylneuraminyl -galactosyl-glucosylceramide. The incorporation of labeled galactose into GSLs was compared in mock-infected and herpes simplex virus type 1-infected BGM cells. Herpes simplex virus type 1 infection resulted in a three- to four-fold increase in galactose incorporation into glucosylceramide and a decrease in galactose incorporation into galactosyl-galactosyl-glucosylceramide and N-acetyl-galactosaminyl-galactosyl-galactosyl-glucosylceramide. The virus-induced alteration in the GSL labeling pattern occurred early in infection, before the release of infectious virus, and was not prevented by the presence of cytosine arabinoside. Treatment of uninfected BGM cells with cycloheximide resulted in alterations in the GSL pattern which were similar to those observed in herpes simplex virus type 1-infected cells. These observations suggest that an early virus function such as inhibition of host cell protein synthesis is responsible for the observed alterations of GSL metabolism. Experiments with a syncytium-producing strain of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus indicated that other herpes viruses altered GSL metabolism in a manner similar to herpes simplex virus type 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Dales S. Biogenesis of poxviruses: glycolipid metabolism in vaccinia-infected cells. Virology. 1978 Jan;84(1):108–117. doi: 10.1016/0042-6822(78)90222-2. [DOI] [PubMed] [Google Scholar]
- Barron A. L., Olshevsky C., Cohen M. M. Characteristics of the BGM line of cells from African green monkey kidney. Brief report. Arch Gesamte Virusforsch. 1970;32(4):389–392. doi: 10.1007/BF01250067. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Steiner S. M., Courtney R. J., Skelly J. Metabolism of galactose in herpes simplex virus-infected cells. Virology. 1976 Jan;69(1):216–228. doi: 10.1016/0042-6822(76)90208-7. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fishman P. H., Bennett V., Cuatrecasas P. Cholera toxin and cell growth: role of membrane gangliosides. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4224–4228. doi: 10.1073/pnas.71.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Barron A. L. Surface antigen produced by herpes simplex virus (HSV). J Immunol. 1972 Mar;108(3):711–718. [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ray E. K., Blough H. A. The effect of herpesvirus infection and 2-deoxy-D-glucose on glycosphingolipids in BHK-21 cells. Virology. 1978 Jul 1;88(1):118–127. doi: 10.1016/0042-6822(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Roizman B., Borman G. S., Rousta M. K. Macromolecular synthesis in cells infected with herpes simplex virus. Nature. 1965 Jun 26;206(991):1374–1375. doi: 10.1038/2061374a0. [DOI] [PubMed] [Google Scholar]
- Rosenfelder G., Young W. W., Jr, Hakomori S. I. Association of the glycolipid pattern with antigenic alterations in mouse fibroblasts transformed by murine sarcoma virus. Cancer Res. 1977 May;37(5):1333–1339. [PubMed] [Google Scholar]
- Ruhlig M. A., Person S. Alterations of neutral glycolipids in cells infected with syncytium-producing mutants of herpes simplex virus type 1. J Virol. 1977 Nov;24(2):602–608. doi: 10.1128/jvi.24.2.602-608.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956 May;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Savage T., Roizman B., Heine J. W. Immunological specificity of the glycoproteins of herpes simplex virus subtypes 1 and 2. J Gen Virol. 1972 Oct;17(1):31–48. doi: 10.1099/0022-1317-17-1-31. [DOI] [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Wherrett J. R., Chatterjee S., Murray R. K. Gangliosides of cultured mouse cells. Partial characterization and demonstration of 14C-glucosamine incorporation. J Biol Chem. 1970 Dec 25;245(24):6718–6725. [PubMed] [Google Scholar]