Abstract
A rat cDNA containing the complete coding sequence for rat tyrosine hydroxylase (tyrosine 3-monooxygenase, EC 1.14.16.2) was isolated from a rat PC12 cDNA library and subcloned in a bacterial expression plasmid, and large amounts of functional enzyme were produced in Escherichia coli. The recombinant enzyme was purified approximately 20-fold to a final specific activity of 1.8 mumol/min per mg of protein, with a yield of 30%. As much as 1 mg of pure protein could be obtained from 1 g of wet bacterial cells. The purified hydroxylase was shown to be homogeneous by denaturing polyacrylamide electrophoresis and isoelectric focusing. Amino acid analysis of the N terminus (25 residues) revealed 100% identity with rat PC12 tyrosine hydroxylase, as deduced from its cDNA sequence. Several of the kinetic properties of the recombinant enzyme resembled those of the native PC12 hydroxylase. However, in contrast to the native enzyme, the purified recombinant hydroxylase was shown to be in an activated form. Phosphorylation with cAMP-dependent protein kinase resulted in stoichiometric incorporation of phosphate, but the kinetic profile of the recombinant enzyme was unaffected. Several clues to these differences are considered that may provide insight into the structural features important to the regulation of tyrosine hydroxylase.
Full text
PDF

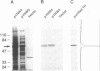
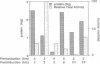
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Joh T. H. Limited proteolysis of rat brain tyrosine hydroxylase defines an N-terminal region required for regulation of cofactor binding and directing substrate specificity. J Mol Neurosci. 1991;2(4):203–215. [PubMed] [Google Scholar]
- Abate C., Smith J. A., Joh T. H. Characterization of the catalytic domain of bovine adrenal tyrosine hydroxylase. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1446–1453. doi: 10.1016/s0006-291x(88)80524-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix T. A., Kuhn D. M., Benkovic S. J. Mechanism of oxygen activation by tyrosine hydroxylase. Biochemistry. 1987 Jun 16;26(12):3354–3361. doi: 10.1021/bi00386a016. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P. F., Chlumsky L. J., Daubner S. C., O'Malley K. L. Expression of rat tyrosine hydroxylase in insect tissue culture cells and purification and characterization of the cloned enzyme. J Biol Chem. 1990 Feb 5;265(4):2042–2047. [PubMed] [Google Scholar]
- Frankel S., Sohn R., Leinwand L. The use of sarkosyl in generating soluble protein after bacterial expression. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1192–1196. doi: 10.1073/pnas.88.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginns E. I., Rehavi M., Martin B. M., Weller M., O'Malley K. L., LaMarca M. E., McAllister C. G., Paul S. M. Expression of human tyrosine hydroxylase cDNA in invertebrate cells using a baculovirus vector. J Biol Chem. 1988 May 25;263(15):7406–7410. [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik J., Andersson K. K., Petersson L., Flatmark T. Soluble tyrosine hydroxylase (tyrosine 3-monooxygenase) from bovine adrenal medulla: large-scale purification and physicochemical properties. Biochim Biophys Acta. 1988 Mar 23;953(2):142–156. doi: 10.1016/0167-4838(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Hoeldtke R., Kaufman S. Bovine adrenal tyrosine hydroxylase: purification and properties. J Biol Chem. 1977 May 25;252(10):3160–3169. [PubMed] [Google Scholar]
- Katz I. R., Yamauchi T., Kaufman S. Activation of tyrosine hydroxylase by polyanions and salts. An electrostatic effect. Biochim Biophys Acta. 1976 Mar 11;429(1):84–95. doi: 10.1016/0005-2744(76)90032-2. [DOI] [PubMed] [Google Scholar]
- Katz I., Lloyd T., Kaufman S. Studies on phenylalanine and tyrosine hydroxylation by rat brain tyrosine hydroxylase. Biochim Biophys Acta. 1976 Oct 11;445(3):567–578. doi: 10.1016/0005-2744(76)90111-x. [DOI] [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. Evidence for the lack of direct phosphorylation of bovine caudate tyrosine hydroxylase following activation by exposure to enzymatic phosphorylating conditions. Biochem Biophys Res Commun. 1975 Oct 6;66(3):907–917. doi: 10.1016/0006-291x(75)90726-3. [DOI] [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. The stimulation of partially purified bovine caudate tyrosine hydroxylase by phosphatidyl-L-serine. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1262–1270. doi: 10.1016/0006-291x(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. A RAPID AND SIMPLE RADIOASSAY FOR TYROSINE HYDROXYLASE ACTIVITY. Anal Biochem. 1964 Sep;9:122–126. doi: 10.1016/0003-2697(64)90092-2. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Nelson T. J., Kaufman S. Interaction of tyrosine hydroxylase with ribonucleic acid and purification with DNA-cellulose or poly(A)-sepharose affinity chromatography. Arch Biochem Biophys. 1987 Aug 15;257(1):69–84. doi: 10.1016/0003-9861(87)90544-3. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Mitchell L. G., Merril C. R., Rasband W. S. Use of image analysis to quantitate changes in form of mitochondrial DNA after x-irradiation. Appl Theor Electrophor. 1989;1(3):163–167. [PubMed] [Google Scholar]
- Pollock R. J., Kapatos G., Kaufman S. Effect of cyclic AMP-dependent protein phosphorylating conditions on the pH-dependent activity of tyrosine hydroxylase from beef and rat striata. J Neurochem. 1981 Oct;37(4):855–860. doi: 10.1111/j.1471-4159.1981.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro P., Pigeon D., Kaufman S. The hydroxylation of phenylalanine and tyrosine by tyrosine hydroxylase from cultured pheochromocytoma cells. J Biol Chem. 1991 Aug 25;266(24):16207–16211. [PubMed] [Google Scholar]
- Richtand N. M., Inagami T., Misono K., Kuczenski R. Purification and characterization of rat striatal tyrosine hydroxylase. Comparison of the activation by cyclic AMP-dependent phosphorylation and by other effectors. J Biol Chem. 1985 Jul 15;260(14):8465–8473. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]