Abstract
Objectives:
To compare muscle and bone health markers in adult males (aged 20-59 yrs) with and without muscular dystrophy (MD).
Methods:
Participants included 11 Fascioscapulohumeral (FSH), 11 Becker’s (Be), 9 limb girdle (LG), 11 Duchenne (DMD), and 14 non-dystrophic controls (CTRL). Physical activity was assessed using Bone (BPAQ) and disability specific (PASIPD) questionnaires. Bone QUS provided T- and Z scores from the Distal Radius (DR) and Mid-shaft tibia (MST). Tibialis anterior cross sectional area (TAACSA) was measured using B-mode ultrasound. Grip strength was measured in all but DMD.
Results:
Physical activity was lower in DMD, FSH and BeMD than CTRL (P<0.05), and lower in DMD than other MDs (P<0.01). T and Z scores were lower in DMD and Be than CTRL (DR, P<0.05); and lower in DMD than CTRL, LG, and FSH (MST, P<0.01). TAACSA and grip strength was 35-59% and 50-58% smaller in MD than CTRL, respectively (P<0.01). Within MD, BPAQ correlated with bone QUS measures (r=0.42-0.38, P<0.01). PASIPD correlated with grip strength (r=0.65, P<0.01) and TAACSA (r=0.46, P<0.01).
Conclusion:
Muscle size, strength, and bone health was lower in adult males with MD compared to adult males without MD, the extent of this is partially determined by physical activity.
Keywords: Muscular Dystrophy, Bone Health, Muscle Cross Sectional Area, Physical Activity, Ultrasound
Introduction
Muscular dystrophy (MD) is a term given to a heterogeneous group of a neuromuscular disorders characterised by a genetic predisposition to an absence or reduction in a variety of proteins within and around the sarcolemma e.g., the sarcoglycan complex[1]. Some of these conditions have well established genetic and molecular characteristics; for example, Duchenne Muscular Dystrophy (DMD) and Becker’s Muscular Dystrophy (Be) are characterised by an absence or reduction in dystrophin, respectively. Limb Girdle Dystrophy (LG) is classically characterised by a deficiency or loss of the sarcoglycan proteins (β, δ or ϒ[2]); however there have now been over 20 types of LG MD identified, each with distinct sarcolemmal proteins being affected[3]. Within Facioscapulohumeral (FSH) Dystrophy, light has recently been shed on the genetic origins of the condition with the aberrant expression of the DUX4 gene in skeletal muscle recently identified[4].
Similar to the distinct locus of impairment within the various proteins of the sarcolemma, the functional outcomes of these conditions are distinct in terms of the progression of the condition, and the pattern of impairment[1]. They are however similar in that they are all associated with a loss of physical function[5]. Despite the established neuromuscular impairments of the muscular dystrophies, the direct comparison of in vivo muscle size and function between conditions, and with age matched adult control groups is limited. In mice with a form of dystrophy (MDX mice), muscles appear larger but weaker[6], and muscle architecture is altered consistent with an increase in muscle size[7]. Until recently, descriptive in vivo data on whole muscle size and function remained limited to a single study reporting muscle strength and size in DMD[8]. However, this involved a convenience sample of twelve individuals aged between 7-18 yrs old, compared to a control population of five individuals aged between 14-45 yrs old. Regardless of these limitations, it represents the only evidence that muscle size is initially increased in DMD, a similar pattern to that which is observed in MDX mice[9]. In contrast, our recent data from adults with DMD was at odds with the data from children, with significant atrophy observed in the Gastrocnemius of adults with the condition[10]. Data on muscle size from other muscle dystrophies remains unreported from an adult population.
A reduction in physical activity, and an associated decrease in loading and muscle mass is known to impact on bone health through reductions in bone mineral density, changes to bone architecture[11]. It has previously been established that children with DMD (mean age = 10 yrs) have a lower bone density than children without the condition[12], and that this is likely to contribute to the greater incidence of long bone fractures in adults with DMD and Be MD 13. However, as with descriptive data on muscle size and strength (two of the main covariables of muscle mass), there is no extant data on appendicular bone health in adult males with muscular dystrophy, and no comparison between the ambulatory and non-ambulatory forms of muscular dystrophy. It is possible that the lack of available data may be due to two predominate factors that of the cost and availability of peripheral quantitative computed tomography (pQCT) and dual-energy X-ray absorptiometry (DXA) scanners, alongside joint mobility issues that may be experienced when positioning individuals with muscle dystrophy during the scan. In contrast, portable quantitative ultrasound scanners (QUS) provide an estimate of bone mineral density based on speed of sound through regions of interest in the appendicular skeleton, without radiation, and allowing the analysis of individuals with limited mobility to be tested in situ. Although these QUS techniques are not suitable for providing a definitive clinical diagnosis of osteopenia, they show good agreement with pQCT measures of bone mineral density and measures of a bone’s material properties[14]; and provide a valid method for comparing appendicular bone health between population groups[15].
Therefore, the aim of the present study was to describe the bone health in the radius and tibia in adult males with four forms of human muscular dystrophy, compared to adult males without the dystrophic condition.
Material and methods
Participants
Fifty-six adult males volunteered for this study. Participants were grouped based on their dystrophic condition. The participant characteristics of each muscular dystrophy group and the non-dystrophic control group are described in Table 1. Participants with muscular dystrophy were recruited from, and tested at, The Neuromuscular Centre (Winsford, UK). All dystrophy participants were receiving weekly physiotherapy treatment involving passive mobility activities lasting approximately 1 hour. All control participants self-reported as being sedentary undertaking less than 1 hour of recreational physical activity; none of the participants were undertaking any structured, or regular exercise training regimes.
Table 1.
Participant demographics.
| CTRL | LG | FSH | Be | DMD | |
|---|---|---|---|---|---|
| n | 14 | 9 | 11 | 11 | 11 |
| Age (yrs) | 27.9 (10.8) | 36.7 (12.9) | 46.3 (10.3)C | 38.2 (11.7) | 25.4 (5.4)F |
| Age range (yrs) | 19-54 | 18-59 | 33-58 | 22-59 | 20-38 |
| Stature (m) | 1.82 (0.09) | 1.77 (0.10) | 1.84 (0.08) | 1.80 (0.08) | 1.67 (0.10)C,F,B |
| Mass (kg) | 83.3 (11.5) | 90.1 (20.5) | 91.5 (20.6) | 87.3 (15.6) | 67.0 (17.5)F,B,L |
| Grip strength (Kg) | 46.9 (9.1) | 19.8 (11.2)C | 23.6 (15.5)C | 21.5 (19.2)C | - |
| PASIPD | - | 11.7 (7.8) | 12.2 (11.8) | 18.4 (21.0) | 1.1 (0.6)F,B,L |
| Ambulatory/ non-ambulatory (n) | 14/0 | 4/5 | 9/2 | 7/4 | 0/11 |
CTRL = Control, LG = Limb Girdle, FSH = fascioscapulohumeral, Be = Becker’s, DMD = Duchenne. PASIPD = Physical activity score for individuals with physical disability.
denotes significant difference from CTRL,
denotes significant difference from FSH,
denotes significant difference from Be and,
denotes significant difference from LG. Data are presented as Mean (SD).
All procedures conformed to the standards set by the latest revision of the Declaration of Helsinki[16], and were approved by the local Ethics Committee of Manchester Metropolitan University, Cheshire. Written informed consent was obtained from all participants prior to inclusion in this study.
Procedures
Participants with muscular dystrophy were tested during a single visit to the Neuromuscular Centre. All tests were carried out prior to their regular physiotherapy session. Control participants were tested in the Muscle Function Lab (MMU-Cheshire, UK). During the single testing session, stature and mass were measured using a wall mounted stadiometer (Harpenden, Holtain Crymych, UK) and digital scales (Seca model 873, Seca, Germany) respectively. In the Dystrophic groups if standing stature could not be measured, arm span was recorded. In order to account for the known discrepancy between standing height and arm span measures, a correction was applied consistent with regression data from adult Caucasian males, the known error of making this correction is 3.5%[17]. Participant height is presented as this corrected value. Where necessary, digital seated scales were adopted for the non-ambulatory participants (6875, Detecto, Webb City, MO, USA). Measures of muscle size and bone health were obtained with all participants in a seated position. All measurements were taken from the participant’s self-reported dominant side. All participants completed a bone specific physical activity questionnaire (BPAQ) consistent with Weeks and Beck[18]. The BPAQ data was analysed using freeware developed by Weeks and Beck, and is presented as the “Total” BPAQ, a combination of current and past physical activity known to be significant predictors of bone strength and health[18]. The individuals with muscular dystrophy also completed a disability specific physical activity questionnaire (PASIPD – Physical Activity Score for Individuals with Physical Disabilities) to account for reduction in physical activity associated with various physical disabilities[19]. This encompasses a lower activity threshold and has subsequently been validated as a reliable outcome measure for physical activity in people with disability[20]. Aside from measures of mass and stature (as described above), all participants were tested using the same equipment regardless of testing location.
Corticosteroid therapy
As previously demonstrated, long-term steroid therapy (>30 months) is known to adversely affect bone mineral density (BMD)[21]. Based on self-report, none of the individuals of the DMD group were currently on long-term steroid therapy, with 2 individuals having discontinued long-term steroid therapy at least two years prior to enrolment on the study. No individuals of any other group were using, or had previously been prescribed, long-term steroid therapy for a period of more than 1 year.
Bone measurements
Bone ratio data (T and Z scores) were obtained using the Sunlight Omnisense™ ultrasound scanner (Sunlight Omnisense 8000p device, Tel Aviv, Israel), at two sites: the Distal Radius (DR), and at the Mid Shaft Tibia (MST).
Distal radius QUS scans were taken at 50% of forearm length, measured from the point of the elbow (olecranon) to the tip of the middle finger, with the wrist straight and the fingers extended. DR scans were recorded with the participant seated, elbow flexed at 90°, with a neutral hand rotation. The forearm was raised from the flat, resting surface using a foam support, and scans were recorded over the anterior region of the radius. MST scans were conducted at 50% of lower limb length, measured as the distance from the base of the heel (calcaneus) to the tip of the patella (proximal side), with the participants in a seated position. QUS scans were recorded over the medial anterior aspect of the tibia.
QUS measurements were taken by tilting the probe repeatedly over the surface of the bone, above the region of interest, in a 140° approximate arc, each arc taking approximately 5s. QUS uses fixed-point transmission techniques to measure amplitude-dependent speed of sound (SOS); QUS data is presented as T-score values, which can be calculated as: T-score (subject measurement - peak young adult mean)/(SD peak young adult mean). Z-Scores are calculated relative to age-matched normative data.
Tibialis anterior ACSA
TA muscle size is known to be associated with tibial bone density[11], therefore TA anatomical cross sectional area (TAACSA the area of the muscle at right angles to the longitudinal axis of the muscle) was measured following bone QUS scans. TAACSA was recorded using B-mode ultrasonography (myLab 25, Esaote, Genoa, Italy), using a linear array probe (7.5 MHz, 6 cm width). Scans were recorded at a TA length consistent with the previously established maximum ACSA of the TA, equivalent to 1/3rd of muscle length from the proximal origin of the muscle[22]. TA length was measured as the linear distance from the proximal origin (identified as the distal edge of the lateral tibial condyle using ultrasonography) to the distal TA myotendinous junction, identified through sagittal plane ultrasonography. TAACSA was recorded with the participant seated and their leg extended and supported under the ankle. At 1/3rd of TA muscle length echo-absorptive markers were placed in parallel, at intervals of 30 mm, from the lateral to the medial edge of the TA muscle. The ultrasound probe was held perpendicular to the TA muscle in the axial plane. The ultrasound probe was moved steadily over the echo-absorptive markers from the lateral to the medial edge of the muscle. Constant, light pressure was placed on the muscle during scanning to avoid compression of the muscle. The images were recorded in real time at a rate of 25 frames per second (DartfishPro, Fribourg, Switzerland). Digital imaging software was used to reconstruct image captures of the TA between the echo-absorptive markers (GNU Image Manipulation Program, www.gimp.org), from which the entire TA muscle was reconstructed on a digital canvas. Digital analysis software (ImageJ 1.45, National Institutes of Health, USA) was used to measure TAACSA. This method of calculating ACSA has previously been accepted as reliable and valid when compared to MRI in the Vastus Lateralis, with a reported interclass correlation between 0.998 and 0.999[23]. It should also be noted that in a number of the dystrophy participants digital reconstruction was unnecessary as the entire TAACSA was visible in a single ultrasound image.
Grip strength
Due to the complexity of the wrist and finger musculature, it was not possible to obtain suitable muscle size measurements using ultrasonography. Therefore, based on previous associations between grip strength and upper limb bone density[24], those individuals who were capable, completed a grip strength protocol.
An analogue handgrip dynamometer (TKK 5101 Grip-D, Takey, Tokyo, Japan) was used to assess grip strength following the bone and TA ultrasound scans. Of those participants (i.e. 33 of 56) that could produce a measureable grip force, three maximal attempts were made. Participants performed three, 5 second, maximal grip efforts in a seated position with the dominant hand, with the hand held in a relaxed position by their side. All three tests were separated by a one-minute duration, and the peak reading recorded. Test-retest reliability for this protocol has previously been reported as high, with low measurement error[25].
Statistics
IBM SPSS Statistics 21 software was used to analyse the data. All variables showed homogeneity of variance (Levene’s test, P>0.05), except for BPAQ and Washburn scores. Washburn scores and Age violated the parametric assumption of normal distribution (Shapiro-Wilk test, P<0.05), all other variables were normally distributed. Differences between the groups was analysed using a one-way between groups ANOVA with Bonferroni post hoc. Variables that violated normal distribution were compared between groups using the Kruskal Wallis test, with post-hoc Mann-Whitney U pairwise comparisons, where appropriate.
Pearson’s correlations were carried out to highlight any relationships between specific variables (e.g. Grip strength and T score DR; Grip strength and Z score DR; TA area and T score MST; TA area and Z score MST). Following significant correlations being observed for BPAQ with all T- and Z-scores, and Age with MST T- and Z-scores; one way ANCOVA was conducted to determine whether: 1) a difference between participant groups T- and Z scores remained whilst controlling for BPAQ and 2) Age has a significant influence on any of the group comparisons for MST.
Due to the fact that some values are close to zero, relative differences were not calculated for T and Z scores. All data are reported as means (SD). Statistically significant differences and/or associations were accepted at α≤0.05
Results
Participants
FSH participants were significantly older than CTRL and DMD participants (P<0.01, Table 1). When age was included as a covariable, only MST Z-scores were significantly influenced as a covariable (ANCOVA, P<0.05), and this is discussed in the bone section below. DMD participants had shorter stature than FSH, Be and CTRL participants (Table 1, P<0.01) and had significantly less body mass than LG, and FSH participants (Table 1, P<0.05).
Physical activity and disability
BPAQ was higher in CTRL compared to FSH, DMD and Be MD (Figure 1, P<0.05). DMD had lower physical activity levels than all other groups (Figure 1, P<0.05). There was no difference between the BPAQ of other muscular dystrophy groups.
Figure 1.
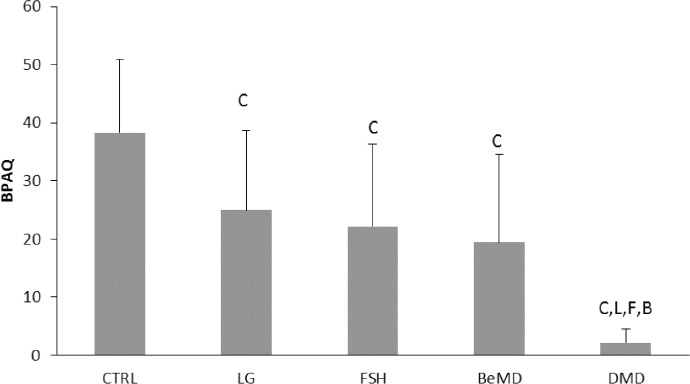
Bone Physical Activity Questionnaire (BPAQ) scores with and without muscular dystrophy. CTRL=Control, LG=Limb Girdle, FSH=fascioscapulohumeral, Be=Becker’s, DMD=Duchenne. C denotes significant difference from CTRL, F denotes significant difference from FSH, B denotes significant difference from Be and L denotes significant difference from LG (P<0.05). Data are presented as Mean (SD).
PASIPD was lower in DMD than all other Dystrophy groups (P<0.01, Table 1). FSH, LG and Be MD participants showed no difference in PASIPD.
Muscle size and strength
TAACSA was 35, 56, 55 and 59% smaller in Be, DMD, FSH and LG compared to CTRL (Figure 2, P<0.01). In addition, LG, FSH and DMD had a smaller TAACSA than Be (Figure 2, P<0.05). There was no difference in TAACSA between LG, FSH and DMD groups.
Figure 2.

Tibialis anterior Anatomical Cross sectional area (TAACSA) in adult males with and without muscular dystrophy. CTRL=Control, LG=Limb Girdle, FSH=fascioscapulohumeral, Be=Becker’s, DMD=Duchenne. C denotes significant difference from CTRL, B denotes significant difference from Be, P<0.05. Data are presented as Mean (SD).
Compared to the CTRL participants Grip strength was 50, 58 and 54% lower in FSH, LG and Be participants, respectively (Table 1, P<0.01). DMD participants were unable to produce a measureable grip force, and were excluded from further grip strength analysis. There was no difference between FSH, LG and Be participants in terms of grip strength.
In terms of physical activity data in the dystrophy participants, BPAQ was not correlated with TAACSA or grip strength, whereas the PASIPD showed significant correlations with both TAACSA (r=0.458, P<0.01) and grip strength (r=0.651, P<0.01).
Bone QUS
Across the upper and lower limb, there was no difference in T and Z scores between the CTRL and FSH and LG participants (Figure 3). The distal radius T and Z scores were lower in DMD than the CTRL participants, and were also lower in the DMD participants than FSH, LG and Be MD (P<0.05, Figure 3A & B).
Figure 3.
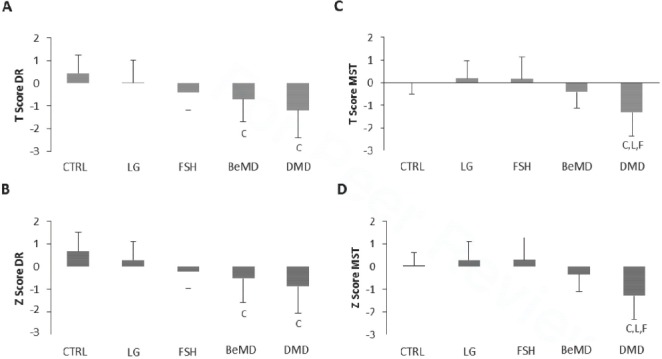
QUS bone health data in adult males with and without muscular dystrophy. Figure A=T score distal radius, B=Z score distal radius, C=T score Mid shaft tibia, D=Z score MST. CTRL=Control, LG=Limb Girdle, FSH=fascioscapulohumeral, Be=Becker’s, DMD=Duchenne. C denotes significant difference from CTRL, F denotes significant difference from FSH, B denotes significant difference from Be and L denotes significant difference from LG. Data are presented as Mean (SD).
There was a significant effect of age on MST T- and Z-scores (r=0.360, and 0.398 respectively, both P<0.01). ANCOVA showed a significant influence of Age as a co-variable for MST Z-scores only (P<0.05), however group difference remained when age was controlled for (P<0.001).
When controlling for BPAQ, ANCOVA showed no significant difference between groups for any of the measures using bone QUS (Z & T-scores from MST and DR were all >0.05). When all dystrophy participants were pooled (n=42) BPAQ was found to correlate significantly with bone QUS at both the DR and MST (Table 2). PASIPD did not correlate with any of the bone QUS data in the dystrophy participants.
Abbreviations
| TAACSA = Tibialis anterior Anatomical cross sectional area |
| FSH = Fascioscapulohumeral |
| Be = Becker’s |
| DMD = Duchenne muscular dystrophy |
| LG = Limb Girdle |
| MD = Muscle dystrophy |
| CTRL = Control |
| BPAQ = Bones specific physical activity questionnaire |
| PASIPD = Physical Activity Score for Individuals with Physical Disabilities |
| DR = Distal radius |
| MST = mid-shaft tibia |
| QUS = quantitative ultrasound |
Table 2.
Bone QUS, muscle size and strength and physical activity in participants with muscular dystrophy.
| Variable | Activity Questionnaire | Pearson’s Correlation | P |
|---|---|---|---|
| DR T-score | BPAQ | 0.407 | <0.01 |
| DR Z-Score | BPAQ | 0.423 | <0.01 |
| MST T-score | BPAQ | 0.390 | <0.05 |
| MST Z-score | BPAQ | 0.387 | <0.05 |
| TAACSA | PASIPD | 0.458 | <0.01 |
| Grip strength* | PASIPD | 0.651 | <0.01 |
Bone QUS recorded from the Distal radius (DR) and Mid-shaft tibia (MST) in the pooled dystrophy participants (n = 42). Tibialis Anterior Cross sectional area (TAACSA).
Duchenne muscular dystrophy participants omitted.
Due to the mixed ambulatory status of some of the dystrophy groups, bone QUS data is also presented as ambulatory compared to non-ambulatory participant groups. Significantly lower T- and Z- scores are observed in the DR of non-ambulatory participants, compared to CTRL and Ambulatory participants with MD (Figure 4, P<0.05). There was no difference in MST between ambulatory, non-ambulatory and CTRL participants (Figure 4).
Figure 4.
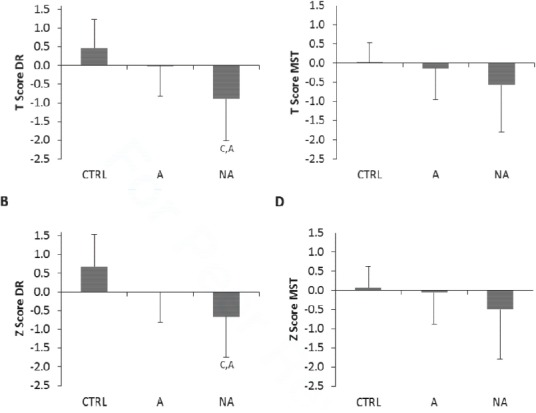
QUS bone health in adult males with and without (CTRL) muscular dystrophy who are ambulatory (A, n=22) and Non-Ambulatory (NA, n=20). Figure A=T score DR, B=Z score DR, C=T score MST, D=Z score MST. C denotes significant difference from CTRL P<0.05, A denotes significant difference from Ambulatory P<0.05. Data are presented as Mean (SD).
Discussion
The main findings of the present study showed that bone health assessed using QUS was negatively affected in adult males with Becker’s and Duchenne muscle dystrophies. Furthermore, Z- and T scores of the MST and DR are influenced by physical activity (BPAQ) rather than ambulatory status; indeed when included as a covariable, BPAQ removed all group differences between the dystrophy groups. An additional novel finding from our adult males with muscular dystrophy, was that significant atrophy previously observed in some, but not all childhood dystrophies, is present in the TA of adult males with FSH, Be, LG and Duchenne muscular dystrophy.
The present understanding of bone health in muscular dystrophies is primarily focused on DMD[12,21], and is understandably mainly from children; there are presently no studies to date that includes adult participants, and only a few with participants up to 18 yrs old[26]. As would be expected given the severity of the impairments experienced by children with DMD, Z-scores are consistently lower than CTRLs and for the femur range from -1.6 to 3.9, depending on the ambulatory status of the participants[27]. Consistent with previous data, the present study has observed significantly lower T- and Z- values from the DR and MST in adults with DMD compared to controls. Further to previous reports on DMD, the present data has also observed lower T- and Z-scores from the distal radius of adult males with Becker’s muscle dystrophy. In contrast to the more impaired Be MD and DMD participants, the present data showed FSH and LG dystrophies to be no different in bone QUS from CTRL.
The importance of childhood physical activity on bone health is well established in non-dystrophic populations[18]. Consistent with previous[18], we have shown an influence of BPAQ on QUS T- and Z-scores. Specifically, the present study has shown that when physical activity is used as a covariant, there is no significant difference in DR and MST T- and Z-scores between CTRLs and the FSH, LG, Be and Duchenne muscular dystrophies; and that significant correlations exist between BPAQ and T- and Z-scores from the MST and DR in the pooled dystrophy populations.
As previously mentioned, there is limited bone health data from adult muscular dystrophy populations; however, there are some studies that have reported higher bone fracture risks in adults with muscular dystrophy[28]. The present data would be consistent with the increased likelihood of fracture incidence, such that our observations of lower muscle mass (discussed below), and lower bone health scores in the least active dystrophies (DMD and BeMD) may be influential to fracture risk. There are numerous variables that contribute to fracture risk in muscle dystrophy[13]; the relationship between BPAQ and bone health data from the present study, would suggest that promoting bone loading through physical activity if, or whilst this is possible, may act to preserve bone health and reduce fracture risk as the dystrophic condition develops. Indeed, in the present data, we observed no association between current physical activity (PASIPD-7 day assessment of physical activity) and T- or Z- scores; whereas we see significant associations between lifespan physical activity (assessed through BPAQ), associated with T- and Z scores in the muscle dystrophies. This would certainly suggest the importance of some form of physical activity in adolescence on lower limb bone health in individuals with muscular dystrophy.
In addition to physical activity, muscle mass in proximity to the bone is known to be influential to BMD through the mechanostat[11]. In the present study we observed no correlations between lower limb bone health scores and TAACSA. It is possible that the confounding influence of pseudohypertrophy in dystrophic populations and the unique patterns of loading and physical activity within this population, may interfere with the previously observed relationship between lower leg muscle mass and MST bone health[11]. Similar to the TAACSA and MST T- and Z-scores, in the present study there was found to be no association between grip strength and DR T- and Z-scores in the Be, FSH and LG participants. Previously, grip strength and bone health have been associated[24], in the present dystrophic population groups the high coefficient of variation from the grip data (e.g. 90% in BeMD vs 19% in CTRL), and low absolute strength (approximately 54% weaker), may render grip strength redundant as a predictive measure of bone health in adult males with muscular dystrophy. Regardless of the high variability, the only previously published strength comparisons in dystrophic populations are from the knee extensors of 4-10 yr old boys with DMD[29]. Our data shows that grip strength is lower by ~55% in adult males with FSH, LG and Be muscle dystrophies, compared to non-dystrophic CTRLs. Grip strength is employed for its ease of use and accessibility, particularly in clinical population groups[30], however its relevance to functional mobility/day-to-day physical independence, may be questioned above more meaningful measures of lower limb strength. It is nonetheless notable that although there is no present data from grip strength in muscular dystrophy, it has previously been shown to be associated with mobility, lower limb strength and muscle mass in the elderly[31]. Furthermore, our observed correlation between grip strength and current physical activity (assessed through PASIPD) in our participants with muscle dystrophy, suggest that, as with muscle atrophy in ageing[31], grip strength may be a cost effective, and easily accessible measure of the progression of the dystrophic condition, and the general day-to-day functional capacity of the individual.
Whilst we observed no association between MST T- and Z scores with TAACSA (see earlier in discussion), the TAACSA was found to be significantly smaller in the present adult males with muscular dystrophy. There is surprisingly limited data on appendicular muscle mass in adults with muscular dystrophy, such that the only extant, direct comparisons of muscle mass between adults with and without muscular dystrophy is from the GM in DMD[10]. Similar to the GM, the present data shows significant atrophy in the TA in FSH, Be, LG and Duchenne muscular dystrophies. We have previously discussed the novelty of these findings in context of the apparent increase in muscle size or “pseudohypertrophy” observed in children with DMD[10]. As with other atrophic conditions e.g. bed rest[32] and ageing[33], the present muscle size data from the MD participants, is positively associated with physical activity assessed through the PASIPD questionnaire. It would be too speculative to categorically state that increased physical activity is beneficial to muscle mass given the complex nature of each of the muscular dystrophies assessed within the present study. However, given the possible contraindications, and equivocal findings of some physical training studies in MD[34], investigating standing time, and breaks in sedentary behaviour, may be more meaningful in those MD conditions in which weight bearing is possible. Indeed, breaks in sedentary behaviour and increased daily physical activity, have been suggested to be independent (though closely associated) modulators the health of otherwise sedentary individuals[35].
Despite the significant findings within the present study, it was somewhat surprising that, given the physical impairments experienced by these participants (the present participants scored in the lowest 45% of disabled participants tested for PASIPD[19]), only BeMD and DMD participants showed significantly lower bone health scores. There are two limitations to consider within the present study that may have influenced the extent to which bone health scores were impaired compared to controls; these are participant numbers and measurement technique. Group sizes of between 9-14 participants are unlikely to be a factor in the present study, as post hoc power analysis revealed that all bone health scores were suitably powered (0.91-0.94, for T and Z scores of DR and MST) with large effects sizes (0.57-0.59, G*Power, University of Kiel, Germany). It is likely however, that within the present study the sensitivity of discerning lower bone health would be enhanced with a more sensitive and direct measure of bone mineral density. As mentioned previously, QUS techniques show good agreement with pQCT measures of bone mineral density and measures of a bone’s material properties[14], and provide a valid method for comparing appendicular bone health between population groups[15]. QUS cannot however, provide an absolute measure of bone mineral density, bone mineral content or bone geometry, and cannot distinguish cortical or trabecular bone, such as with DXA or PQCT[36]. Therefore, we would expect that the use of these latter techniques would confirm our observations from BeMD and DMD, but may identify further clinical observations in LG and FSH MD groups not identified using QUS. Further studies using PQCT and DXA would be essential in providing greater understanding of fracture risks in these participant groups[28].
Conclusion
The present study, has provided novel adult data comparisons of bone and muscle parameters in four types of muscular dystrophies against a non-dystrophic control group. It can be concluded that, muscle size, strength, and bone health is lower in adult males with muscle dystrophy compared to adult males without MD; and the extent of this is likely to be determined by previous physical activity.
Acknowledgements
Mr J Smith received a postgraduate studentship from Dream it, Believe It, Achieve it (Northwich, UK. Registered Charity Number: 1153116).
Footnotes
The authors have no conflict of interest.
Edited by: F. Rauch
References
- 1.Emery AE. The muscular dystrophies. Lancet. 2002;9307:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Bushby K. The limb-girdle muscular dystrophies: diagnostic guidelines. Eur J Paediatr Neurol. 1999;2:53–58. doi: 10.1053/ejpn.1999.0182. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;9869:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 4.Tawil R, Kissel JT, Heatwole C, et al. Evidence-based guideline summary: Evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy. Neurology. 2015;4:357–364. doi: 10.1212/WNL.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lue Y-J, Su C-Y, Yang R-C, et al. Development and validation of a muscular dystrophy-specific functional rating scale. Clin Rehab. 2006;9:804–817. doi: 10.1177/0269215506070809. [DOI] [PubMed] [Google Scholar]
- 6.Dellorusso C, Crawford RW, Chamberlain JS, et al. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;5:467–75. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- 7.Lovering RM, Shah SB, Pratt SJ, et al. Architecture of healthy and dystrophic muscles detected by optical coherence tomography. Muscle Nerve. 2013;4:588–90. doi: 10.1002/mus.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones DA, Round JM, Edwards RH, et al. Size and composition of the calf and quadriceps muscles in Duchenne muscular dystrophy. A tomographic and histochemical study. J Neurol Sci. 1983;2:307–22. doi: 10.1016/0022-510x(83)90071-0. [DOI] [PubMed] [Google Scholar]
- 9.Lynch GS, Hinkle RT, Chamberlain JS, et al. Force and power output of fast and slow skeletal muscles from mdx mice 6-28 months old. J Physiol. 2001;Pt 2:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse CI, Smith J, Denny A, et al. Gastrocnemius medialis muscle architecture and physiological cross sectional area in adult males with Duchenne muscular dystrophy. J Musculoskelet Neuronal Interact. 2015;2:154–160. [PMC free article] [PubMed] [Google Scholar]
- 11.Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;2:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi ML, Mazzanti A, Galbiati E, et al. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos Int. 2003;9:761–7. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Glerup H, Steffensen BF, et al. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J Rehabil Med. 2001;4:150–5. [PubMed] [Google Scholar]
- 14.Lee SC, Coan BS, Bouxsein ML. Tibial ultrasound velocity measured in situ predicts the material properties of tibial cortical bone. Bone. 1997;1:119–25. doi: 10.1016/s8756-3282(97)00076-8. [DOI] [PubMed] [Google Scholar]
- 15.Cook RB, Collins D, Tucker J, et al. The ability of peripheral quantitative ultrasound to identify patients with low bone mineral density in the hip or spine. Ultrasound Med Biol. 2005;5:625–32. doi: 10.1016/j.ultrasmedbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;20:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 17.Reeves S, Varakamin C, Henry C. The relationship between arm-span measurement and height with special reference to gender and ethnicity. Eur J Clin Nutr. 1996;6:398–400. [PubMed] [Google Scholar]
- 18.Weeks BK, Beck BR. The BPAQ: a bone-specific physical activity assessment instrument. Osteoporos Int. 2008;11:1567–1577. doi: 10.1007/s00198-008-0606-2. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, Zhu W, McAuley E, et al. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;2:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 20.van der Ploeg H, Streppel K, van der Beek A, et al. The physical activity scale for individuals with physical disabilities: test-retest reliability and comparison with two accelerometers. Arch Phys Med Rehabil. 2005:41–56. doi: 10.1123/jpah.4.1.96. [DOI] [PubMed] [Google Scholar]
- 21.Crabtree NJ, Roper H, McMurchie H, et al. Regional changes in bone area and bone mineral content in boys with Duchenne muscular dystrophy receiving corticosteroid therapy. J Pediatric. 2010;3:450–455.e1. doi: 10.1016/j.jpeds.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga T, Roy R, Shellock F, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;6:926–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- 23.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;1:116–8. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji S, Tsunoda N, Yata H, et al. Relation between grip strength and radial bone mineral density in young athletes. Arch Phys Med Rehabil. 1995;3:234–238. doi: 10.1016/s0003-9993(95)80607-5. [DOI] [PubMed] [Google Scholar]
- 25.Shechtman O, Gestewitz L, Kimble C. Reliability and validity of the DynEx dynamometer. J Hand Ther. 2005;3:339–47. doi: 10.1197/j.jht.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Khatri IA, Chaudhry US, Seikaly MG, et al. Low bone mineral density in spinal muscular atrophy. J Clin Neur Dis. 2008;1:11–17. doi: 10.1097/CND.0b013e318183e0fa. [DOI] [PubMed] [Google Scholar]
- 27.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop. 2000;1:71–4. [PubMed] [Google Scholar]
- 28.Pouwels S, de Boer A, Leufkens H, et al. Risk of fracture in patients with muscular dystrophies. Osteoporos Int. 2014;2:509–518. doi: 10.1007/s00198-013-2442-2. [DOI] [PubMed] [Google Scholar]
- 29.Marden FA, Connolly AM, Siegel MJ, et al. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;3:140–148. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;5:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 32.Rittweger J, Frost HM, Schiessl H, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;6:1019–29. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;2:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 34.van der Kooi EL, Vogels OJ, van Asseldonk RJ, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology. 2004;4:702–8. doi: 10.1212/01.wnl.0000134660.30793.1f. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay MS, Colley RC, Saunders TJ, et al. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;6:725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 36.Dowthwaite JN, Flowers PP, Scerpella TA. Agreement between pQCT-and DXA-derived indices of bone geometry, density, and theoretical strength in females of varying age, maturity, and physical activity. J Bone Miner Res. 2011;6:1349–1357. doi: 10.1002/jbmr.322. [DOI] [PMC free article] [PubMed] [Google Scholar]


