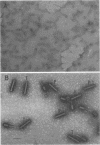
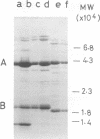
Abstract
The tail of bacteriophage PS17 of Pseudomonas aeruginosa was shown to be bactericidal, and its properties were compared with those of pyocin R1. Temperature-sensitive mutants were isolated from PS17, and the products at nonpermissive temperature were morphologically characterized. Bactericidal substances were found in the lysates of such mutants that were defective in the head formation but not in the tail formation. Phage tails were purified from the lysate of one such mutant, and its chemical and biological properties were studied. Isolated tails killed sensitive cells by a single-hit process and repressed the uptake of leucine in sensitive cells. These results were consistent with the previous findings on the serological and morphological relationship between PS17 and pyocin R1. However, certain differences were also shown between them in shape and protein composition.
Full text
PDF

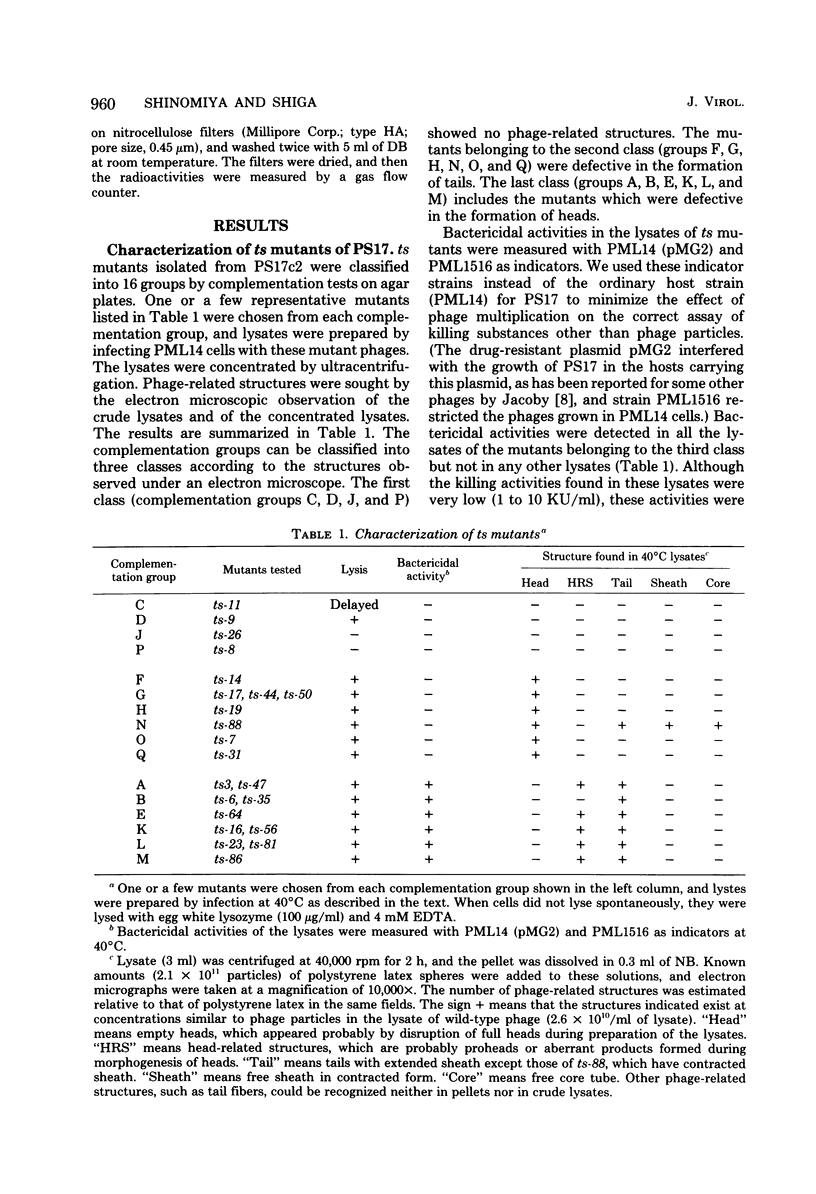
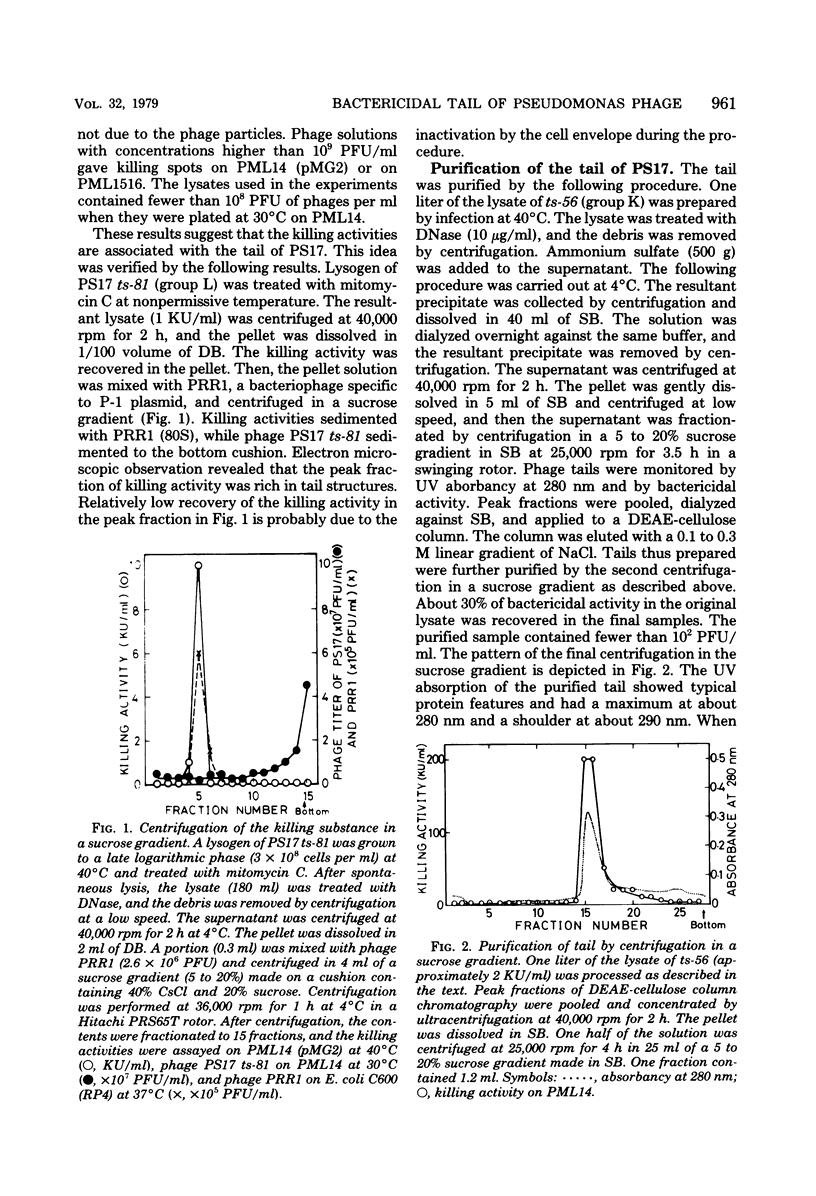


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- Iijima M. Mode of action of pyocin R1. J Biochem. 1978 Feb;83(2):395–402. doi: 10.1093/oxfordjournals.jbchem.a131926. [DOI] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGEYAMA M. STUDIES OF A PYOCIN. I. PHYSICAL AND CHEMICAL PROPERTIES. J Biochem. 1964 Jan;55:49–53. doi: 10.1093/oxfordjournals.jbchem.a127839. [DOI] [PubMed] [Google Scholar]
- Kageyama M., Shinomiya T., Aihara Y., Kobayashi M. Characterization of a bacteriophage related to R-type pyocins. J Virol. 1979 Dec;32(3):951–957. doi: 10.1128/jvi.32.3.951-957.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaziro Y., Tanaka M. Studies on the mode of action of pyocin. I. Inhibition of macromolecular synthesis in sensitive cells. J Biochem. 1965 May;57(5):689–695. [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Osumi M., Kageyama M. Defective pyocin particles produced by some mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1975 Dec;124(3):1508–1521. doi: 10.1128/jb.124.3.1508-1521.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T. Studies on biosynthesis and morphogenesis of R-type pyocins of Pseudomonas aeruginosa. 3. Subunits of pyocin R and their precipitability by anti-pyocin R serum. J Biochem. 1972 Sep;72(3):499–510. doi: 10.1093/oxfordjournals.jbchem.a129929. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Kageyama M. A fluorescent probe response to the interaction of pyocin R1 with sensitive cells. J Biochem. 1977 Feb;81(2):333–341. doi: 10.1093/oxfordjournals.jbchem.a131463. [DOI] [PubMed] [Google Scholar]
- Yui C. Structure of pyocin R. I. Isolation of sheath from pyocin R by alkali treatment and its properties. J Biochem. 1971 Jan;69(1):101–110. doi: 10.1093/oxfordjournals.jbchem.a129437. [DOI] [PubMed] [Google Scholar]