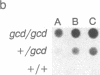Abstract
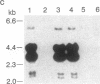
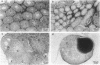
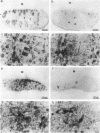
A genetic analysis is necessary to gain a greater understanding of the complex developmental processes in mammals. Toward this end, an insertional transgenic mouse mutant has been isolated that results in abnormal germ-cell development. This recessive mutation manifests as infertility in both males and females and is specific for the reproductive organs, since all other tissues examined were histologically normal. A developmental analysis of the gonadal tissues demonstrated that the germ cells were specifically depleted as early as day 11.5 of embryonic development, while the various somatic cells were apparently unaffected. Therefore, the mutated locus must play a critical role in the migration/proliferation of primordial germ cells to the genital ridges of developing embryos. In addition, females homozygous for the mutation could potentially be a valuable animal model of a human syndrome, premature ovarian failure. This mutation has been named germ-cell deficient, gcd.
Full text
PDF
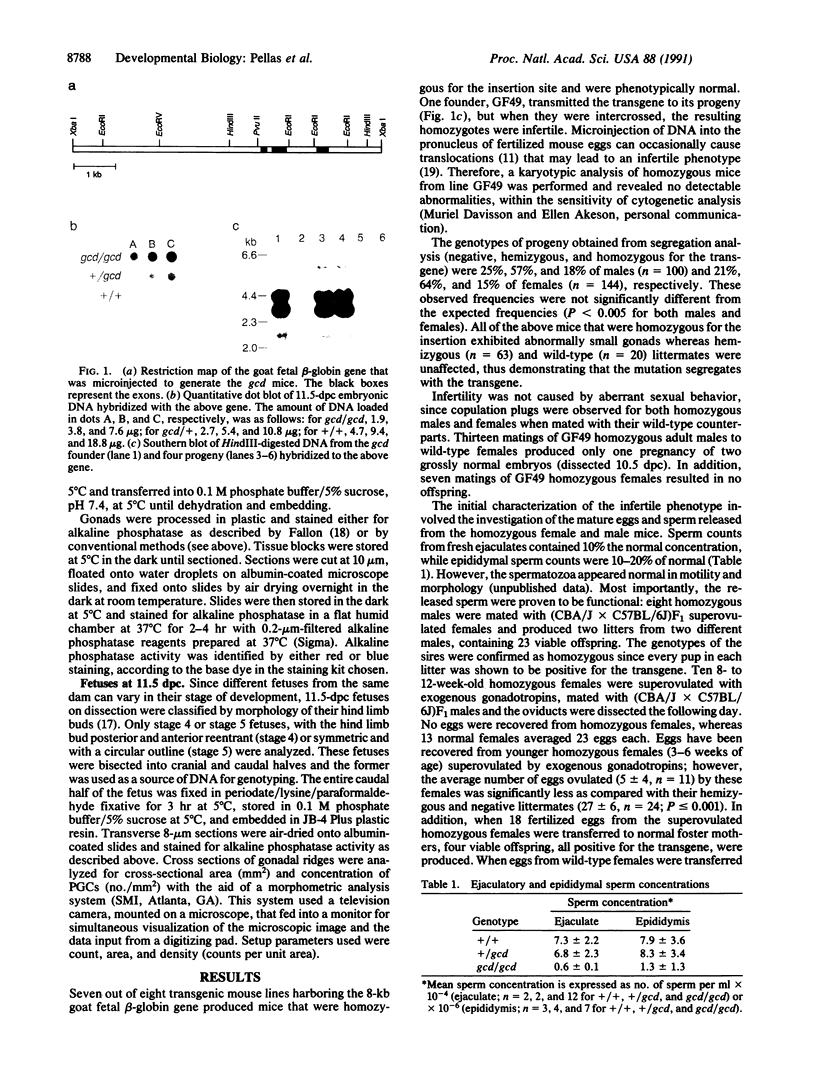
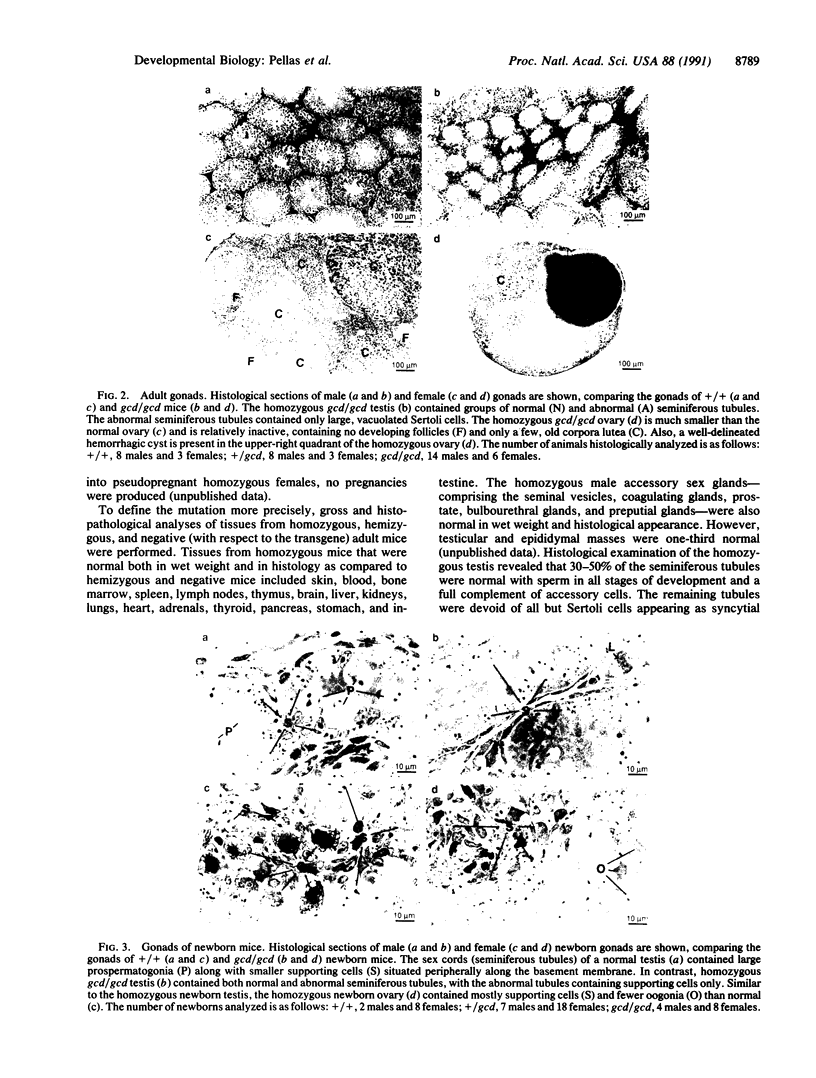
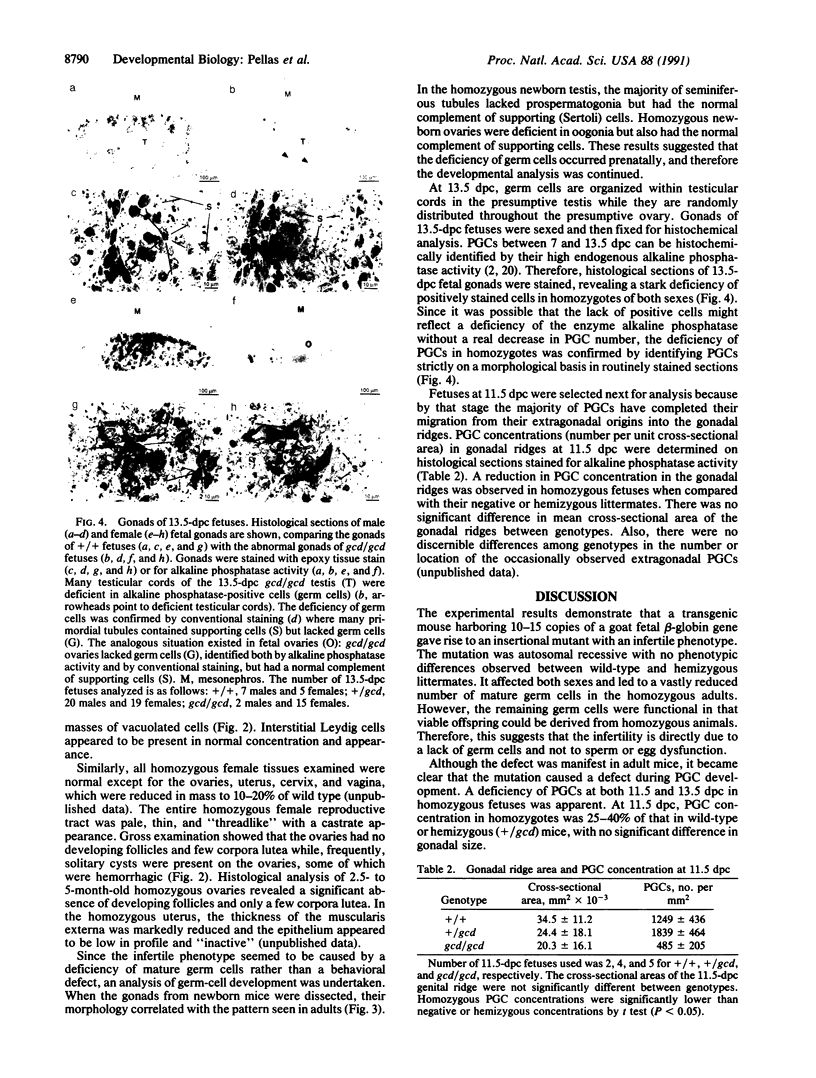

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiman J., Smentek C. Premature ovarian failure. Obstet Gynecol. 1985 Jul;66(1):9–14. [PubMed] [Google Scholar]
- Al-Shawi R., Burke J., Jones C. T., Simons J. P., Bishop J. O. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol. 1988 Nov;8(11):4821–4828. doi: 10.1128/mcb.8.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh A. Morphological signs of a direct effect of experimental cryptorchidism on the Sertoli cells in rats irradiated as fetuses. Biol Reprod. 1981 Feb;24(1):145–152. doi: 10.1095/biolreprod24.1.145. [DOI] [PubMed] [Google Scholar]
- Donovan P. J., Stott D., Cairns L. A., Heasman J., Wylie C. C. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986 Mar 28;44(6):831–838. doi: 10.1016/0092-8674(86)90005-x. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Snow M. H., McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990 Oct;110(2):521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Pravtcheva D., Poorman P. A., Moses M. J., Brock W. A., Ruddle F. H. Association of foreign DNA sequence with male sterility and translocation in a line of transgenic mice. Somat Cell Mol Genet. 1989 Nov;15(6):569–578. doi: 10.1007/BF01534918. [DOI] [PubMed] [Google Scholar]
- Handel M. A., Eppig J. J. Sertoli cell differentiation in the testes of mice genetically deficient in germ cells. Biol Reprod. 1979 Jun;20(5):1031–1038. doi: 10.1095/biolreprod20.5.1031. [DOI] [PubMed] [Google Scholar]
- Hekman A. C., Trapman J., Mulder A. H., van Gaalen J. L., Zwarthoff E. C. Interferon expression in the testes of transgenic mice leads to sterility. J Biol Chem. 1988 Aug 25;263(24):12151–12155. [PubMed] [Google Scholar]
- JONES E. C., KROHN P. L. The relationships between age, numbers of ocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961 Feb;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Jones E. C. The ageing ovary and its influence on reproductive capacity. J Reprod Fertil Suppl. 1970 Mar;12:17–30. [PubMed] [Google Scholar]
- MINTZ B., RUSSELL E. S. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957 Mar;134(2):207–237. doi: 10.1002/jez.1401340202. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Russell L. D., Van Beek M. E., Hanten G. R., Kovac M. J., Kozak C. A., Meistrich M. L., Overbeek P. A. Symplastic spermatids (sys): a recessive insertional mutation in mice causing a defect in spermatogenesis. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5016–5020. doi: 10.1073/pnas.87.13.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Kuroda H., Onoue H., Fujita J., Nishimune Y., Matsumoto K., Nagano T., Suzuki F., Kitamura Y. Studies of Sl/Sld in equilibrium with +/+ mouse aggregation chimaeras. II. Effect of the steel locus on spermatogenesis. Development. 1988 Jan;102(1):117–126. doi: 10.1242/dev.102.1.117. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985 Aug;75(2):385–392. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Wilkie T. M., Chen H. Y., Brinster R. L. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell. 1984 Apr;36(4):869–877. doi: 10.1016/0092-8674(84)90036-9. [DOI] [PubMed] [Google Scholar]
- Portuondo J. A., Neyro J. L., Benito J. A., de los Rios A., Barral A. Familial 46,XX gonadal dysgenesis. Int J Fertil. 1987 Jan-Feb;32(1):56–58. [PubMed] [Google Scholar]
- Russell E. S., McFarland E. C., Peters H. Gametic and pleiotropic defects in mouse fetuses with Hertwig's macrocytic anemia. Dev Biol. 1985 Aug;110(2):331–337. doi: 10.1016/0012-1606(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Cleary M. L., Haynes J. R., Lingrel J. B. Structure and evolution of goat gamma-, beta C- and beta A-globin genes: three developmentally regulated genes contain inserted elements. Cell. 1981 Dec;27(2 Pt 1):359–369. doi: 10.1016/0092-8674(81)90419-0. [DOI] [PubMed] [Google Scholar]
- Xiang X., Benson K. F., Chada K. Mini-mouse: disruption of the pygmy locus in a transgenic insertional mutant. Science. 1990 Feb 23;247(4945):967–969. doi: 10.1126/science.2305264. [DOI] [PubMed] [Google Scholar]