Abstract
For a long time, carbon monoxide (CO) was known for its toxic effect on organisms. But there are still many things left to discover on that molecule. CO is formed directly in the body by the enzymatic activity of heme oxygenase (HO). CO plays an important role in many physiological processes, such as cell protections (against various stress factors), and the regulation of metabolic processes. Recent research proves that CO also operates in the female reproductive system. At the centre of interest is the importance of CO for gestation. During the gestation period, CO is an important element affecting the proper function of the feto-placental unit and generally affects fetal survivability rates. Gestation is one of the most important processes of successful reproduction, although there are more relevant processes that need to be researched. While already proven that CO influences steroidogenesis and the corpus luteum survivability rate, our knowledge concerning the function and importance of CO in the reproductive system is still relatively limited. As an example, our knowledge of CO function in an oocyte, the most important cell for reproduction, is almost non-existent. The aim of this review is to summarize our current knowledge concerning the function of CO in the female reproductive system.
Keywords: Carbon monoxide, heme oxygenase, reproduction, oocyte, gestation
Introduction
For decades, only peptides, proteins, lipid derivatives and nucleic acid were considered factors regulating physiological functions. Gaseous molecules, except for oxygen, were considered unimportant. In the eighties it was demonstrated that the factor responsible for vascular smooth muscle cell relaxation (endothelial cell derived relaxing factor, EDRF) is nitric oxide (NO) [1,2]. The significance of the discovery of the nitric oxide signalling-function had a huge impact on biomedical research. Soon after proving the signalling function of NO, it was discovered that carbon monoxide (CO) also has an important role in the proper function of the organism.
The fact that the organism produces CO as a by-product of heme degradation catalysed by the microsomal enzyme heme oxygenase (HO) was known long before the discovery of the NO signalling function [3,4]. Endogenously produced CO was considered only a waste product, with negative effects in high concentrations [5]. Shortly after the demonstration of the NO signalling function, the understanding of CO’s importance to the organism changed, because the physiological role of CO in neurotransmission [6] and the relaxation of the anal sphincter [7] was identified.
Recently it was discovered that low levels of CO are an important factor for the proper functioning of the body. It is now known that the HO/CO system regulates many cellular functions and contributes to cellular protection from the negative effects of stress [8]. Problems in the function of the HO/CO system lead to health complications and even reproductive failure. However, the significance of HO/CO in reproduction is not yet fully understood.
Endogenous production of CO
The reaction catalysed by HO is the main endogenous source of CO. HO catalyses first and is the rate-limiting step in the oxidative degradation of heme [3,4] (Figure 1).
Figure 1.
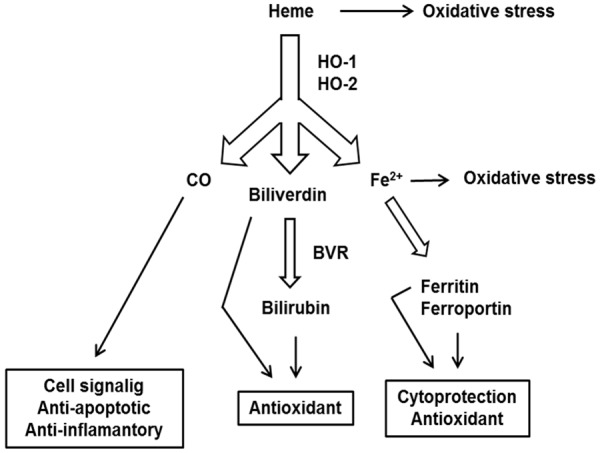
HO catabolic reactions. Both HO isoforms catalyse the oxidative degradation of heme, giving rise to CO, Fe2+ and biliverdin. Biliverdin is subsequently reduced to bilirubin by the enzyme biliverdin reductase (BVR). Excess of heme and Fe2+ induces oxidative stress. Each product of heme catabolism has a different function in the cell. CO influences a variety of signalling pathways, generally has cytoprotective, antiapoptotic and anti-inflammatory properties. Biliverdin and bilirubin are important antioxidants. Free Fe2+ induces the expression of ferritin and ferroportin, which remove redox-active Fe2+.
There are two known isoforms of HO, HO-1 and HO-2. Both catalyse identical biochemical reactions of heme transformation to biliverdin-Ixα. Both proteins contain a highly conserved sequence of 24 amino acids, which are considered to be a binding site for heme [9,10] and both isoforms share a similar hydrophobic region, which serves for the membrane attachment [11,12]. However, both isoforms differ in their enzyme kinetics, thermostability and immunoreactivity [13].
Inducible isoform HO-1 (~32 kDa) is a protein attached to the endoplasmic reticulum membrane [14]. In response to stressors, changes in HO-1 location may occur, because HO-1 is translocated to lipid rafts (caveolae), mitochondria and nucleus [15]. Redistribution of HO-1 has a signal function, e.g. in the nucleus it is involved in the regulation of transcription factors (e.g. nuclear factor erythroid 2-related factor 2), and in cell protection against oxidative stress [16].
Under physiological conditions, HO-1 is mainly expressed in tissues which participate in the degradation of erytrocytes, such as the spleen or bone marrow. In other tissues, the level of HO-1 is usually low, but its expression can be stimulated by a wide range of stressors (e.g. oxidative and thermal stress and hypoxia), the increased expression of which has a cytoprotective effect [17].
Induction of HO-1 expression is controlled by several signalling pathways, e.g. mitogen-activated protein kinases (MAPK) or nuclear factor κB [18]. Also the enzymatically inactive form of HO-1 has an ability to suppress oxidative stress [19]. The mechanism of action of enzymatically inactive HO-1 is not yet fully understood, but it is known that HO-1 can directly bind to other proteins and thereby alter their activity [20].
HO-2 (~32 kDa) is a constitutively expressed isoform with its highest expression in the brain and testes [10]. Similarly to HO-1, HO-2 is also bound to the endoplasmic reticulum membrane. HO-2 can also be localised in outer nuclear membranes [21] and endosomes [22]. HO-2 does not respond to transcriptional activation by stress factors, and the only known activators of HO-2 gene expression are glucocorticoids [10,23]. HO-2 is responsible for the stable production of CO and creates a barrier against cell damage e.g. by oxidative stress [24]. It is assumed that HO-2 also operates as an oxygen level sensor that is involved in the protective response of cells to hypoxia [25].
HO-3 was an additionally described isoform besides HO-1 and HO-2. However, HO-3 is probably not expressed in an in vivo condition and, due to the high homology of HO-3 and HO-2 and lack of introns in the HO-3 gene, HO-3 is accepted as a pseudogene from the HO-2 transcript [26].
HO/CO signalling pathway
Carbon monoxide affects cell function by binding structures containing transition metals (e.g. Fe, Cu) [27], of which the best known is heme. A large number of proteins (e.g. soluble guanylyl cyclase (sGC), cyclooxygenase, cytochrome P450, cytochrome c oxidase, inducible nitric oxide synthase (iNOS)) contain the heme molecule and hence there is also a wide range of potential targets for CO [28]. Conformational changes occur after the binding of CO to ferrous ion in hemoprotein [29]. Figure 2 summarize HO/CO signalling pathway.
Figure 2.
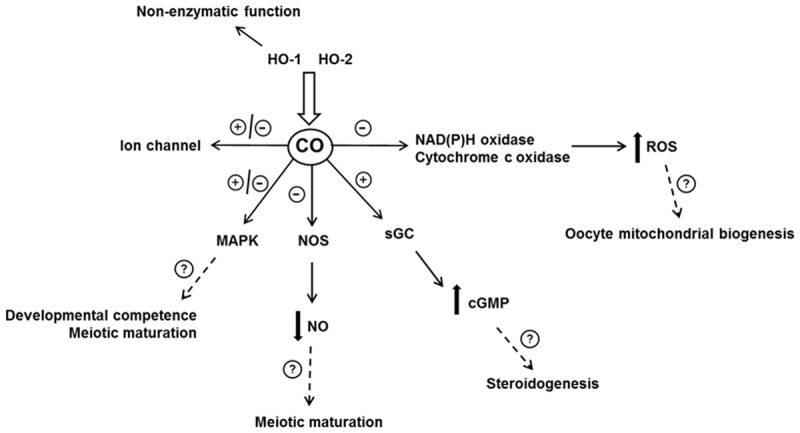
HO/CO signaling pathway. In the cell, HO/CO acts through several mechanisms. Besides catalytic function, HO has also a non-enzymatic function. Binding of HO to other protein (e.q. transcription factors) modulate their activity. CO operates through activation/inhibition of several pathways. By modulation of these pathways CO could affect various function of the female reproductive system, for example functions in relation to the oogenesis. See text for details.
Soluble guanylyl cyclase is a common target for CO and NO. Binding of CO or NO to the heme group of sGC leads to increased formation of 3’,5’-cyclic guanosine monophosphate (cGMP). A change in cGMP levels consequently affects signalling pathways [30,31]. CO is involved in regulation of vascular tone and neurotransmission through the sGC/cGMP signalling pathway [17].
In the case of NO in the reproductive system, e.g. NO by sGC/cGMP signalling pathway inhibits ovarian steroidogenesis [32,33] and contributes to the maintenance of oocytes at the first meiotic block [34]. To date, there are no known sGC/cGMP mediated effects of CO on the female reproductive system, but e.g. because it is known that HO/CO affect steroidogenesis [35], this CO effect may be mediated through sGC/cGMP. However, CO is a considerably weaker sGC activator compared with NO and therefore CO is considered rather an endogenous modulator of the NO/sGMP signalling pathway [30,36].
The interaction between CO and NO not only takes place in the competitive binding to effector proteins, but also in the direct regulation of NOS or HO activity. Binding of CO to the heme group of iNOS leads to reduced iNOS activity and hence to the reduced production of NO [37]. On the other hand, NO increases the expression of HO [38]. Due to the interaction between HO/CO and NO/NOS, CO is considered as a feedback inhibitor of NOS that reduces the overproduction of NO and the associated oxidative stress. Changes in NO levels regulate oocyte meiotic maturation.
The correct course of meiotic maturation is crucial for the formation of fertilisable and developmentally competent oocytes. Low levels of NO stimulate meiotic maturation and, conversely, high levels of NO maintain meiotic block and further lead to developmental disorders [39,40]. The HO/CO system may be a controller of oocyte NO levels and therefore also a regulator of meiotic maturation. However, this possibility still awaits clarification.
CO increases ROS production by binding to mitochondrial cytochrome c oxidase and/or to the plasma membrane NAD(P)H oxidase. Low levels of ROS produced in this way act as an important second messenger [41]. Electron leaks from the electron transport chain with the subsequent ROS formation are caused by binding of CO to cytochrome c oxidase. ROS can then affect different signalling pathways [42,43].
CO-induced ROS inhibits smooth muscle cells’ proliferation through inhibition of ERK 1/2 kinases and by reducing the expression of cyclin D [43]. In addition, for example, CO-induced ROS increases mitochondria biogenesis via activation of transcription factors’ nuclear respiratory factor-1 (Nrf-1), Nrf-2 and gamma-coactivator-1α. Mitochondrial biogenesis allows cells to replace damaged mitochondria and cope with periods of increased metabolic demands [44,45]. In the case of oocyte, the reduced number of mitochondria is associated with decreased fertilisation ability [46].
The question arises whether the exogenous application of CO could improve fertilisation percentages through ROS/mitochondrial biogenesis. The answer still awaits clarification. The overall effect of a temporary slight increase of ROS production is positive, because it initiates a series of processes, such as induction of antioxidant enzymes and activation of cytoprotective genes. This leads to the overall increase in cell resistance against the effects of stressors [47]. ROS are also involved in the regulation of the meiotic cycle. The slight increase in ROS levels promotes meiotic maturation and, conversely, the cell-permeable antioxidants inhibit meiotic maturation [48]. It is necessary to clarify whether CO affects meiotic maturation.
It is proven that CO acts on cell function through MAPK (p38, ERK 1/2 and JNK). Modulation of the MAPK signalling pathway via CO is responsible for cytoprotective, anti-inflammatory, anti-apoptotic and anti-proliferative properties of CO [17,49,50]. Because the CO is unable to directly bind to MAPK protein, it must influence MAPK activity via other proteins or signalling pathways. For example, in the case of p38, it is assumed that CO up-regulates p38 by transient increase of ROS level [51] and/or via the sGC/cGMP signalling pathway [52].
In mammalian oocyte, the p38 is involved in spindle apparatus assembly and function, whereby defects in spindle apparatus lead to cell cycle arrest [53]. p38 is also involved in maintenance of the second meiotic block [54]. In the case of in vitro conditions, it is demonstrated that transient delay of meiotic maturation resumption leads to improved developmental competence [55,56]. In accordance with those facts, CO could be a promising regulator of meiotic maturation.
The functions of a wide variety of ion channels for K+, Na+ and Ca2+ are regulated by CO. Activation or inhibition of ion channels via CO regulates various physiological functions. For example, CO causes vasodilation by increasing activity of large conductance calcium-activated potassium channels (BKCa) and has neuroprotective effects due to inhibition of potassium voltage-gated channel subfamily B member 1 (KCNB1) [57,58].
In the uterine muscle, BKCa contributes to the maintenance of uterine quiescence during pregnancy, and CO is also important in the transition to a more contractile state at the onset of labour [59]. CO, through activation of BKCa may contribute to the maintenance of pregnancy as well as regulate the onset of labour.
HO/CO and the female reproductive system
The presence of HO is demonstrated in the ovaries [35], uterus and placenta [60-62], in which the distribution of isoforms differs according to the cell type. In the ovary, both isoforms are localised in the corpus luteum (CL) and follicular cells. In the ovarian stroma, only HO-2 is present [35,63,64]. The presence of HO-1 mRNA is shown in mice oocytes [65]. In porcine oocytes, HO-1 mRNA as well as HO-2 mRNA and also HO-1 and HO-2 proteins are localised (our unpublished results).
In the ovaries and uterus, as well as in other tissues, exposure to stressors (e.g. oxidative stress, excessive accumulation of free heme) leads to the increased expression of HO-1 [63,66-68]. Expression of HO is also affected by changes in hormone levels and, therefore, expression of HO fluctuates during the oestrous cycle and pregnancy. In the uterus, HO-1 expression positively correlates with increased levels of oestrogens and progesterone. Expression of HO-2 positively correlates only with a level of progesterone [67-69].
During the mouse oestrous cycle, significant increase in expression of HO-1 occurs in the oestrus phase, when a surge of progesterone occurs [68]. If HO activity is inhibited by HO inhibitor chromium mesoporphyrin (CrMP), there is a significantly reduced occurrence of the oestrus phase of the oestrous cycle [70]. The reason for the increased expression of HO-1 in the uterus is probably to protect the embryo from an excess of free heme and an improper inflammatory response, as both processes have proven deleterious effects [68,71,72]. This assumption is confirmed by the fact that the increased expression of HO-1 in the uterus correlates with a decrease in the level of free heme. For these reasons, HO-1 activity is considered as crucial for the maintenance of an optimal environment for nidation and implantation [68].
Changes in HO activity during the oestrous cycle can affect the development and survivability of follicles. In the ovaries, activation of inflammatory processes and the release of heme are associated with ovulation [71,73] and, as well as in the uterus, an increase of HO-1 activity may lead to protection against excessive development of these harmful processes in the ovaries and in the oestrus phase of the oestrous cycle [72,74]. Indeed, in the case of HO-1-deficient mice, the decreased production of oocytes is demonstrated, which indicates a disturbance in the process of ovulation.
Oocytes obtained from HO-1-deficient mice have decreased fertilisation ability after in vitro fertilisation. Increased level of CL cell apoptosis also occurs in HO-1-deficient mice [74] when such an increase leads to a decreased ability to produce progesterone [75]. Decreased production of progesterone is also observed in case HO activity is inhibited by CrMP [35]. Up-regulation of HO-1 leads to an increased production of CO [76] that may subsequently activate the signalling pathway with cytoprotective effects [8]. Conversely, the state of HO-1 deficiency leads to the decreased production of CO [77], causing increased apoptosis [78]. The HO/CO pathway is therefore important in preventing functional disturbances of the ovary.
Fully functional granulosa cells surrounding oocyte are important for the development of the ability of oocyte to be fertilised. The rate of HO expression fluctuates along with the state of granulosa cells. In healthy follicles, there is a low level of HO-1 expression, but the level of HO-1 expression significantly increases in atretic follicles [64]. In the case of HO-2, there is an opposite trend in the level of expression. In healthy follicles, a significant level of HO-2 expression is detected, but in atretic follicles, it is low. High levels of HO-1 expression in granulosa cells from atretic follicles are considered to be a consequence of the action of stress factors [64]. The fact that the action of stressors leads to an increased level of HO-1 expression is often used as a marker for the exposure of cells to e.g. oxidative stress.
Bergandi et al. [79] used the level of HO-1 expression in combination with the level of iNOS expression as a marker of oocytes’ competence to be fertilised. In cumulus cells from unfertilised oocytes, a higher level of expression of both HO-1 and iNOS was observed. The authors explain the results as that of oxidative stress affecting granulosa cells, causing an increased level of HO-1 and iNOS expression, as well as the decreased fertilisation ability of oocyte. Pfeiffer et al. [65] also used HO-1 as one of the markers whose differential content size in the oocyte may affect the quality of embryos.
The importance of HO for the proper function of female reproductive organs is obvious (Table 1) summarize the female reproductive functions which may have relationship with HO/CO) and therefore the question arises whether exogenous HO up-regulation or CO delivery could have a positive effect. Clinical application in treatment of e.g. the various inflammatory conditions in the body is now being intensively studied [17]. The relationship between HO activity and steroidogenesis is supported by several works.
Table 1.
The female reproductive system functions which may have relationship with HO/CO based on so far published results. The table does not include functions related to pregnancy
| Processes | References |
|---|---|
| Steroidogenesis | 35, 67-69, 70, 74, 95 |
| CL maintenance | 74 |
| Oestrous cycle | 35, 68, 70, 74, 80 |
| Ovulation | 74 |
| Fertilization ability | 74 |
| Protection against stress factors | 74, 79, 80 |
| Function granulosa cells | 64, 79 |
Application of hemin (HO activator) to rats leads to increased synthesis of androstenedione and oestradiol. Treatment by an HO inhibitor leads to reduced synthesis of progesterone and androstenedione. The synthesis of oestrogens remains unchanged. The reason for various changes in steroidogenesis may be a different mode of action of HO/CO on different steroidogenesis pathways. Together with changes in hormone levels after the application of an HO inhibitor, changes in the oestrous cycle also occur simultaneously, leading to shortened length of the oestrus phase of the oestrous cycle [35,70].
In contrast, HO-1 deficiency in mice does not lead to changes in the level of progesterone or oestrogens during pregnancy. Although increased activity of HO (HO-1 isoform) occurs during the oestrous cycle in response to oestrogens, HO-1 deficiency in mice simply does not affect levels of sex hormones [80]. The possible reason for the various effects of HO-1 deficiency and HO inhibitor may be that CrMP is a nonspecific inhibitor of both HO isoforms. Also, the significance of HO-1 during the oestrous cycle or pregnancy may vary. In fact, HO-1 deficiency enhances apoptosis of the CL cell, also suggesting a reduced ability to produce progesterone [74].
However, it is unlikely that modulation of HO activity would be used as an effective method of affecting the oestrous cycle. In accordance with the fact that the formation of oocyte which is fertilisable and developmentally competent is essential for reproduction, the question arises whether it would be possible to influence oocyte quality by modulating HO activity.
Role of HO/CO during pregnancy
For the successful development of the fetus, it is necessary for hemodynamic and immune changes to occur during pregnancy. If these changes do not occur correctly, the risk of failures in feto-maternal communication and connection is increased. Finally, these complications result in impaired fertility. Compared with other processes of the female reproductive system, the HO/CO system is best studied in pregnancy and several reviews summarize the link between HO/CO and pregnancy [81-85]. Figure 3 summarizes significance of HO/CO during pregnancy.
Figure 3.
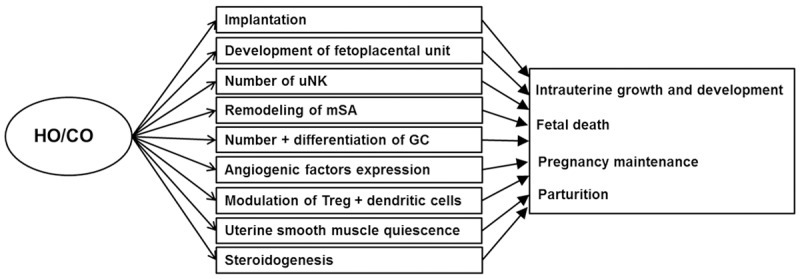
Significance of HO/CO during pregnancy. Full-fledged intrauterine development and subsequent parturition is result from interplay of many processes. It is proved that HO/CO is important for the right function of many processes influencing the intrauterine development. See text for details.
Localisation of the HO isoform is different in distinct placental cell types [67,82]. In human placenta, HO-1 is mainly localised in syncytiotrophoblast. Conversely, in cytotrophoblast a smaller amount is found. Syncytiotrophoblast is directly exposed to maternal blood and hence a potential immunological reaction or inflammatory stress. Inducible HO-1 located in the syncytiotrophoblast can therefore directly respond to these processes [86].
Changes in HO expression occur in response to various pathologies of pregnancy. Both spontaneous and artificially induced mice abortions are associated with reduced expression of HO [87,88]. Reduced HO expression also occurs in the pathologies of pregnancy such as pre-eclampsia, fetal growth retardation or H-mole [89,90]. Although it is unclear whether decreased HO expression triggers pathology or is merely an accompanying signal, it is clearly demonstrated that HO/CO is crucial for the normal course of pregnancy.
HO-1 deficiency is associated with infertility [91], but in HO-1-/- mice, it is shown that in fact conception occurs, but all fetuses die in utero [80]. Also, the inhibition of both HO isoforms by CrMP leads to fetal intrauterine deaths [70]. Already partial HO-1 deficiency (HO-1+/-) leads to an increased fetal loss and hence smaller litter size [80,92].
Implantation is a process in which HO-1 has an important role. HO-1 deficiency in mice leads to delayed establishment of a connection between blastocyst and uterine epithelial cells [80]. Delayed implantation has a negative impact on placentation, development of the fetal-placental unit and subsequent fetal growth [93]. HO-1 deficiency leads to an overall reduction in weight of the fetal-placental unit. Disturbances in placental function lead to intrauterine fetal growth restriction (IUGR) and the low birth weight of successfully derived offspring [80,92,94,95].
HO-1 is important during placentation for the survivability of trophoblast and also for trophoblast differentiation into mature phenotype. Differentiation of trophoblastic stem cells to the so-called giant cells (GCs) takes place during placentation. HO inhibition by nonspecific inhibitor zinc protoporphyrin (ZnPP) affects this process, because it reduces the trophoblastic stem cells’ viability and also suppresses their differentiation into GCs. Also placenta from HO-1-deficient mice shows a reduced number of GCs and an increased rate of apoptosis.
The ability to reverse this negative effect by CO exogenous application indicates the significance of CO [80,92]. If CO is applied to HO-1- deficient mice, positive changes) take place in placenta, such as placental enlargement, increased amount of GC, reduced pathological changes and decreased fetal mortality [80,82].
During pregnancy, there is a significant increase of blood flow in the uteroplacental junction, which is facilitated by the growth and remodelling of the maternal spiral uterine artery (SA) system [84]. Uterine natural killer cells (UNKs) are important cells that regulate remodelling of maternal uterine vasculature [96]. In placenta of HO-1-deficient mice, a smaller number of UNKs and lower expression of cytokine IL-15 are detected, which is important for UNKs’ differentiation and their retention in the fetoplacental junction. The reduced quantity of UNKs is associated with a decrease in production of angiogenic factors [94,97] and an increase in synthesis of anti-angiogenic factors [98].
Overall, HO-1 deficiency leads to a significant deterioration in SA remodelling, reduced size of fetoplacental unit and IUGR [94,97]. The negative effect of HO-1 deficiency may be reversed by exogenous CO application, which leads to an increase of UNK number, production of angiogenic factors and normalisation of fetoplacental unit size [94]. For these reasons, the overall significance of the HO/CO system for the proper course of gestation is obvious.
Modulation of regulatory T cells (Treg) and dendritic cells is an important mechanism by which HO-1 protects the fetus against abortion. HO-1 is involved in the maintenance of dendritic cells in an immature state (tolerogenic), which is important for the protection of the fetus against harmful immune responses. HO-1 inhibition by ZnPP leads to a decreased amount of Treg in the fetoplacental junction, which leads to fetal allograft rejection. On the other hand, up-regulation of HO-1 by CoPP maintains tolerogenic dendritic cells and leads to an increased Treg quantity, thereby preventing fetal allograft rejection [99-101].
On the basis of the aforementioned works, we may consider the possibility of using the HO/CO system in the treatment/prevention of pregnancy disorders. Activation of HO-1 or exogenous CO application, leads to a decrease in fetal deaths [80,102] and has a positive effect on fetal growth [80,103]. The HO/CO system may also participate in fetal protection against the harmful effects of pathogens. Listeria monocytogenes infection leads to reduced HO-1 expression and, conversely, exogenous induction of HO-1 by cobalt protoporphyrin (CoPP) results in the inhibition of the onset of abortion [104]. A similar effect is also found in the case of Brucella abortus infection, when the application of CoPP also suppresses abortion [105].
Conclusion and future perspectives
Deleterious processes, such as oxidative stress or inflammatory reaction, result in disorders of the reproductive system. HO/CO belongs to the signalling pathways that are involved in protecting cells from these processes. Methods of assisted reproduction and reproduction biotechnology are connected to in vitro conditions, which, in comparison to in vivo conditions, are more stressful for cells.
Consequently, this leads to deterioration in the quality of gametes and embryos. HO/CO could prevent this deterioration by activation of cytoprotective mechanisms and thus improve the methods used thus far. Likewise, reduced function of HO/CO may impair reproduction and exogenous HO activation or CO supplementation could reverse the negative effect of HO deficiency.
Because of the significance of HO/CO in oogenesis being an unexplored area, there is a need to focus on this key reproductive process. In the case of H2S and NO, it is known that these gasotransmitters are important for the course of meiotic maturation and the prevention of post-ovulatory aging. Gasotransmitters interact among themselves and therefore it is important to know the effect of CO. Only then is it possible to obtain a comprehensive view of the effect of gasotransmitters on oogenesis. Together with the evaluation of the effect of HO deficiency/activation or exogenous CO donation on the course of oogenesis, as well as overall reproduction, it is necessary to examine the cross-talk between CO/NO/H2S.
A large portion of experiments is aimed only at HO-1. Despite the importance of HO-1, this is insufficient. HO-1 and HO-2 have a distinct significance, but they can also partially replace each other. Therefore, for the understanding of HO/CO significance, it is important to study both isoforms.
Acknowledgements
Supported by the Internal Grant Agency of the Czech University of Life Sciences, Prague (CIGA) (Project No. 20152022).
Disclosure of conflict of interest
None.
References
- 1.Palmer RJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 3.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 5.Stewart RD. The effect of carbon monoxide on humans. Annu Rev Pharmacol. 1975;15:409–423. doi: 10.1146/annurev.pa.15.040175.002205. [DOI] [PubMed] [Google Scholar]
- 6.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 7.Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: Heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:799–804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 9.Rotenberg MO, Maines MD. Characterization of a cDNA-encoding rabbit brain heme oxygenase-2 and identification of a conserved domain among mammalian heme oxygenase isozymes: possible heme-binding site? Arch Biochem Biophys. 1991;290:336–344. doi: 10.1016/0003-9861(91)90549-x. [DOI] [PubMed] [Google Scholar]
- 10.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 11.McCoubrey WK, Maines MD. Domains of rat heme oxygenase-2: the amino terminus and histidine 151 are required for heme oxidation. Arch Biochem Biophys. 1993;302:402–408. doi: 10.1006/abbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 12.Shibahara S, Muller R, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985;82:7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 14.Yoshida T, Kikuchi G. Purification and properties of heme oxygenase from pig spleen microsomes. J Biol Chem. 1978;253:4224–4229. [PubMed] [Google Scholar]
- 15.Dunn LL, Midwinter RG, Ni J, Hamid HA, Parish CR, Stocker R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid Redox Signal. 2014;20:1723–1742. doi: 10.1089/ars.2013.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Zang G, Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryter SW, Choi AK. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 2016;167:7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YM, Pae HO, Park JE, Lee YC, Woo JM, Kim NH, Choi YK, Lee BS, Kim SR, Chung HT. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;14:137–167. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori R, Kashiba M, Toma T, Yachie A, Goda N, Makino N, Soejima A, Nagasawa T, Nakabayashi K, Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 20.Dennery PA. Signaling function of heme oxygenase proteins. Antioxid Redox Signal. 2014;20:1743–1753. doi: 10.1089/ars.2013.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma N, Ding X, Doi M, Izumi N, Semba R. Cellular and subcellular localization of heme oxygenase-2 in monkey retina. J Neurocytol. 2004;33:407–415. doi: 10.1023/B:NEUR.0000046571.90786.6e. [DOI] [PubMed] [Google Scholar]
- 22.West AR, Oates PS. Subcellular location of heme oxygenase 1 and 2 and divalent metal transporter 1 in relation to endocytotic markers during heme iron absorption. J Gastroen Hepatol. 2007;23:150–158. doi: 10.1111/j.1440-1746.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu N, Wang X, McCoubrey WK, Maines MD. Developmentally regulated expression of two transcripts for heme oxygenase-2 with a first exon unique to rat testis: control by corticosterone of the oxygenase protein expression. Gene. 2000;241:175–183. doi: 10.1016/s0378-1119(99)00439-4. [DOI] [PubMed] [Google Scholar]
- 24.Turkseven S, Drummond G, Rezzani R, Rodella L, Quan S, Ikehara S, Abraham NG. Impact of silencing HO-2 on EC-SOD and the mitochondrial signaling pathway. J Cell Biochem. 2007;100:815–823. doi: 10.1002/jcb.21138. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Sánchez J, Chánez-Cárdenas ME. A review on hemeoxygenase-2: focus on cellular protection and oxygen response. Oxid Med Cell Longev. 2014;2014:604981. doi: 10.1155/2014/604981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Boczkowski J, Poderoso JJ, Motterlini R. CO-metal interaction: vital signaling from a lethal gas. Trends Biochem Sci. 2006;31:614–621. doi: 10.1016/j.tibs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Tsiftsoglou AS, Tsamadou AI, Papadopoulou LC. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol Mol Biol Rev. 2004;68:453–473. doi: 10.1128/MMBR.68.3.453-473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 31.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 32.Ishimaru R, Leung K, Hong L, LaPolt PS. Inhibitory effects of nitric oxide on estrogen production and cAMP levels in rat granulosa cell cultures. J Endocrinol. 2001;168:249–255. doi: 10.1677/joe.0.1680249. [DOI] [PubMed] [Google Scholar]
- 33.Grasselli F, Ponderato N, Basini G, Tamanini C. Nitric oxide synthase expression and nitric oxide/cyclic GMP pathway in swine granulosa cells. Domest Anim Endocrin. 2001;20:241–252. doi: 10.1016/s0739-7240(01)00096-0. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz KR, Pires PR, Mesquita LG, Chiaratti MR, Leal CL. Effect of nitric oxide on the cyclic guanosine monophosphate (cGMP) pathway during meiosis resumption in bovine oocytes. Theriogenology. 2014;81:556–564. doi: 10.1016/j.theriogenology.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Alexandreanu IC, Lawson DM. Heme oxygenase in the rat ovary: immunohistochemical localization and possible role in steroidogenesis. Exp Biol Med (Maywood) 2003;228:59–63. doi: 10.1177/153537020322800108. [DOI] [PubMed] [Google Scholar]
- 36.Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Loughran PA, Billiar TR. Carbon monoxide decreases the level of iNOS protein and active dimer in IL-1β-stimulated hepatocytes. Nitric Oxide. 2008;18:256–265. doi: 10.1016/j.niox.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- 39.Bu S, Xia G, Tao Y, Lei L, Zhou B. Dual effects of nitric oxide on meiotic maturation of mouse cumulus cell-enclosed oocytes in vitro. Mol Cell Endocrinol. 2003;207:21–30. doi: 10.1016/s0303-7207(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 40.Tichovská H, Petr J, Chmelíková E, Sedmíková M, Tůmová L, Krejčová M, Dorflerova A, Rajmon R. Nitric oxide and meiotic competence of porcine oocytes. Animal. 2011;5:1398–1405. doi: 10.1017/S1751731111000565. [DOI] [PubMed] [Google Scholar]
- 41.Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 42.D’Amico G, Lam F, Hagen T, Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J Cell Sci. 2006;119:2291–2298. doi: 10.1242/jcs.02914. [DOI] [PubMed] [Google Scholar]
- 43.Taillé C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 44.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 46.Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 47.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 48.Combelles CH, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–880. doi: 10.1016/s1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 50.Kim HP, Ryter SW, Choi AK. Co as a cellular signaling molecule. Annu Rev Pharmacol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 51.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, Costa d’Avila J, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Carbon monoxide orchestrates a protective response through PPARγ. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Wang R, Wang Z, Wu L, Hanna ST, Peterson-Wakeman R. Reduced vasorelaxant effect of carbon monoxide in diabetes and the underlying mechanisms. Diabetes. 2001;50:166–174. doi: 10.2337/diabetes.50.1.166. [DOI] [PubMed] [Google Scholar]
- 53.Ou XH, Li S, Xu BZ, Wang ZB, Quan S, Li M, Zhang QH, Ouyang YC, Schatten H, Xing FQ, Sun QY. P38α MAPK is a MTOC-associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle. 2014;9:4130–4143. doi: 10.4161/cc.9.20.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyagaki Y, Kanemori Y, Tanaka F, Baba T. Possible role of p38 MAPK-MNK1-EMI2 cascade in metaphase-II arrest of mouse oocytes. Biol Reprod. 2014;91:45–53. doi: 10.1095/biolreprod.113.116962. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod. 2003;69:2045–2052. doi: 10.1095/biolreprod.103.021105. [DOI] [PubMed] [Google Scholar]
- 56.Vanhoutte L, Nogueira D, Gerris J, Dhont M, De Sutter P. Effect of temporary nuclear arrest by Phosphodiesterase 3-Inhibitor on morphological and functional aspects of in vitro matured mouse oocytes. Mol Reprod Dev. 2008;75:1021–1030. doi: 10.1002/mrd.20851. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011;589:3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peers C, Boyle JP, Scragg JL, Dallas ML, Al-Owais MM, Hettiarachichi NT, Elies J, Johnson E, Gamper N, Steele DS. Diverse mechanisms underlying the regulation of ion channels by carbon monoxide. Brit J Pharmacol. 2015;172:1546–1556. doi: 10.1111/bph.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorca RA, Prabagaran M, England SK. Functional insights into modulation of BKCa channel activity to alter myometrial contractility. Front Physiol. 2014;5:289. doi: 10.3389/fphys.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odrcich MJ, Graham CH, Kimura KA, McLaughlin BE, Marks GS, Nakatsu K, Brien JF. Heme oxygenase and nitric oxide synthase in the placenta of the guinea-pig during gestation. Placenta. 1998;19:509–516. doi: 10.1016/s0143-4004(98)91044-x. [DOI] [PubMed] [Google Scholar]
- 61.Ihara N, Akagi R, Ejiri K, Kudo T, Furuyama K, Fujita H. Developmental changes of gene expression in heme metabolic enzymes in rat placenta. FEBS Lett. 1998;439:163–167. doi: 10.1016/s0014-5793(98)01324-6. [DOI] [PubMed] [Google Scholar]
- 62.Kreiser D, Kelly DK, Seidman DS, Stevenson DK, Baum M, Dennery PA. Gestational pattern of heme oxygenase expression in the rat. Pediatr Res. 2003;54:172–178. doi: 10.1203/01.PDR.0000072516.83498.07. [DOI] [PubMed] [Google Scholar]
- 63.Murphy BJ, Laderoute KR, Short SM, Sutherland RM. The identification of heme oxygenase as a major hypoxic stress protein in Chinese hamster ovary cells. Brit J Cancer. 1991;64:69–73. doi: 10.1038/bjc.1991.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harada T, Koi H, Kubota T, Aso T. Haem oxygenase augments porcine granulosa cell apoptosis in vitro. J Endocrinol. 2004;181:191–205. doi: 10.1677/joe.0.1810191. [DOI] [PubMed] [Google Scholar]
- 65.Pfeiffer MJ, Taher L, Drexler H, Suzuki Y, Makałowski W, Schwarzer C, Wang B, Fuellen B, Boiani M. Differences in embryo quality are associated with differences in oocyte composition: a proteomic study in inbred mice. Proteomics. 2015;15:675–687. doi: 10.1002/pmic.201400334. [DOI] [PubMed] [Google Scholar]
- 66.Maines MD, Kutty RK. Differential response of testicular and ovarian heme oxygenase activity to metal ions. Arch Biochem Biophys. 1983;226:134–144. doi: 10.1016/0003-9861(83)90278-3. [DOI] [PubMed] [Google Scholar]
- 67.Cella M, Farina MG, Keller Sarmiento MI, Chianelli M, Rosenstein RE, Franchi AM. Heme oxygenase-carbon monoxide (HO-CO) system in rat uterus: effect of sexual steroids and prostaglandins. J Steroid Biochem Mol Biol. 2006;99:59–66. doi: 10.1016/j.jsbmb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Zenclussen ML, Casalis PA, Jensen F, Woidacki K, Zenclussen AC. Hormonal fluctuations during the estrous cycle modulate heme oxygenase-1 expression in the uterus. Front Endocrinol (Lausanne) 2014;5:32. doi: 10.3389/fendo.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acevedo CH, Ahmed A. Hemeoxygenase-1 inhibits human myometrial contractility via carbon monoxide and is upregulated by progesterone during pregnancy. J Clin Invest. 1998;101:949–955. doi: 10.1172/JCI927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alexandreanu IC, Lawson DM. Effects of chronic administration of a heme oxygenase substrate or inhibitor on progression of the estrous cycle, pregnancy and lactation of Sprague-Dawley rats. Life Sci. 2002;72:153–162. doi: 10.1016/s0024-3205(02)02166-5. [DOI] [PubMed] [Google Scholar]
- 71.Vinatier D, Dufour P, Tordjeman-Rizzi N, Prolongeau JF, Depret-Moser S, Monnier JC. Immunological aspects of ovarian function: role of the cytokines. Eur J Obstet Gynecol Reprod Biol. 1995;63:155–168. doi: 10.1016/0301-2115(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Tang J, Sun Y, Wu J, Yu P, Wang G. Upregulation of HO-1 attenuates LPS-stimulated proinflammatory responses through downregulation of p38 signaling pathways in rat ovary. Inflammation. 2015;38:1085–1092. doi: 10.1007/s10753-014-0074-0. [DOI] [PubMed] [Google Scholar]
- 73.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 74.Zenclussen ML, Jensen F, Rebelo S, El-Mousleh T, Casalis PA, Zenclussen AC. Heme oxzgenase-1 expression in the ovary dictates a proper oocyte ovulation, fertilization, and corpora lutea maintenance. Am J Reprod Immunol. 2012;67:376–382. doi: 10.1111/j.1600-0897.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- 75.Devoto L, Vega M, Kohen P, Castro O, Carvallo P, Palomino A. Molecular regulation of progesterone secretion by the human corpus luteum throughout the menstrual cycle. J Reprod Immunol. 2002;55:11–20. doi: 10.1016/s0165-0378(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 76.Vreman HJ, Zentner AR, Wong RJ, Stevenson DK. Carbon monoxide production and upregulation of heme oxygenase activity in mice after heme administration. Pediatr Res. 1999;4:231A–231A. [Google Scholar]
- 77.Chen B, Guo L, Fan C, Bolisetty S, Joseph R, Wright MM, Agarwal A, George JF. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AK, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergandi L, Basso G, Evangelista F, Canosa S, Dalmasso P, Aldieri E, Revelli A, Benedetto C, Ghigo D. Inducible nitric oxide synthase and heme oxygenase 1 are expressed in human cumulus cells and may be used as biomarkers of oocyte competence. Reprod Sci. 2014;21:1370–1377. doi: 10.1177/1933719114525268. [DOI] [PubMed] [Google Scholar]
- 80.Zenclussen ML, Casalis PA, El-Mousleh T, Rebelo S, Langwisch S, Linzke N, Volk HD, Fest S, Soares MP, Zenclussen AC. Haem oxygenase-1 dictates intrauterine fetal survival in mice via carbon monoxide. J Pathol. 2011;225:293–304. doi: 10.1002/path.2946. [DOI] [PubMed] [Google Scholar]
- 81.Bainbridge SA, Smith GN. HO in pregnancy. Free Radical Bio Med. 2005;38:979–988. doi: 10.1016/j.freeradbiomed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Levytska K, Kingdom J, Baczyk D, Drewlo S. Heme oxygenase-1 in placental development and pathology. Placenta. 2013;34:291–298. doi: 10.1016/j.placenta.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 83.George EM, Granger JP. Heme oxygenase in pregnancy and preeclampsia. Curr Opin Nephrol Hypertens. 2013;22:156–162. doi: 10.1097/MNH.0b013e32835d19f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zenclussen ML, Linzke N, Schumacher A, Fest S, Meyer N, Casalis PA, Zenclussen AC. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Front Pharmacol. 2015;5:291. doi: 10.3389/fphar.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schumacher A, Zenclussen AC. Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance. Front Pharmacol. 2015;5:288. doi: 10.3389/fphar.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshiki N, Kubota T, Aso T. Expression and localization of heme oxygenase in human placental villi. Biochem Bioph Res Co. 2000;276:1136–1142. doi: 10.1006/bbrc.2000.3551. [DOI] [PubMed] [Google Scholar]
- 87.Zenclussen AC, Joachim R, Hagen E, Peiser C, Klapp BF, Arck PC. Heme oxygenase is downregulated in stress-triggered and interleukin-12-mediated murine abortion. Scand J Immunol. 2002;55:560–569. doi: 10.1046/j.1365-3083.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 88.Zenclussen AC, Sollwedel A, Zambon Bertoja AZ, Gerlof K, Zenclussen ML, Woiciechowsky C, Volk HD. Heme oxygenase as a therapeutic target in immunological pregnancy complications. Int Immunopharmacol. 2005;5:41–51. doi: 10.1016/j.intimp.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Zenclussen AC, Lim E, Knoeller S, Knackstedt M, Hertwig K, Hagen E, Klapp BF, Arck PC. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50:66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 90.Lash GE, McLaughlin BE, MacDonald-Goodfellow SK, Smith GN, Brien JF, Marks GS, Nakatsu K, Graham CH. Relationship between tissue damage and heme oxygenase expression in chorionic villi of term human placenta. Am J Physiol Heart C Physiol. 2003;284:H160–H167. doi: 10.1152/ajpheart.00738.2002. [DOI] [PubMed] [Google Scholar]
- 91.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1994;98:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao H, Wong RJ, Kalish FS, Nayak NR, Stevenson DK. Effect of heme oxygenase-1 deficiency on placental development. Placenta. 2009;30:861–868. doi: 10.1016/j.placenta.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A 2 α is crucial for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2789–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 94.Linzke N, Schumacher A, Woidacki K, Croy BA, Zenclussen AC. Carbon monoxide promotes proliferation of uterine natural killer cells and remodeling of spiral arteries in pregnant hypertensive heme oxygenase-1 mutant mice. Hypertension. 2014;63:580–588. doi: 10.1161/HYPERTENSIONAHA.113.02403. [DOI] [PubMed] [Google Scholar]
- 95.Solano ME, Kowal MK, O’Rourke GE, Horst AK, Modest K, Plösch T, Barikbin R, Remus CC, Berger RG, Jago C, Ho H, Sass G, Parker VJ, Lzdon JP, DeMayo FJ, Hecher K, Karimi K, Arck PC. Progesterone and HMOX-1 promote fetal growth by CD8 T cell modulation. J Clin Invest. 2015;125:1726–1738. doi: 10.1172/JCI68140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hatta K, Carter AL, Chen Z, Leno-Duran E, Ruiz-Ruiz C, Olivares EG, Tse MY, Pagn SC, Croy BA. Expression of the vasoactive proteins AT1, AT2, and ANP by pregnancy-induced mouse uterine natural killer cells. Reprod Sci. 2011;18:383–390. doi: 10.1177/1933719110385136. [DOI] [PubMed] [Google Scholar]
- 97.Zhao H, Azuma J, Kalish F, Wong RJ, Stevenson DK. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol Reprod. 2011;85:1005–1012. doi: 10.1095/biolreprod.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devery LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 99.Sollwedel A, Bertoja AZ, Zenclussen ML, Gerlof K, Lisewski U, Wafula P, Sawityki B, Woiciechowsky C, Volk HD, Zenclussen AC. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of Bag-1 and Neuropilin-1 at the fetal-maternal interface. J Immunol. 2005;175:4875–4885. doi: 10.4049/jimmunol.175.8.4875. [DOI] [PubMed] [Google Scholar]
- 100.Schumacher A, Wafula PO, Teles A, El-Mousleh T, Linzke N, Zenclussen ML, Langwisch S, Heinze K, Wollenberg I, Casalis PA, Volk HD, Fest S, Zenclussen AC. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One. 2012;7:e42301. doi: 10.1371/journal.pone.0042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zenclussen AC, Schumacher A, Zenclussen ML, Wafula P, Volk HD. Immunology of pregnancy: cellular mechanisms allowing fetal survival within the maternal uterus. Exprt Rev Mol Med. 2007;9:1–14. doi: 10.1017/S1462399407000294. [DOI] [PubMed] [Google Scholar]
- 102.Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, Sollwedel A, Volk HD, Ritter T, Zenclussen AC. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69:35–52. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, Abraham N, Dennery PA. Heme oxygenase-1 modulates fetal growth in the rat. Lab Invest. 2002;82:687–692. doi: 10.1097/01.lab.0000017167.26718.f2. [DOI] [PubMed] [Google Scholar]
- 104.Tachibana M, Hashino M, Nishida T, Shimizu T, Watarai M, Bereswill S. Protective role of heme oxygenase-1 in Listeria monocytogenes-induced abortion. PLoS One. 2011;6:e25046. doi: 10.1371/journal.pone.0025046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tachibana M, Watanabe K, Yamasaki Y, Suzuki H, Watarai M. Expression of heme oxygenase-1 is associated with abortion caused by Brucella abortus infection in pregnant mice. Microb Pathogenesis. 2008;45:105–109. doi: 10.1016/j.micpath.2008.04.002. [DOI] [PubMed] [Google Scholar]


