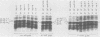Abstract
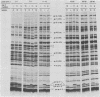
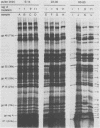
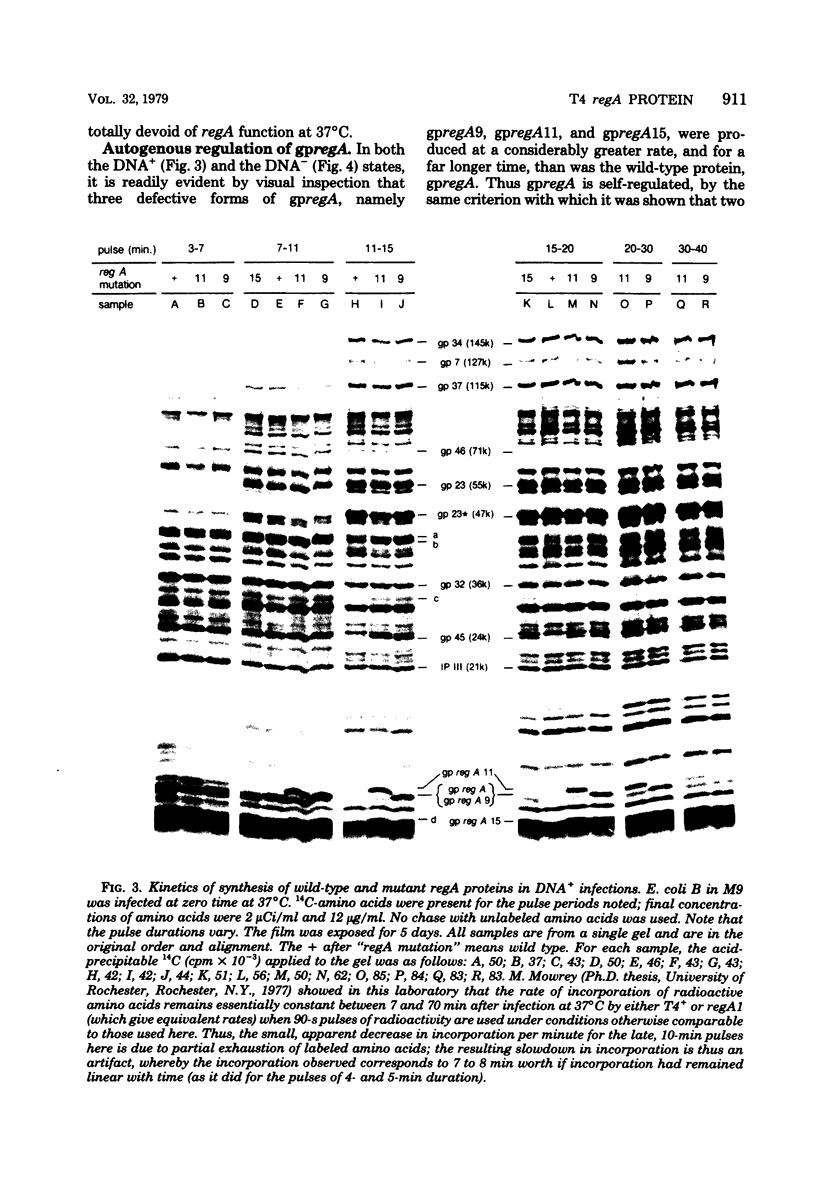
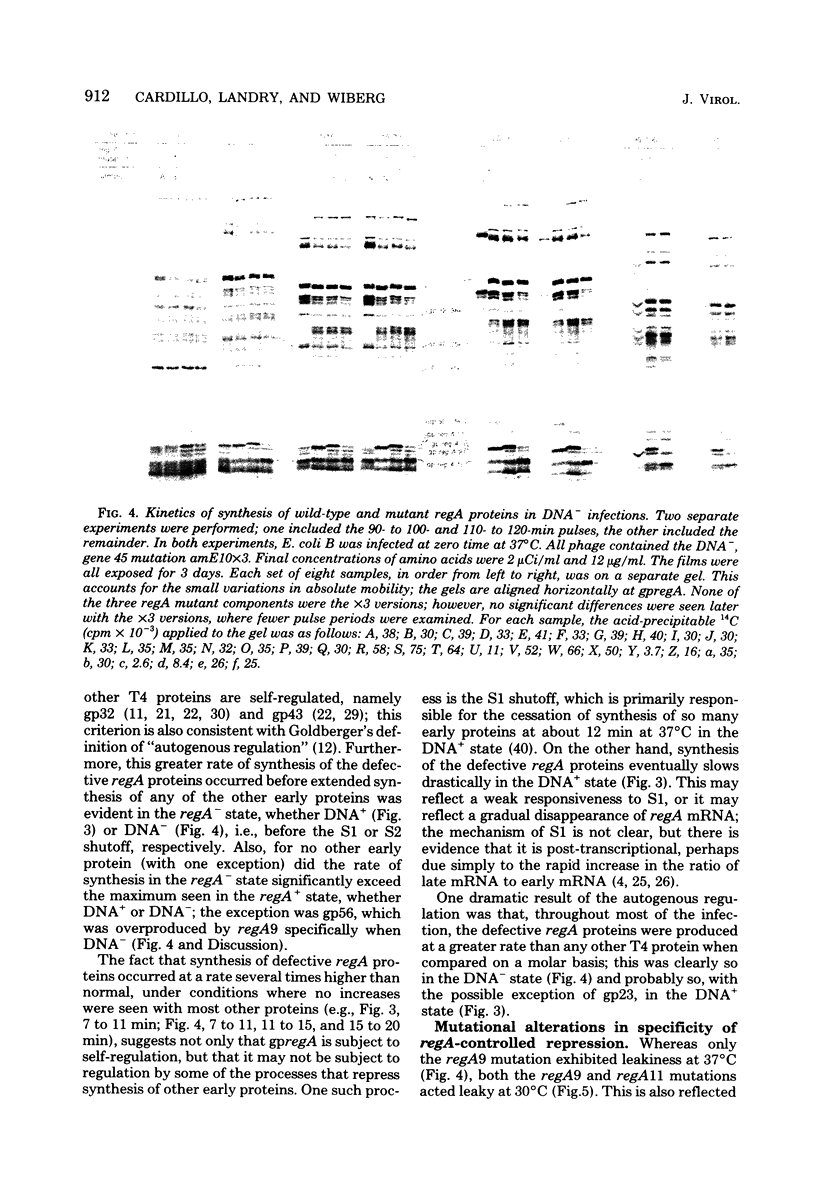
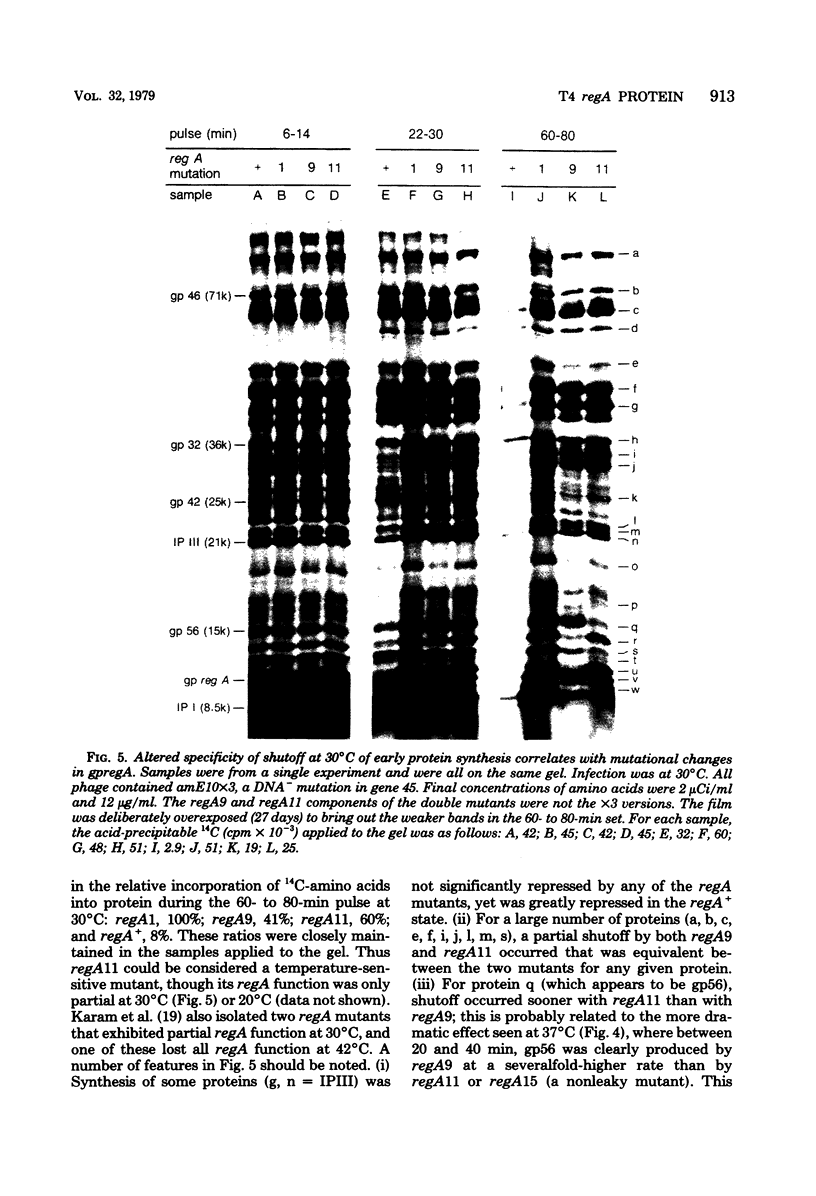
Proteins labeled with 14C-amino acids after infection of Escherichia coli B by T4 phage were examined by electrophoresis in the presence of sodium dodecyl sulfate. Four regA mutants (regA1, regA8, regA11, and regA15) failed to make a protein having a molecular weight of about 12,000, whereas mutant regA9 did make such a protein; regA15 produced a new, apparently smaller protein that was presumably a nonsense fragment, whereas regA11 produced a new, apparently larger protein. We conclude that the 12,000-dalton protein was the product of the regA gene. The molecular weight assignment rested primarily on our finding that the regA protein had the same mobility as the T4 gene 33 protein, which we identified by electrophoresis of whole-cell extracts of E. coli B infected with a gene 33 mutant, amE1120. Synthesis of wild-type regA protein occurred from about 3 to 11 min after infection at 37 degrees C in the DNA+ state and extended to about 20 min in the DNA- state. However, synthesis of the altered regA proteins of regA9, regA11, and regA15 occurred at a higher rate and for a much longer period in both the DNA+ and DNA- states; thus, the regA gene is autogenously regulated. At 30 degrees C, both regA9 and regA11 exhibited partial regA function by eventually shutting off the synthesis of many T4 early proteins; the specificity of this shutoff differed between these two mutants. We also obtained evidence that the regA protein is not Stevens's "polypeptide 3." As a technical point, we found that, when quantitating acid-precipitable radioactivity in protein samples containing sodium dodecyl sulfate, it was necessary to use 15 to 20% trichloroacetic acid; use of 5% acid, e.g., resulted in loss of over half of the labeled protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. H., Agrawal H. C., Moore B. W. Effect of N,N,N',N'-tetramethylethylenediamine on the migration of proteins in SDS polyacrylamide gels. Anal Biochem. 1974 Apr;58(2):592–601. doi: 10.1016/0003-2697(74)90228-0. [DOI] [PubMed] [Google Scholar]
- Belfort M. Anomalous behavior of bacteriophage lambda polypeptides in polyacrylamide gels: resolution, identification, and control of the lambda rex gene product. J Virol. 1978 Oct;28(1):270–278. doi: 10.1128/jvi.28.1.270-278.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolund C. Influence of gene 55 on the regulation of synthesis of some early enzymes in bacteriophage T4-infected escherichia coli. J Virol. 1973 Jul;12(1):49–57. doi: 10.1128/jvi.12.1.49-57.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G. Effect of prolonged 100 degrees C heat treatment in sodium dodecyl sulfate upon peptide bond cleavage. Anal Biochem. 1976 Mar;71(1):300–303. doi: 10.1016/0003-2697(76)90040-3. [DOI] [PubMed] [Google Scholar]
- Dirksen M. L., Wiberg J. S., Koerner J. F., Buchanan J. M. EFFECT OF ULTRAVIOLET IRRADIATION OF BACTERIOPHAGE T2 ON ENZYME SYNTHESIS IN HOST CELLS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1425–1430. doi: 10.1073/pnas.46.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Pero J. New phage-SPO1-induced polypeptides associated with Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2761–2765. doi: 10.1073/pnas.71.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gold L., O'Farrell P. Z., Russel M. Regulation of gene 32 expression during bacteriophage T4 infection of Escherichia coli. J Biol Chem. 1976 Nov 25;251(22):7251–7262. [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Geballe A. P., Snopek T. J., Sugino A., Cozzarelli N. R. Bacteriophage T4 RNA ligase: preparation of a physically homogeneous, nuclease-free enzyme from hyperproducing infected cells. Nucleic Acids Res. 1977 Sep;4(9):3175–3186. doi: 10.1093/nar/4.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Studies of mutations in T4 control genes 33 and 55. Genetics. 1975 Mar;79(3):349–360. doi: 10.1093/genetics/79.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Black L. W., Tsugita A. Complete aimino acid sequence of bacteriophage T4 internal protein I and its cleavage site on virus maturation. J Mol Biol. 1977 Feb 15;110(1):165–177. doi: 10.1016/s0022-2836(77)80104-6. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J. Electron microscopical studies of phage multiplication. II. Production of phage-related structures during multiplication of phages T2 and T4. Virology. 1957 Apr;3(2):256–274. doi: 10.1016/0042-6822(57)90092-2. [DOI] [PubMed] [Google Scholar]
- Karam J. D., Bowles M. G. Mutation to overproduction of bacteriophage T4 gene products. J Virol. 1974 Feb;13(2):428–438. doi: 10.1128/jvi.13.2.428-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J., McCulley C., Leach M. Genetic control of mRNA decay in T4 phage-infected Escherichia coli. Virology. 1977 Feb;76(2):685–700. doi: 10.1016/0042-6822(77)90251-3. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Van Houwe G., Belin D., Gibbs W., Epstein R. H. Regulation of the expression of bacteriophage T4 genes 32 and 43. Virology. 1977 May 1;78(1):87–98. doi: 10.1016/0042-6822(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Russel M. Control of bacteriophage T4 DNA polymerase synthesis. J Mol Biol. 1973 Sep 5;79(1):83–94. doi: 10.1016/0022-2836(73)90271-4. [DOI] [PubMed] [Google Scholar]
- Russel M., Gold L., Morrissett H., O'Farrell P. Z. Translational, autogenous regulation of gene 32 expression during bacteriophage T4 infection. J Biol Chem. 1976 Nov 25;251(22):7263–7270. [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Control of gene function in bacteriophage T4. IV. Post-transcriptional shutoff of expression of early genes. J Virol. 1973 Sep;12(3):538–547. doi: 10.1128/jvi.12.3.538-547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Söderberg B. O. Thioredoxin induced by bacteriophage T4: crystallization and preliminary crystallographic data. J Mol Biol. 1976 Jan 25;100(3):415–419. doi: 10.1016/s0022-2836(76)80072-1. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney J. B., Vande Woude G. F., Bachrach H. L. Sodium dodecylsulfate-dependent anomalies in gel electrophoresis: alterations in the banding patterns of foot-and-mouth disease virus polypeptides. Anal Biochem. 1974 Apr;58(2):337–346. doi: 10.1016/0003-2697(74)90201-2. [DOI] [PubMed] [Google Scholar]
- Thompson S., Wiberg J. S. Late effect of bacteriophage T4D on the permeability barrier of Escherichia coli. J Virol. 1978 Feb;25(2):491–499. doi: 10.1128/jvi.25.2.491-499.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley Level of specific prereplicative mRNA's during bacteriophage T4 regA-, 43- and T4 43- infection of Escherichia coli B. J Virol. 1976 Feb;17(2):538–549. doi: 10.1128/jvi.17.2.538-549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S. L., Warner V., Aldrich C., Cardillo T. S. Genetic mapping of regA mutants of bacteriophage T4D. J Virol. 1977 Jun;22(3):742–749. doi: 10.1128/jvi.22.3.742-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Gratzer W. B. Limitations of the detergent-polyacrylamide gel electrophoresis method for molecular weight determination of proteins. J Chromatogr. 1971 Apr 22;57(1):121–125. doi: 10.1016/0021-9673(71)80013-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]