Abstract
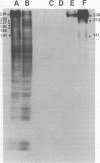
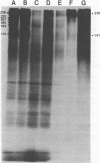
A correlation between Epstein-Barr virus membrane antigen (MA) and three surface glycoproteins has been established on the basis of radio-immunoprecipitation and immunoabsorption experiments. For radio-immunoprecipitation, Epstein-Barr virus-infected cells were radiolabeled either with neuraminidase-galactose oxidase tritiated borohydride, a procedure highly specific for surface glycoproteins, or with a general tritiated amino acid mixture. Intact cells were incubated with MA(−) or MA(+) human sera, washed free of unbound immunoglobulins, and then lysed with Nonidet P-40. The antigen-antibody complexes were bound to protein A-Sepharose and after elution with sodium dodecyl sulfate were analyzed by acrylamide gel electrophoresis in sodium dodecyl sulfate. MA(+) sera specifically precipitated three glycoproteins with molecular weights of 236,000, 212,000, and 141,000 from B95-8 cells induced with 12-O-tetradecanoylphorbal-13-acetate (TPA) and from Raji cells superinfected with P3HR-1 virus. These glycoproteins were not detected on Epstein-Barr virus-negative Ramos cells treated with TPA or on B95-8 cells treated simultaneously with TPA and phosphonoacetic acid. Soybean lectin-Sepharose bound all three glycoproteins, and lectin-Sepharose-bound glycoproteins from TPA-induced P95-8 cells absorbed MA-specific antibody from MA(+) human sera. The data strongly suggest that either all three glycoproteins have MA determinants or they are part of a complex in which one or more of the components constitute the reactive antigen.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggar W. D. Effect of phorbol myristate acetate on cellular metabolism and lysozyme release from alveolar macrophages and polymorphonuclear leukocytes. Infect Immun. 1978 Aug;21(2):669–671. doi: 10.1128/iai.21.2.669-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dolyniuk M., Wolff E., Kieff E. Proteins of Epstein-Barr Virus. II. Electrophoretic analysis of the polypeptides of the nucleocapsid and the glucosamine- and polysaccharide-containing components of enveloped virus. J Virol. 1976 Apr;18(1):289–297. doi: 10.1128/jvi.18.1.289-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Quantitative correlation between in vitro and in vivo activities of phorbol esters. Cancer Res. 1979 Mar;39(3):714–719. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Henle W., Guerra A., Henle G. False negative and prozone reactions in tests for antibodies to Epstein-Barr virus-associated nuclear antigen. Int J Cancer. 1974 Jun 15;13(6):751–754. doi: 10.1002/ijc.2910130603. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Pearson G., Nadkarni J. S., Nadkarni J. J., Klein E., Henle G., Henle W., Clifford P. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968 Nov 1;128(5):1011–1020. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer R. H., Rabin H., Brown A. M. Selective stimulation and differentiation of early antigens in lymphoblastoid cell lines producing Epstein-Barr-like viruses. J Gen Virol. 1978 Nov;41(2):295–301. doi: 10.1099/0022-1317-41-2-295. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Henle G., Henle W. Production of antigens associated with Epstein-Barr virus in experimentally infected lymphoblastoid cell lines. J Natl Cancer Inst. 1971 Jun;46(6):1243–1250. [PubMed] [Google Scholar]
- Pearson G. R., Orr T. W. Antibody-dependent lymphocyte cytotoxicity against cells expressing Epstein-Barr virus antigens. J Natl Cancer Inst. 1976 Mar;56(3):485–488. doi: 10.1093/jnci/56.3.485. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Qualtiere L. F. Papain solubilization of the Epstein-Barr virus-induced membrane antigen. J Virol. 1978 Oct;28(1):344–351. doi: 10.1128/jvi.28.1.344-351.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Epstein-Barr virus-induced membrane antigens: immunochemical characterization of Triton X-100 solubilized viral membrane antigens from EBV-superinfected Raji cells. Int J Cancer. 1979 Jun 15;23(6):808–817. doi: 10.1002/ijc.2910230612. [DOI] [PubMed] [Google Scholar]
- Rabin H., Neubauer R. H., Hopkins R. F., Levy B. M. Characterization of lymphoid cell lines established from multiple Epstein-Barr virus (EBV)-induced lymphomas in a cotton-topped marmoset. Int J Cancer. 1977 Jul 15;20(1):44–50. doi: 10.1002/ijc.2910200109. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., O'Conor G. T., Lorenz D. E., Kirschstein R. L., Legallais F. Y., Tralka T. S. Lymphoid cell-culture line derived from lymph node of marmoset infected wtih Herpesvirus saimiri--preliminary report. J Natl Cancer Inst. 1971 May;46(5):1099–1109. [PubMed] [Google Scholar]
- Silvestre D., Kourilsky F. M., Klein G., Yata Y., Neauport-Sautes C., Levy J. P. Relationship between the EBV-associated membrane antigen on Burkitt lymphoma cells and the viral envelope, demonstrated by immunoferritin labelling. Int J Cancer. 1971 Sep 15;8(2):222–233. doi: 10.1002/ijc.2910080206. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Neubauer R. H., Rabin H. Biochemical identification of primate lymphoid cell-surface glycoproteins. Int J Cancer. 1979 Jan 15;23(1):76–81. doi: 10.1002/ijc.2910230114. [DOI] [PubMed] [Google Scholar]
- Svedmyr A., Demissie A., Klein G., Clifford P. Antibody patterns in different human sera against intracellular and membrane-antigen complexes associated with Epstein-Barr virus. J Natl Cancer Inst. 1970 Mar;44(3):595–610. [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]