Abstract
An elevated serum prostate-specific antigen (PSA) level alone cannot distinguish between local-regional recurrences and distant metastases after treatment with curative intent. With available salvage treatments, it has become important to localize the site of recurrence. 11C-Acetate PET/CT was performed in patients with rising PSA, with statistical analysis of detection rates, sites/location of detection, PSA kinetics and comparison with other tracers (FDG and Choline). Correlation to biopsy, subsequent imaging and PSA response to focal treatment was also performed. 88% (637) of 721 11C-Acetate PET/CT scans performed were positive. There was a statistically significant difference in PSA values between the positive and negative scans (P < 0.001 for mean difference) with the percentage of positive scans and PSA having a positive correlation. A PSA of 1.09 ng/mL was found to be an optimal cutoff. PSAdT was significantly correlated with a positive scan only when the PSA was < 1.0 ng/mL. For this subgroup, a PSAdT of < 3.8 months appeared significant (P < 0.05) as an optimal cutoff point. 11C-Acetate PET/CT demonstrates a high detection rate for the site of recurrence/metastasis in biochemical relapsed prostate cancer (88% overall detection rate, PPV 90.8%). This analysis suggests an optimal PSA threshold of > 1.09 ng/mL or a PSAdT of < 3.8 months when the PSA is below 1.0 ng/mL as independent predictors of positive findings.
Keywords: Prostate cancer, carbon-11 acetate, positron emission tomography, PSA recurrence
Introduction
Prostate cancer is the second most common cancer in American men with the American Cancer Society 2015 estimates for prostate cancer of about 220,800 new cases of prostate cancer diagnosed and 27,540 deaths from prostate cancer. About 1 man in 6 will be diagnosed with prostate cancer during his lifetime and death from prostate cancer in American men lags only behind lung cancer.
Regardless of the type of primary treatment for prostate cancer, a significant proportion of patients will experience relapse, occurring in 35-40% after radical prostatectomy [1,2] and external beam radiotherapy [3-5]. In these patients, evidence of residual or recurrent disease is heralded by detectable or increasing serum prostate-specific antigen (PSA) [6-11]-also referred to as “biochemical relapse”. In many cases, there is no or minimal evidence of disease on standard imaging studies such as MRI, CT, ultrasound and technetium bone scans. A rising PSA causes significant anxiety for both the patient and treating doctors. Subsequent treatment decisions rely critically on distinguishing between loco-regional relapse in the prostate bed and adjacent soft tissues, loco-regional relapse in lymph nodes, and distant metastases. Imaging with 18F fluorodeoxyglucose (FDG) positron emission tomography (PET) is successful in other types of cancer, but does not appear useful for prostate adenocarcinoma, primarily because prostate adenocarcinoma does not routinely exhibit a glycolytic phenotype [12,13]. Additionally, FDG tracer excretion through the kidneys into the bladder significantly obscures evaluation of the prostate bed.
Over the past few years, novel PET tracers have been introduced to assist with the evaluation of prostate adenocarcinoma. In addition to 11C or 18F-choline, 11C-Acetate appears to be highly promising. Various groups have tested the potential of 11C-Acetate PET imaging in prostate adenocarcinoma [14-20] providing encouraging results. We retrospectively evaluated the performance of 11C-Acetate PET/CT at our institution in men with biochemical recurrence after definitive treatment.
Methods
The study was approved by our institutional review board for all patients undergoing 11C-Acetate PET/CT imaging at our institution. Male patients with histologically proven prostate adenocarcinoma and PSA biochemical recurrence were imaged with 11C-Acetate PET/CT from May 2011 through December 2015. Recurrence in the post-surgical setting was defined as a PSA level greater than 0.2 ng/mL on the 2 most recent PSA assessments. For patients who received external beam radiation or brachytherapy, recurrence was defined by three consecutive rises in PSA values after a nadir PSA after treatment.
PSA kinetics results were calculated using the latest PSA values available for each patient over the 12 months prior to 11C-Acetate imaging. PSA doubling time was calculated using the best-line fit method popularized by Memorial Sloan Kettering Cancer Center with the formula used to calculate PSAdT in months as PSAdT = ln(2)/b.
In which ln(2) is the natural logarithm of 2, and b is the slope of all the log-scale PSA measurements [21]. The calculation of PSA velocity (PSAvel), in ng/mL/year was defined by the slope of the least squares regression of the PSA values.
Patients & characteristics
Of 887 patients imaged with 11C-Acetate PET/CT, 721 met the criteria for biochemical relapse for which PSA doubling time (PSAdT) and velocity (PSAvel) could be calculated. In 161 patients, the PSA was ≤ 1.0 ng/mL (mean, 0.6; median 0.6). In 234 patients, the PSA was > 1.0 and ≤ 3.0 ng/mL. Detailed characteristics of the study population are summarized in Table 1.
Table 1.
Patient Characteristics
| Entire Cohort | |
|---|---|
| No. patients | 721 |
| Age, yrs | |
| Range | 41-91 |
| Mean | 70 |
| Gleason score | |
| 5 | 5 (< 1%) |
| 6 | 89 (12%) |
| 7 | 332 (46%) |
| 8 | 135 (18%) |
| 9 | 127 (18%) |
| 10 | 9 (1%) |
| Unknown | 24 (3%) |
| Prior Treatment | |
| Prostatectomy | 213 (30%) |
| Duration since treatment (yrs) | |
| Range | 0.2-27 |
| Mean/median | 5.98/4 |
| Radiation* | 251 (35%) |
| Duration since treatment (yrs) | |
| Range | 0.3-23 |
| Mean/median | 6.86/6 |
| Prostatectomy + Radiation** | 229 (32%) |
| Duration since treatment (yrs) | |
| Range | 0.2-27 |
| Mean/median | 8.8/7 |
| ADT¥ | 18 (2%) |
| Cyrotherapy | 5 (< 1%) |
| HIFUΩ | 5 (< 1%) |
| PSA at imaging, ng/mL | |
| Range | 0.22-148 |
| Mean/median | 5.9/2.6 |
| PSA Doubling Time, months | |
| Range | 0.2-151 |
| Mean/median | 6.7/4.24 |
| PSA Velocity, ng/mL/yr | |
| Range | 0.01-34.4 |
| Mean/median | 0.68/0.22 |
External beam radiation and/or Brachytherapy.
Adjuvant or Salvage external beam radiation.
Androgen Deprivation Therapy.
High Intensity Focused Ultrasound.
Radiopharmaceutical synthesis and PET/CT imaging
[11C] CO2 gas was produced from the cyclotron (PETtrace series 800, General Electric) and reacted with methylmagnesium bromide to produce 11C-Acetate. Our typical yield of 11C-Acetate was 1.4 Curies with a total synthesis time of 10 minutes.
11C-Acetate PET/CT imaging was performed on an integrated PET/CT scanner (Siemans Biograph 6 TruePoint or Biograph 16; Malvern, PA USA). Patients were positioned on the camera and then 740-1480 MBq C11-Acetate (half-life 20.3 minutes) was administered as a bolus intravenous injection. A CT topogram was obtained from the vertex through the pelvis. On the basis of the topogram, the tube current for the CT scan was adjusted utilizing a Care doseTM application to minimize exposure. The tube voltage was 130 kVp. After the CT scan, emission images beginning at the pelvis and proceeding cranially were obtained (3-7 minutes post injection, mean 4.25). Images were reconstructed with iterative reconstruction (2 iterations, 8 subsets, matrix 168, Gaussian filter). The administration of 11C-Acetate was well tolerated by all patients and no adverse events were encountered.
Imaging interpretation and analysis
All images were interpreted by a physician board certified by the American Board of Nuclear Medicine, with more than 15 years of experience with PET/CT interpretation. Detected lesions were defined as moderate to intense focal areas of increased metabolic activity over background in the prostate, prostatic bed, nodes and bone. Equivocal studies were considered as negative in this analysis. Increased metabolic activity in the inguinal nodes and mediastinum were encountered often and were considered as physiologic background, as prostate adenocarcinoma metastatic disease to these regions would be rare. For consideration as metastases, metabolic nodes in these regions were required to be pathologically enlarged or enlarging compared to prior imaging studies. In the pelvic and retroperitoneal nodal regions, sub-centimeter mildly metabolic nodes where considered positive if they were rounded and showed substantially higher metabolic activity than similarly sized physiologic nodes in the inguinal region.
A patient-based analysis of sensitivity and positive predictive value (PPV) was performed. PPV was calculated based on correlation to biopsy results, subsequent imaging or PSA response to focal treatment with Radiation Therapy (RT) or Cryotherapy. With regard to a patient-based analysis, calculating specificity and NPV is not possible as we assumed that all patients included in this analysis had recurrent disease or persistent primary tumor and that our cohort therefore did not include true-negative cases.
Chi-squares, 2-sample t tests, multivariate and univariate logistic regression analyses (SPSS v22, SigmaXL v6.2) were used to determine whether there was a relationship between PSA levels, PSA kinetics and the rate of detection of the site of relapse. Age, stage, Gleason score, PSA at diagnosis, and type of initial therapy (surgery versus radiation) were also considered in the logistic regression analyses. Other forms of initial therapy (HIFU, Cryotherapy, ADT) where not considered further in the analysis as there were too few of these for meaningful comparison. PSA and PSA kinetics were considered as independent and combined variables. Gleason score was considered both as a continuous variable and in categorical risk subgroups as low (Gs 5-6), intermediate (Gs 7) and high (Gs 8-10).
Results
637 (88%) of the 721 11C-Acetate PET/CT scans included in the analysis were considered positive. The duration from initial treatment to imaging was similar for post prostatectomy and post radiation (mean 5.98 and 6.86 years respectively), while there was a statistically significant longer duration since primary treatment in those with both prior prostatectomy and radiation (mean 8.8, P < 0.0001). There was a statistically significant difference in PSA values between the positive and negative scans (positive result mean 6.405, negative result mean 2.036, P < 0.001, mean difference 4.369). The percentage of positive scans and PSA value had a positive correlation. Logistic regression and ROC analysis demonstrated that a PSA of 1.09 ng/mL was an optimal cutoff point with a sensitivity of 80% (AUC 0.724, P < 0.0001).
Differences in PSA doubling time (PSAdT) were only found to be significantly correlated with a positive scan when the PSA was < 1.0 ng/mL (P < 0.05). A doubling time of < 3.8 months appeared as an optimal cutoff point with 85% sensitivity. Figure 1 and Table 2 provide additional subgroup analysis.
Figure 1.
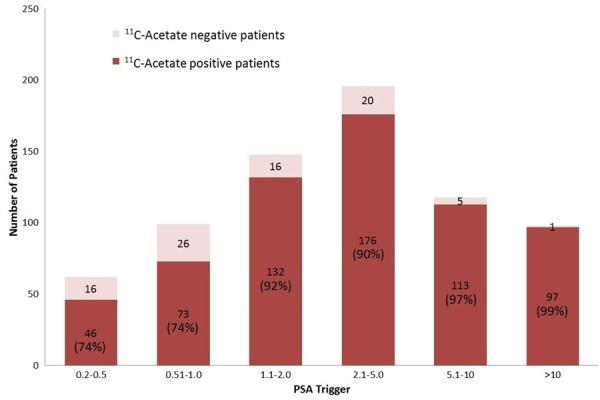
PSA subgroup 11C-acetate PET/CT detection rate.
Table 2.
Doubling time influence on detection
| PSAdT | PSA ≤ 1 | PSA > 1 |
|---|---|---|
| ≤ 3 months | 90% | 92% |
| > 3 ≤ 10 months | 72% | 94% |
| > 10 months | 50% | 90% |
PSA velocity was not found to be a significant predictor of positivity. On multivariate analysis, age, stage, Gleason score, PSA at diagnosis and type of initial treatment were also not found to be independent predictors of positive scans.
True positive scan findings could be correlated (non-overlapping) in 325 studies. Positive correlation was found by histologic correlation from biopsy in 96 of 105 studies, subsequent imaging (bone scan, CT and/or MRI) in 130 of 145, or PSA response to either focal treatment with RT or Cryotherapy alone (a > 30% decrease in PSA value from the pretreatment value) in 69 of 75. The true positives were seen in 295 of 325 cases, yielding a combined PPV of 90.8%.
In the 105 patients where histologic confirmation was available, biopsy sites included the prostate gland (38), post prostatectomy prostate bed or seminal vesicle bed regions (12), pelvic/abdominal lymph nodes (25), supraclavicular nodes (6), thoracic node (2), neck node (1), bone (5), liver (1), colon (1), urinary bladder (2), kidney (1), pancreas (1), perineum (1) and lung (9). There were 6 negative prostate/bed biopsies, 2 negative pelvic node biopsies and one negative hilar node biopsy. Ninety-one biopsies confirmed prostate adenocarcinoma with a resulting histologic PPV of 91%.
Five of the biopsies where performed in areas of concern for other malignancies (i.e. at the time of 11C-acetate imaging, increased focal uptake was present in areas not suspected to be prostate cancer, but highly concerning for other malignancies). These biopsies where all positive and revealed renal cell carcinoma, pancreatic adenocarcinoma, urinary bladder carcinoma, and non-Hodgkin’s lymphoma (in two).
Focal lesions were detected in the prostate or bed in 26% while in 23%, only focal pelvic nodal lesions were detected. Lesions were detected in both the prostate/bed and pelvic nodes in 9%. Distant sites were detected in 209 studies (42%). Of these, 49% were in bone only and 31% were bone plus soft tissue lesions. 20% of the distant lesions were also detected in other areas such as the lungs, mediastinal nodes or in supraclavicular nodes (particularly on the left). For lymph nodes, detected lesions where ≥ 5 mm in short axis dimension.
Figures 2, 3, 4 and 5 provide examples of 11C-Acetate imaging seen in our study. Figure 2 shows a patient with a post treatment rising PSA, with detection of an involved small pelvic lymph node and a single right pelvic bony lesion. Figures 3 and 4 demonstrate variations on the detection of locally recurrent disease with an apical fossa focus and right seminal vesicle bed lesion respectively. In both of those cases, only locally recurrent disease was found and salvage radiation therapy lead to durable PSA remissions. Figure 5 shows a case of PSA recurrence after prostatectomy, but complicated by underlying ulcerative colitis. Small nodes where detected in the pelvis with no other lesions demonstrated. The 11C-Acetate study allowed for targeted proton radiation therapy, also leading to a durable PSA response and with no side effects.
Figure 2.
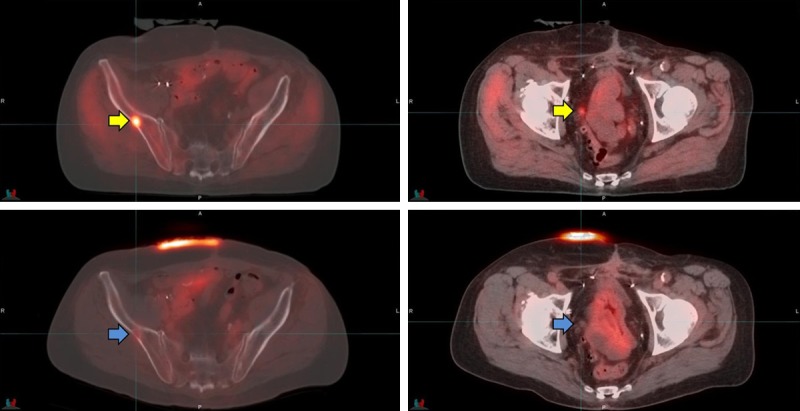
The patient in the Figure was Gleason 7, post RT 11 years previously now with a rising PSA, 11.15 ng/mL. The top row images show the 11C-Acetate PET with a metabolic focus in right ilium (SUVmax 9.1) and a small right pelvic node (yellow arrows). The lower row images show the 18F-FDG PET in the same patient performed within 23 days. On the 18F-FDG study the lesion in the right ilium is minimally apparent (SUVmax 2.2) and the node in right pelvis is negative for FDG activity above background (blue arrows).
Figure 3.
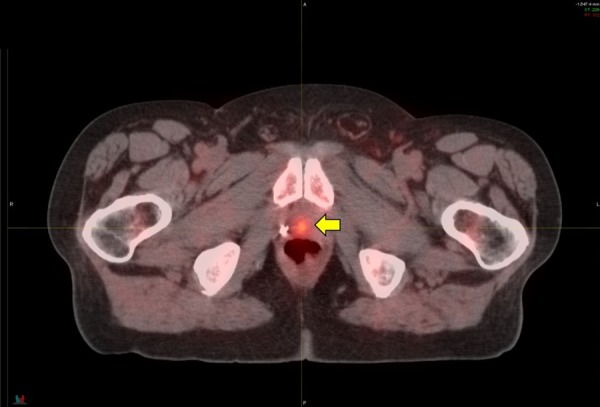
76-year-old with Prostate Adenocarcinoma (PCa), Gleason score was 8 (4 + 4) and PSA was 4.0. He underwent prostatectomy with PSA remaining < 0.1 for several years. His PSA then began to rise, 3.3 ng/mL and PSA doubling at a rate of 10.9 months. 11C-Acetate PET/CT imaging showed a small recurrence of cancer in the prostate bed (yellow arrow). No involved lymph nodes were detected and no lesions were seen on the study to suggest distant metastatic disease. Based on the results of the 11C-Acetate imaging study, the patient proceeded with Intensity Modulated Radiation Therapy (IMRT) to the prostate bed. The radiation therapy plan was modified from the standard “blind” application of radiation to this region. Instead, the area of recurrent cancer identified on the imaging study was targeted by the radiation and less radiation was then given to the surrounding areas, including the urinary bladder and rectum. After radiation treatment, the PSA fell to < 0.1 ng/mL and has remained stable at that level for 4 years. He experienced no side effects from the radiation treatment and no additional treatment has thus far been necessary.
Figure 4.
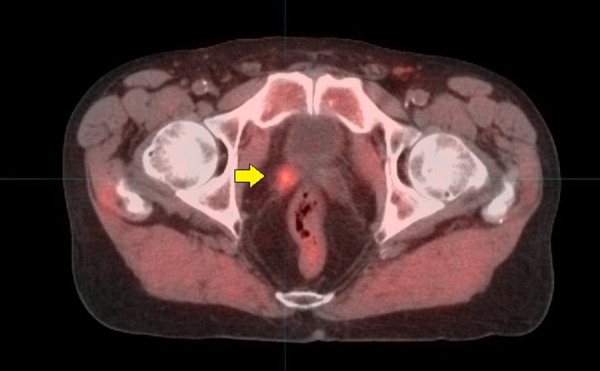
69-year-old with Gleason 8 (4 + 4) and PSA 4.8 ng/mL. He underwent a prostatectomy at which time extracapsular extension was found. After surgery his PSA was initially undetectable, but within a year had risen to 0.5 ng/mL, with a PSA doubling time of 3.18 months. A technetium bone scan was negative for bone metastasis. 11C-Acetate PET/CT imaging showed a small focus of increased metabolism in the right seminal vesicle bed (yellow arrow), indicating locally residual cancer. There were no involved lymph nodes on the scan and no lesions were seen on the study to suggest distant metastatic disease to the bone or elsewhere. Based on the 11C-Acetate imaging study, the patient underwent IMRT. Radiation therapy was performed to the entire prostate bed region but also with a radiation “boost” targeted to the focal area identified on the imaging study. After radiation therapy, PSA fell to < 0.1 ng/mL and has remained stable at that level for 2.5 years so far.
Figure 5.
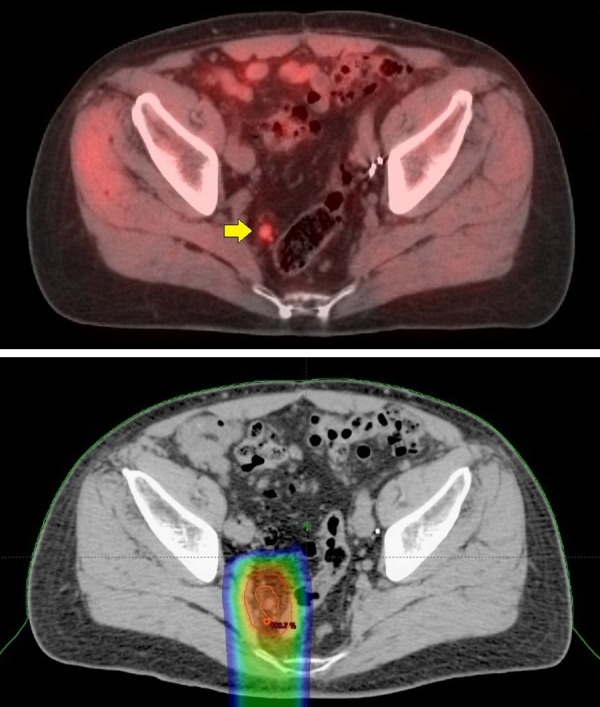
72-year-old with Gleason 9 (5 + 4) and PSA 10.8. He underwent a prostatectomy and his PSA remained < 0.1 ng/mL for 7 years. PSA then began to rise, 0.63 ng/mL with a PSA doubling time of 9.3 months. An abdominal and pelvic CT as well as technetium bone scans where negative. 11C-Acetate PET/CT imaging showed a metabolic 9 mm right peri-rectal lymph node (top image, yellow arrow). A small 5 mm metabolic node was also seen higher up in the left pelvis (not shown). No metabolic lesions were seen in the prostate bed and no lesions were seen on the study to suggest distant metastatic disease. Radiation treatment to the prostate bed with radiation extending to the pelvic lymph nodes is technically viable. This case was complicated, however, by a history of ulcerative colitis, making standard radiation problematic. The patient opted to undergo Intensity Modulated Proton Therapy. The proton therapy was administered to the pelvic lymph nodes detected on the 11C-Acetate imaging alone. The 11C-Acetate images were electronically integrated into the treatment plan to help guide the proton therapy. The bottom image shows the targeting of the proton beam treatment (color areas), which is narrow and mostly avoids the colon. Two years following treatment, the PSA has remained < 0.1 ng/mL. He experienced no side effects from the radiation treatment and importantly, has not required hormonal therapy or experience exacerbation of his ulcerative colitis.
There was no difference in the detected distribution of lesions in those with higher risk Gleason scoring (> 8) compared to men with lower Gleason scores. There was no significant difference in PSA and PSAdT between studies localizing lesions to the prostate/bed and pelvic lymph nodes, nor was there a difference in the PSAdT between local, pelvic nodal and distant sites. The PSA value however was significantly higher with detection of distant sites compared to both prostate/bed and pelvic nodal sites (P < 0.0001). See Table 3.
Table 3.
PSA (ng/mL), PSAdT (months) and locations of detected lesions
| Prostate/Bed | Pelvic Nodes | Distant Sites | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| PSA | PSAdT | PSA | PSAdT | PSA | PSAdT | |
| Range | 0.34-45 | 0.3-56 | 0.22-82.5 | 0.8-35.2 | 0.22-148 | 0.2-151 |
| Mean | 4.41 | 7.4 | 4.63 | 6.69 | 8.89 | 4.98 |
| Median | 2.8 | 6.0 | 2.1 | 5.3 | 3.78 | 3.0 |
Discussion
Conventional imaging methods, such as CT and MRI, have been shown to be of limited value in detecting recurrent prostate cancer lesions. They often fail to characterize lymph nodes smaller than 1 cm in diameter and offer no reliable differentiation between malignant and nonmalignant lymph node enlargement. 18F-FDG PET/CT also appears to have limited value for detecting early recurrence and metastases. In our own comparison study, 18 men with biochemically recurrent PCa and negative conventional imaging were sequentially imaged with 11C-Acetate and 18F-FDG PET/CT [12]. The detection rate for recurrent/metastatic disease with 18F-FDG PET/CT was only 11%, while with 11C-Acetate PET/CT it was 78%. See Figure 2.
The timing of imaging after tracer injection of 11C-Acetate appears to have an influence on performance. In a prior study from our institution, early-versus-late imaging was compared in forty patients. Early post tracer injection PET/CT imaging was performed (3-7 minutes, mean 4.25), with subsequent later pelvic/lower abdominal imaging (21-31 minutes, mean 26.6). In that study, lesions involving the lymph nodes, peri-prostate soft tissues and bone were all more visually conspicuous on the early imaging as compared to the later imaging and demonstrated statistically significant higher maximal SUVs and lesion to background ratios (P < 0.001) [22].
Overall, prior studies evaluating 11C-Acetate PET have demonstrated a wide range of detection rates. However, when reviewed in context of technique (PET alone versus PET/CT, timing of imaging after injection-shorter being preferred, and PSA), generally good detection rates are seen, and are comparable to our study findings. See Table 4.
Table 4.
Prior 11C-acetate PET/CT studies
| Author | Year | Camera | n | Delay | PSA ng/mL | Detection rate |
|---|---|---|---|---|---|---|
| Kotzerke [17] | 2002 | PET | 31 | 5 min | 0.1-150.6 (mean 10.4) | 83% |
| < 2.0 | 63% | |||||
| Frickle [15] | 2003 | PET | 25 | 2 min | 0.3-400 (mean 50) | 83% |
| Oyama [20] | 2003 | PET | 46 | 10-20 min | 0.3-47.5 (mean 5.2) | 59% |
| Sandblom [37] | 2006 | PET | 20 | 10 min | Median 2.0 | 75% |
| Vees [38] | 2006 | PET | 11 | 2 min | < 0.8 | 55% |
| Wachter [39] | 2006 | PET | 50 | 15 min | 0.5-24.9 | 64% |
| Albrecht [40] | 2007 | PET | 17 (RT) | 2 min | 2.6-30.2 | 82% |
| 15 (RP) | 0.08-4.8 | 60% | ||||
| Dusing [41] | 2010 | PET/CT | 20 | 5-10 min | Unknown | 85% |
| Yu [42] | 2011 | PET | 8 | 2 min | 6.3-2,012 | 100% |
| Haseebuddin [43] | 2013 | PET/CT | 107 | 10-15 min | 1.4-225.4 | 68% |
| Dusing [26] | 2014 | PET/CT | 120 | Unknown | Mean 7.6 | 68% |
The results of our study demonstrate that the positivity of 11C-Acetate PET/CT imaging is strongly correlated with the PSA value at the time of image acquisition. Our calculated optimal PSA threshold value for obtaining a 11C-Acetate PET/CT scan is 1.09 ng/mL, with a selected ROC sensitivity cutoff of 80%. Our results are similar to the findings of European studies that used 11C-Choline or 18F-Choline where the PSA is greater than 1.0-2.0. A large-scale investigation of 11C-Choline by Giovacchini [23] calculated a PSA threshold value of 1.4 ng/mL, with a sensitivity and specificity of 73% and 72%, respectively. Castellucci [24] found a higher optimal PSA threshold value of 2.43 ng/mL when examining 11C-Choline for imaging, with sensitivity and specificity of 73% and 69%, respectively. Similarly, Graute [25] established a PSA threshold value of 1.74 ng/mL for 18F-choline that was associated with a sensitivity of 82%. In a recent study by Dusing [26] with 11C-Acetate PET/CT, a PSA cut-off value of 1.24 ng/mL was found to have a sensitivity of 86.6%.
The detection rate for cancer recurrence or metastatic disease in our analysis of 11C-Acetate was 88%, which compares to 42-74% reported for 11C-Choline and 18F-Choline [25,27-29]. A recent comparative analysis by Yu et al [30] also found 11C-Acetate tended to be advantageous over 11C-Choline in detecting local recurrence and lymph node lesions.
Lesion detection at lower PSA levels (< 1.0 ng/mL) are of more particular clinical interest. It is at this low range where many treatment decisions are made, such as otherwise “blind” radiation to the prostate bed or initiation of hormone therapy. Determining the optimal time to perform a 11C-Acetate PET/CT scan in patients with biochemical recurrent prostate cancer would be expected to increase the likelihood of localizing recurrent tumor sites at a point in time early enough to allow for more successful use of salvage therapies. Disease-free survival is significantly improved for salvage radiotherapy for example when PSA levels are < 2.0 ng/mL [31-33]. Choline performance in this range is suboptimal, with a reported detection rate of 44% [28]. In our study, a large segment of patients (161) were evaluated with a PSA of < 1.0 ng/mL which afforded analysis in this PSA range not feasible in most prior smaller studies. 11C-Acetate PET/CT performance in this low PSA range was seen to be significantly higher than that reported for Choline, with a 11C-Acetate detection rate of 74%.Additionally, when 11C-Acetate imaging was performed when the PSA was < 1.0 ng/mL and with a short PSA doubling time (< 3 months), the detection rate was 90%.
Our study overcomes some of the limitations sited in prior small statistically under powered studies. To our knowledge, this is the largest published experience with 11C-Acetate PET/CT in North America. Data was available for comparative and logistic regression analysis in 721 patients of which 161 (22%) had PSA values < 1.0 ng/mL at the time of referral for imaging. Histology, subsequent imaging and radiation/cryotherapy treatment response for true positive analysis was available in a large number (325) of patients.
There are some limitations in our study. Treatment regimens were not standardized between patients. Some patients had multiple types of therapies before the imaging and still met our inclusion criteria. It is possible that patients who failed one form of treatment might be more likely to develop recurrence and may have higher rates of 11C-Acetate positivity. Although fairly well balanced, our study also included a large group of patients with radiation as the primary treatment or those who failed both prostatectomy and salvage bed radiation therapy. In contrast, some of the prior studies with 11C-Choline and 18F-Choline included only those patients with prior prostatectomy. Additional subgroup analysis of our data may provide insights to some of these disparities. There was also a trend for referral for imaging with shorter PSA doubling times (< 10-12 months [87%]) as this was a common clinical trigger for considering changes to treatment, whereas patients with longer doubling times (i.e. > 12 months) are at lower risk [32,34] and were less likely to require intervention. This may have increased our overall detection rate in comparison to other studies. Nevertheless, our cohort likely accurately reflects typical clinical practice, where changes in treatment for higher risk patients with biochemical recurrence would benefit from more precise staging prior to initiating additional treatment.
11C-Acetate shows higher metabolic uptake in tumor foci compared to normal prostate tissue; however, similar to 11C-Choline, there is significant overlap with benign prostate hyperplasia nodules and prostatitis [35,36]. This limits 11C-Acetate’s utility in the primary diagnosis of prostate adenocarcinoma, and may also be an important source of false positives in those previously treated with radiation or focal therapy alone, as BPH and prostatitis may occur in those settings.
A review of the resulting changes to treatment management was not part of this initial analysis - to be addressed along with duration of treatment response in a subsequent patient follow up analysis. Nevertheless, a high level of treatment changes can be ascertained by the 75 patients whereby the findings on 11C-Acetate imaging prompted focal therapy to the identified target(s). An additional 220 patients were treated with focal treatment to 11C-Acetate positive lesions in conjunction with systemic treatment (ADT, ADT + Chemo). Taking just these subsets of patients into consideration, 11C-Acetate imaging contributed to at least a 33% change in management.
In conclusion, 11C-Acetate PET/CT appears to have a high detection rate for the site of recurrence/metastasis in biochemical relapsed prostate cancer (88% overall detection rate, PPV 90.8%). In this large series of patients with early post injection imaging on modern PET/CT equipment, our analysis suggests 11C-Acetate imaging performed when the PSA > 1.09 ng/mL or a with a PSA doubling time of < 3.8 months when the PSA was below 1.0 ng/mL had the highest likelihood of producing positive findings. Particularly evident with 11C-Acetate imaging in our cohort was the high detection of locally recurrent and pelvic nodal disease (58%), both of which are potentially amenable to additional focal therapy with a curative intent.
Acknowledgements
This work was supported by grants from the Almeida Molecular Imaging Foundation and radiopharmacy support from Cardinal Health.
Disclosure of conflict of interest
None.
References
- 1.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170:1872–1876. doi: 10.1097/01.ju.0000091876.13656.2e. [DOI] [PubMed] [Google Scholar]
- 3.Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100:1283–1292. doi: 10.1002/cncr.20093. [DOI] [PubMed] [Google Scholar]
- 4.Rosser CJ, Chichakli R, Levy LB, Kuban DA, Smith LG, Pisters LL. Biochemical disease-free survival in men younger than 60 years with prostate cancer treated with external beam radiation. J Urol. 2002;168:536–541. [PubMed] [Google Scholar]
- 5.Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:629–633. doi: 10.1016/s0360-3016(00)00717-3. [DOI] [PubMed] [Google Scholar]
- 6.Babaian RJ, Troncoso P, Steelhammer LC, Lloreta-Trull J, Ramirez EI. Tumor volume and prostate specific antigen: implications for early detection and defining a window of curability. J Urol. 1995;154:1808–1812. doi: 10.1016/s0022-5347(01)66790-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson JK, Oesterling JE. Patient evaluation if prostate-specific antigen becomes elevated following radical prostatectomy or radiation therapy. Urol Clin North Am. 1994;21:677–685. [PubMed] [Google Scholar]
- 8.Goad JR, Chang SJ, Ohori M, Scardino PT. PSA after definitive radiotherapy for clinically localized prostate cancer. Urol Clin North Am. 1993;20:727–736. [PubMed] [Google Scholar]
- 9.Leibman BD, Dillioglugil O, Wheeler TM, Scardino PT. Distant metastasis after radical prostatectomy in patients without an elevated serum prostate specific antigen level. Cancer. 1995;76:2530–2534. doi: 10.1002/1097-0142(19951215)76:12<2530::aid-cncr2820761219>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. J Urol. 1994;152:1358–1368. doi: 10.1016/s0022-5347(17)32422-9. [DOI] [PubMed] [Google Scholar]
- 11.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 12.Almeida FD, Patino C, Blackwell E. C11-Acetate PET/CT compared to F-18 FDG PET for men with early recurrent prostate adenocarcinoma. Radiological Society of North America Scientific Assembly and Annual Meeting. 2012 [Google Scholar]
- 13.Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18 F] fluorocholine and [18 F] fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168:273–280. [PubMed] [Google Scholar]
- 14.Dimitrakopoulou-Strauss A, Strauss LG. PET imaging of prostate cancer with 11C-acetate. J Nucl Med. 2003;44:556–558. [PubMed] [Google Scholar]
- 15.Fricke E, Machtens S, Hofmann M, van den Hoff J, Bergh S, Brunkhorst T, Meyer GJ, Karstens JH, Knapp WH, Boerner AR. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 16.Hautzel H, Muller-Mattheis V, Herzog H, Roden W, Coenen HH, Ackermann R, Muller-Gartner HW, Krause BJ. [The (11C) acetate positron emission tomography in prostatic carcinoma. New prospects in metabolic imaging] . Urologe A. 2002;41:569–576. doi: 10.1007/s00120-002-0244-9. [DOI] [PubMed] [Google Scholar]
- 17.Kotzerke J, Volkmer BG, Glatting G, van den Hoff J, Gschwend JE, Messer P, Reske SN, Neumaier B. Intraindividual comparison of [11C] acetate and [11C] choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25–30. [PubMed] [Google Scholar]
- 18.Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1380–1384. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 19.Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, Sadato N, Yamamoto K, Okada K. 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181–186. [PubMed] [Google Scholar]
- 20.Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, Kibel AS, Andriole GL, Picus J, Welch MJ. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549–555. [PubMed] [Google Scholar]
- 21.Dotan ZA, Bianco FJ Jr, Rabbani F, Eastham JA, Fearn P, Scher HI, Kelly KW, Chen HN, Schoder H, Hricak H, Scardino PT, Kattan MW. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J. Clin. Oncol. 2005;23:1962–1968. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida FD, Yen CK, Finkelstein SE, Bans LL, Scholz MC, Lam RY. Early imaging improves performance of C11-Acetate PET/CT for recurrent prostate adenocarcinoma. Uro Today Int J. 2013;6 art 66. [Google Scholar]
- 23.Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, Cozzarini C, Di Muzio N, Rigatti P, Fazio F, Messa C. Predictive factors of [(11)C] choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301–309. doi: 10.1007/s00259-009-1253-3. [DOI] [PubMed] [Google Scholar]
- 24.Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, Franceschelli A, Martorana G, Manferrari F, Fanti S. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–1400. doi: 10.2967/jnumed.108.061507. [DOI] [PubMed] [Google Scholar]
- 25.Graute V, Jansen N, Ubleis C, Seitz M, Hartenbach M, Scherr MK, Thieme S, Cumming P, Klanke K, Tiling R, Bartenstein P, Hacker M. Relationship between PSA kinetics and [18F] fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39:271–282. doi: 10.1007/s00259-011-1970-2. [DOI] [PubMed] [Google Scholar]
- 26.Dusing RW, Peng W, Lai SM, Grado GL, Holzbeierlein JM, Thrasher JB, Hill J, Van Veldhuizen PJ. Prostate-specific antigen and prostate-specific antigen velocity as threshold indicators in 11C-acetate PET/CTAC scanning for prostate cancer recurrence. Clin Nucl Med. 2014;39:777–783. doi: 10.1097/RLU.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwee SA, Coel MN, Lim J. Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med. 2012;26:501–507. doi: 10.1007/s12149-012-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308–1313. doi: 10.1016/j.juro.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 29.Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, Hruby G, Fogarty G, Jagavkar R, Kneebone A, Hickey A, Fanti S, Tarlinton L, Emmett L. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 30.Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601. [PMC free article] [PubMed] [Google Scholar]
- 31.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–375. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 32.McLeod DG. The effective management of biochemical recurrence in patients with prostate cancer. Rev Urol. 2005;7(Suppl 5):S29–36. [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, Klein EA, Kupelian PA, Roehrborn CG, Pistenmaa DA, Pacholke HD, Liauw SL, Katz MS, Leibel SA, Scardino PT, Slawin KM. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 34.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 35.Mena E, Turkbey B, Mani H, Adler S, Valera VA, Bernardo M, Shah V, Pohida T, McKinney Y, Kwarteng G, Daar D, Lindenberg ML, Eclarinal P, Wade R, Linehan WM, Merino MJ, Pinto PA, Choyke PL, Kurdziel KA. 11C-Acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. J Nucl Med. 2012;53:538–545. doi: 10.2967/jnumed.111.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarzenbock S, Souvatzoglou M, Krause BJ. Choline PET and PET/CT in primary diagnosis and staging of prostate cancer. Theranostics. 2012;2:318–330. doi: 10.7150/thno.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996–1000. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, Soloviev D, Hany TF, Miralbell R. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (< 1 ng/mL) after radical prostatectomy. BJU Int. 2007;99:1415–1420. doi: 10.1111/j.1464-410X.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 39.Wachter S, Tomek S, Kurtaran A, Wachter-Gerstner N, Djavan B, Becherer A, Mitterhauser M, Dobrozemsky G, Li S, Potter R, Dudczak R, Kletter K. 11C-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J. Clin. Oncol. 2006;24:2513–2519. doi: 10.1200/JCO.2005.03.5279. [DOI] [PubMed] [Google Scholar]
- 40.Albrecht S, Buchegger F, Soloviev D, Zaidi H, Vees H, Khan HG, Keller A, Bischof Delaloye A, Ratib O, Miralbell R. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging. 2007;34:185–196. doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 41.Dusing RW, Drisko JA, Grado GG, Levine M, Holzbeierlein JM, Van Veldhuizen P. Prostate imaging modalities that can be used for complementary and alternative medicine clinical studies. Urol Clin North Am. 2011;38:343–357. doi: 10.1016/j.ucl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, Link JM, Montgomery RB, Higano CS, Eary JF, Mankoff DA. C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: relative findings and response to therapy. Clin Nucl Med. 2011;36:192–198. doi: 10.1097/RLU.0b013e318208f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haseebuddin M, Dehdashti F, Siegel BA, Liu J, Roth EB, Nepple KG, Siegel CL, Fischer KC, Kibel AS, Andriole GL, Miller TR. 11C-acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J Nucl Med. 2013;54:699–706. doi: 10.2967/jnumed.112.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]


