Abstract
We have established a growth selection requirement for a catalytic antibody with modest chorismate mutase activity. Conversion of (-)-chorismate into prephenate is the key step in the biosynthesis of the aromatic amino acids tyrosine and phenylalanine. Strains of the yeast Saccharomyces cerevisiae containing an insertion mutation in the structural gene for the enzyme chorismate mutase (EC 5.4.99.5) require exogenous supplements of these two amino acids for efficient growth. Intracellular expression of the heterologous antibody catalyst in one such strain, identified by random mutagenesis and genetic selection, provides a substantial growth advantage under auxotrophic conditions; complementation was not observed with an unrelated esterolytic antibody. In addition to demonstrating that tailored immunoglobulin catalysts can carry out vital biochemical reactions in vivo, these experiments provide a powerful selection assay for identifying genetic changes within the antibody molecule itself that augment chemical efficiency.
Full text
PDF

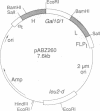
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S. G., Wickner R. B., Cottarel G., Schaus M., Tirtiaux C. Molecular cloning and characterization of ARO7-OSM2, a single yeast gene necessary for chorismate mutase activity and growth in hypertonic medium. Mol Gen Genet. 1986 Nov;205(2):326–330. doi: 10.1007/BF00430446. [DOI] [PubMed] [Google Scholar]
- Bowdish K., Tang Y., Hicks J. B., Hilvert D. Yeast expression of a catalytic antibody with chorismate mutase activity. J Biol Chem. 1991 Jun 25;266(18):11901–11908. [PubMed] [Google Scholar]
- Carlson J. R. A new means of inducibly inactivating a cellular protein. Mol Cell Biol. 1988 Jun;8(6):2638–2646. doi: 10.1128/mcb.8.6.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes J. D., Blacklow S. C., Knowles J. R. Searching sequence space by definably random mutagenesis: improving the catalytic potency of an enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):696–700. doi: 10.1073/pnas.87.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A., Cafferkey R., Bowdish K. Production of antibodies in transgenic plants. Nature. 1989 Nov 2;342(6245):76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- Hilvert D., Carpenter S. H., Nared K. D., Auditor M. T. Catalysis of concerted reactions by antibodies: the Claisen rearrangement. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4953–4955. doi: 10.1073/pnas.85.14.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidheini T., Mösch H. U., Evans J. N., Braus G. Yeast allosteric chorismate mutase is locked in the activated state by a single amino acid substitution. Biochemistry. 1990 Apr 17;29(15):3660–3668. doi: 10.1021/bi00467a011. [DOI] [PubMed] [Google Scholar]
- Schmidheini T., Sperisen P., Paravicini G., Hütter R., Braus G. A single point mutation results in a constitutively activated and feedback-resistant chorismate mutase of Saccharomyces cerevisiae. J Bacteriol. 1989 Mar;171(3):1245–1253. doi: 10.1128/jb.171.3.1245-1253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Catalytic antibodies. Science. 1986 Dec 19;234(4783):1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]