Abstract
Increasing recognition of the importance of brown adipose tissue (BAT) motivates the development of reproducible and quantitative methods for measuring it. Positron emission tomography (PET)/computerized tomography (CT) with 18F-fluorodeoxyglucose (FDG) has become the principal method to non-invasively detect brown adipose tissue (BAT) in humans. Improvements in quantitation and standardization will drive further clinical application. One disorder hypothesized to involve dysregulation in thermoregulation and the processing of pain involving BAT is fibromyalgia syndrome (FMS). This report describes an approach with additional technical standardization to measure cold-inducible, BAT activity (ci-BAT) semi-quantitatively and reliably with minimal operator intervention with the FDG PET/CT technique. Ci-BAT was measured to test whether FMS patients have decreased BAT activation compared to normal controls. Threshold parameters to optimally separate ci-BAT from non-ci-BAT were developed based on the distribution of the pixel-wise parametric data from each merged PET/CT scan for each study session occurring on different days. BAT activity was the same under warm conditions in both control and FMS subjects attesting to reproducibility and reliability. However, considerable variability arose between groups at cool temperatures consistent with other literature. Increases in ci-BAT activity were significantly less in FMS patients than in controls, as hypothesized. Ci-BAT recruitment can be quantified non-invasively using FDG PET/CT using semi-automated techniques in human subjects across different diagnostic groups or within groups undergoing manipulations of interest.
Keywords: Glucose metabolism, Randall cycle, software, obesity, diabetes, fibromyalgia, positron emission tomography, fluorodeoxyglucose, brown adipose tissue
Introduction
This report describes the characteristics and measurement of brown adipose tissue (BAT) in humans using FDG PET/CT. The need to standardize the conditions under which BAT is assayed led to several modifications of the usual approach. In particular, semi-automated image analysis of the PET/CT scans increased efficiency and minimized dependence on operators. As a sample application, these methods were applied to a preliminary study of BAT in fibromyalgia syndrome (FMS).
BAT is characterized by many small fat vacuoles, a large number of mitochondria, uncoupling protein (UCP1), dense sympathetic innervation, and dense tissue vascularization [1]. BAT localizes in humans at supraclavicular, perirenal, paraspinal, and mediastinal regions playing an important role in lipid metabolism, particularly during thermogenesis upon exposure to cold [2]. BAT uncouples oxidative metabolism from producing ATP thereby generating heat [3]. Given current problems with obesity and diabetes, understanding the modulation of BAT using various interventions (exercise, diet, drugs, etc.) becomes of great clinical interest [4]. Therefore, measurement of BAT activity becomes an important dependent variable.
The routine, straightforward, semi-quantitative estimation of BAT by using 18F-fluorodeoxyglucose (FDG) PET/CT involves several steps [5]. First, the subjects incorporate FDG at the ambient temperature with some degree of fasting (e.g., 4-6 h). The glucose concentration is checked to ensure there is no hyperglycemia. After uptake, a PET/CT scan is obtained; a conventional, single energy, fused CT scan is used for attenuation correction. After scan acquisition, the regions of putative fat are identified by their density based on the CT scan, since muscle and bone have clearly different radiographic densities. Since the differences in density between BAT, beige, and white fat are small, routine CT scans do not readily differentiate between these deposits. So, only the fat compartment can be reliably measured with PET/CT. Second, to identify BAT, a threshold of uptake of FDG is empirically determined in the fat compartment. The threshold can be visually estimated. Alternatively, a threshold can be estimated based on routine clinical FDG PET/CT imaging of individuals with negative studies undergoing diagnostic evaluation for other purposes, for example, tumor detection. This threshold is then applied to the scan in question taken under the same conditions to define regions of interest of BAT. BAT activity can then be expressed as the mean or maximal FDG uptake in the region of interest above background adjusted for the injected FDG dose and total or lean body weight (SUV, standardized uptake value).
Several prior reviews have summarized the data on BAT activity as measured with FDG PET/CT in humans as well as discussed the strengths and limitations of different measurement methods [6-9]. Research has highlighted the need for increased standardization of conditions because FDG uptake depends on multiple factors such as age, gender, body mass index (BMI), ambient temperature, outdoor temperature, time of day, and diet [10-15]. Here, we report one such approach among ongoing developments to estimate ci-BAT reliably, semi-quantitatively, and efficiently using FDG PET/CT that despite limitations remains currently the gold standard in BAT quantitation in humans.
A method to estimate BAT activation based upon FDG uptake in a standardized manner is to physiologically either maximally block FDG uptake on a control scan and to stimulate maximally in the activation condition. As noted previously, numerous studies find some subjects can show FDG uptake in BAT during uptake at normal ambient room temperatures particularly in the winter. To establish a control baseline without any FDG uptake, some manipulation must ensure no uptake occurs. Cold exposure in the fasting state has become the standard method to stimulate FDG uptake in humans. The contrast between the two scans provides a defined measure of cold-inducible BAT (ci-BAT). To this end, BAT FDG glucose uptake was physiologically maximized by a low fat, low carbohydrate (LFLC) diet, fasting, and exposure to cold. BAT uptake was minimized through a combination of a high fat, low carbohydrate, protein-sparing (HFLCPS) diet and exposure to warm temperature. The low carbohydrate diet followed by fasting prevents postprandial hyperglycemia avoiding competition with FDG uptake. The HFLCPS diet decreases FDG uptake perhaps through greater dietary free fatty acids relative to glucose [12,13,16]. Through the Randall cycle (glucose-fatty acid cycle), higher concentrations of fatty acids stimulate their oxidation thereby blocking glucose uptake and flux through glycolysis in BAT [17].
Another parameter of importance is the blood glucose concentration. Serum glucose competes with FDG for uptake. Although hyperglycemia is typically excluded by checking with a glucometer before performing FDG PET/CT, variable concentrations of glucose can impact BAT measurements as well as can small differences in injected FDG dose or lean body mass. Brain FDG uptake appears to follow BAT uptake even following a carbohydrate meal [18]. Since brain glucose consumption at rest is physiologically maintained constant, mean total brain FDG uptake was used to further standardized BAT uptake.
Additional standardization was achieved by developing software to semi-quantitatively measure BAT with PET/CT to increase efficiency and reproducibility. Thresholds were defined quantitatively based upon parameter distributions of the total fat compartment and BAT regions with FDG uptake. To this end, scripts were developed that minimized operator intervention. For the scan having FDG uptake blocked, the threshold was selected such that less than 5% of the voxels were above threshold. As an example, these approaches were applied to a clinical problem.
Patients with FMS, a chronic pain syndrome with multiple sites of musculoskeletal tenderness and pain, show several physiological abnormalities. There appears dysregulation between the thermoregulatory and pain modulation systems that share many sites of convergence in the central nervous system [19]. In particular, FMS sufferers show heightened sympathetic activity and impaired thermoregulation with decreased body temperature compared to control subjects. Cold exacerbates the pain. These facts motivate the hypothesis that impaired BAT activation may occur in response to cold stress. The details of the FMS study are not relevant to this report that focuses on imaging; those results will be presented in detail elsewhere. The application to this particular question of the standardized, semi-automated approach described here along with related software should expedite the measurement of BAT in humans and in preclinical models.
Materials and methods
The study was approved by the institutional review board (IRB), and all subjects signed a written informed consent. All subjects were female: 11 adult subjects without significant medical illness were matched with 13 patients with FMS (diagnosed by American College of Rheumatology 2010 criteria) on the basis of body mass index (controls = 24.76 kg/m2; FMS 25.32 kg/m2) and season. Controls available were younger than patients with fibromyalgia (controls = 26 years; FMS = 37 years). They were studied on two separate days on average 18 days apart counterbalancing the order of temperature conditions.
Experimental times were maintained constant across subjects as BAT displays a thermogenic biorhythm [15]. Subjects were not to exercise strenuously before the study. Subjects had an overnight fast followed in counter-balanced manner by either of two early morning breakfasts (8:00-8:30 AM) on different days: 1) HFLCPS diet to minimize FDG uptake in BAT via the Randall cycle; or 2) low fat, low carbohydrate (LFLC) diet to maximize FDG uptake. At 10:30 AM the subjects were clothed in a light hospital gown and were laid on a recliner in a temperature-controlled room [warm, 21°C (75°F) for HFLCPS condition; or cool, 17°C (62°F) for LFLC condition]. Using cooler temperatures consistently led to increased shivering. At 1:00 PM (2.5 hours later) while in the same room, a blood sample was drawn, and the concentration of blood glucose concentration was checked (< 150 mg/dl). The subject was injected over 5-10 s intravenously with a bolus of 10 ml saline containing FDG (Siemens PETNET, Minneapolis, MN) at a dose of 185 MBq (5 mCi)/70 kg. The subject rested in the quiet room with eyes closed and covered along with a noise dampening headset. Isotope uptake occurred from 1:00 PM to 1:45 PM at the assigned temperature. The subject was monitored to ensure wakefulness.
At 1:45 PM after FDG uptake, the subject was then transferred from the temperature controlled room to the PET/CT suite and situated in a Siemens Biograph 12 PET/CT (Siemens, Knoxville, TN) in 3D mode producing an image with eighty-one 2 mm slices. A bismuth-coated latex eye shield (Universal Medical, Norwood, MA) minimized radiation exposure to the eye lens.
The parameters for the CT protocol were spiral acquisition mode; 130 kV; 90 mAs; slice collimation 2 mm; slice width 3 mm; feed 6.6 mm/rotation; pitch 0.55; scan rotation time 1 s; increment 3 mm; field of view 16.2 cm; number of slices 109; scan time 25.6 s; and kernel H31 s. PET parameters were time, 15 min; reconstruction with attenuation correction; FORE OSEM; matrix size 256 × 256; zoom 2; trim factor 2; iterations 6; subset 16; filter Gaussian 2 mm; match CT slice position ON. Each PET scan was corrected for attenuation with the associated fused CT scan. It is noteworthy that it was not possible to precisely duplicate the subject’s position between scanning sessions preventing straightforward image subtraction across imaging sessions. The field of interest for this work sampled from the head to thorax just below the clavicles and required two bed positions. Data were corrected for decay, electronic deadtime, randoms, and scatter. PET and CT scans for each session were merged using FLIRT (FSL, Oxford, UK). To account for differences in dose, body composition, tracer delivery, and to serve as an internal standard, the FDG data were normalized through proportional scaling to whole brain glucose uptake (20,000 counts) which remains relatively constant under physiological conditions. For comparison to other literature, the FDG PET/CT scans show normalized counts (0-14,000; threshold 3,500) and SUV (0-8, threshold SUV = 2 using the kBq/ml quantitation from the DICOM header and calculated based on body weight); if based upon lean body weight, the SUV would be 1.1-1.7 fold greater.
Results
The algorithm and scripts are available upon request and at https://github.com/CNUHub. The procedure and algorithm are outlined in Figure 1. Pertinent details follow. Note that it was not possible to exactly duplicate the positioning across the two scan sessions precluding direct image subtraction. A bounding box was used to isolate the head. A histogram of the extra-cerebral voxel intensities of all CT scans are shown in Figure 2A; the threshold for all fat was set from 887 to 999 HU (~FWHM) disregarding the subtle differences in attenuation between white and brown adipose tissue that were not apparent. This threshold generated a template for all fat that was merged subsequently with the coregistered (fused) PET scan for each individual session on different days (warm, cool). Figure 2 also shows the histograms of voxel activity in bins of differing radioactivity used to isolate BAT at 21°C (75°F; Figure 2B) and at 17°C (62°F; Figure 2C). Subtracting studies in the warm condition from those done in the cool condition shows a rightward shift in distributions (Figure 2D). A probability density as a function of counts indicates that a threshold of 2,745 counts (as shown in Figure 2B-D) excludes 95% of all fat voxels as active under conditions designed to turn off BAT FDG uptake (HFLCPS, warmth; i.e., false positive; not shown). Histograms of activity (FDG uptake) within the template of fat were generated for each temperature (cold, warm) and subject group (control, patient).
Figure 1.

Cartoon of experimental protocol. Timing is shown on left. Black rectangle along timeline indicates FDG uptake period. Scans show subjects exposed to warmth (red; 21°C, 75°F) and high fat, low-carbohydrate, protein sparing (HFLCPS) breakfast on (left); coolness (blue; 17°C, 62°F) and low fat, low carbohydrate (LFLC) breakfast (right). FDG PET scans are placed arbitrarily and are not linked to timeline. Dotted outline of face shows slight differences in positioning (arrows draw attention to the position of the head and mandible that may not be identical across the two scanning sessions) preventing voxel-wise subtraction. Black irregular regions in right scan symbolize FDG uptake of BAT in cold.
Figure 2.
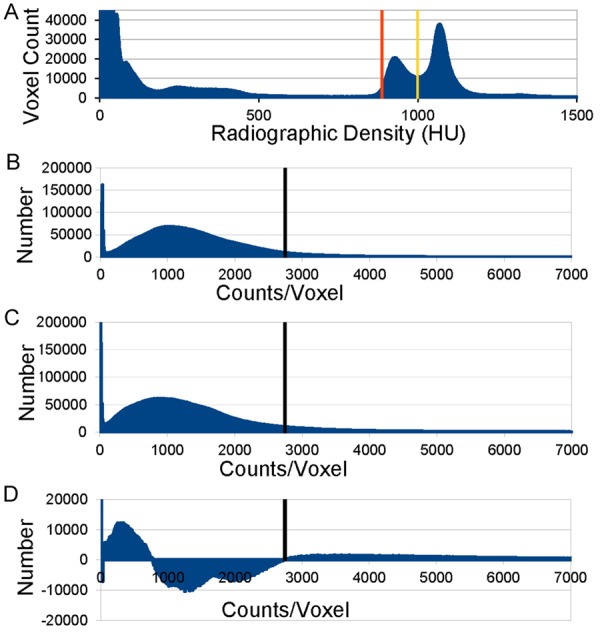
Histogram of number of voxels (voxel count) vs. density or radioactivity. (A) CT density (HU) of all extracerebral voxels from all PET/CT studies. (B) Distribution from all studies performed at 21°C; maximum ordinate 2(10)5. (C) Distribution from all studies performed at 17°C; maximum ordinate 2(10)5. Note rightward shift in comparison to B. (D) Distribution difference between all studies performed at 17°C (B) minus those at 21°C (C); maximum ordinate at 2(10)4. Black vertical bar (B-D) = threshold at 2,745 counts.
Figure 3 shows a representative scan from one subject; the fat template (blue; Figure 3B); FDG PET/CT in warm (Figure 3C) and in cold (Figure 3D). The color scale (same in C and D) was selected to highlight activated BAT. Of note, simple visual inspection of the scans using a color scale covering the entire range of radioactivity measured could not detect consistently differences between temperature conditions. CT, PET, and fused PET/CT demonstrate evidence of BAT in the neck and peri-clavicular region. The CT radiolucency highlighted merges with FDG uptake in the PET scan. It was not possible to directly subtract the images across study sessions; the precise reproduction of the positioning of head, neck and thorax was not possible (see Figure 1). Therefore, each scan session required separate analysis as outlined in Figures 2 and 3. Based on the approach outlined here, the threshold for FDG uptake for BAT was 3,500 counts or SUVtotal bodyweight 2. The latter would be 1.1- to 1.7-fold greater if calculated with lean body weight.
Figure 3.

FDG uptake in a control subject at warm (E102) and cool (E101) ambient temperatures. A. CT scan for warm session. B. Fat template (blue; E101) for thresholds in Figure 1 superimposed on CT scan. C. FDG uptake in PET/CT (E102) at warm temperature. D. FDG uptake in PET/CT (E101) at cool temperature. Note difference in positions of the neck between E101 and E102 (same subject, different scanning sessions). Color scale show counts/SUVtotal body weight; threshold 3,500 counts or SUV 2.
Figure 4 shows the scatter plot of BAT FDG uptake. FDG uptake at warm temperature was the same across groups and showed good reliability as indicated by the small standard error of the mean. The HFLCPS appeared effective at blocking FDG uptake. There was considerable heterogeneity in the cold condition across both groups, but FMS patients showed a decrease in uptake despite a large outlier that was not removed from the data [Wilcoxon Mann-Whitney test: n1 = 10; n2 = 12; U = 86.0; P (one-tail) = 0.05].
Figure 4.
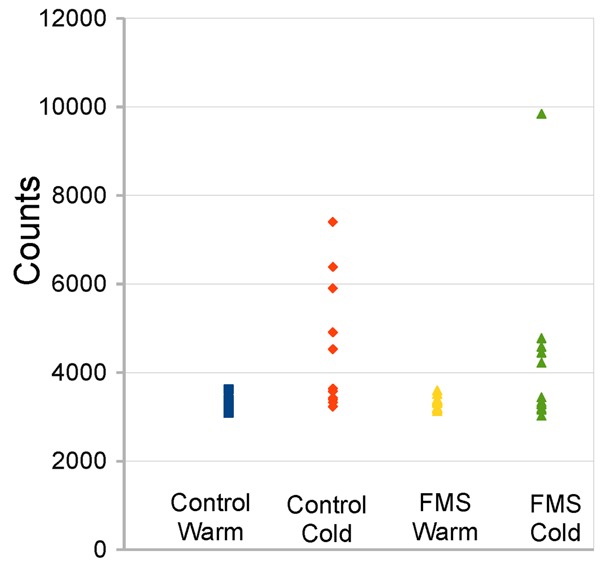
Scatter plot of BAT activity across subject groups at cool vs. warm ambient temperatures. Note distribution is not normal. One probable outlier occurred in the FMS patients in the cool condition. For statistical analysis, this outlier was retained.
Discussion
Several studies using FDG PET/CT have previously assessed BAT activity in humans [6-13,18,20]. These works have highlighted the need for increased standardization of conditions because FDG uptake depends on multiple factors and analysis strategies vary widely. Although FDG PET/CT remains the current gold standard for non-invasive imaging of BAT in humans, recent reviews highlight the need for greater standardization across studies as well as the limitations of the technique [6-9]. There remains a need for additional procedures and general purpose computer software to estimate objectively, reliably, and non-invasively ci-BAT FDG uptake. Such work will likely be of particular use to ongoing studies of BAT for translational application to human metabolism, diabetes, and obesity.
FDG uptake as measured here was highly consistent and homogeneous across subjects under warm conditions. In-vivo tracer studies in humans under carefully controlled conditions do not find significant uptake of glucose or free fatty acids (FFA) under warm conditions in most subjects [21]. Therefore, the FDG uptake measures under warm conditions and HFLCPS diet likely represent background. There was marked heterogeneity in glucose uptake in the cold as reported previously [21,22]. This heterogeneity occurs in normal healthy subjects, and has been hypothesized to arise from the inherent variability in the amount of BAT at baseline across individuals [22]. The variability is likely greater in patient populations such as FMS with dysfunctional regulatory mechanisms that may impair BAT activation itself.
The automated procedures described herein can lead to significant savings in time as well as avoiding operator bias. In practice, the thresholds used here can be used as the initial pass through the data. They can be verified and modified for a specific implementation after several studies are collected, distributions examined, and specific thresholds selected (i.e., scripts: adipose_threshold; ci_bat_threshold; see https://github.com/CNUHub).
Two other studies have reported some automation of the analysis of FDG PET/CT in the assessment of BAT. Ruth et al developed a screening algorithm to flag routine clinical FDG PET/CT scans to converge with a radiologist’s visual score of 0 (no BAT) to 2 (BAT positive) [23]. That approach did not provide specific preparatory conditions, cold exposure, or quantitation; various imaging parameters were not defined based upon parametric analysis of voxel distributions. The automated method of Gifford et al used FDG PET/CT under thermoneutral and cold exposure to develop a mask for activated BAT in order to examine MRI properties of activated BAT [24]. Critically, identical positioning was required for both PET/CT sessions which, when implemented in practice, produced some misalignment between sessions. As noted above, subtle misalignments can produce significant artifacts without deploying diffeomorphic methods. The development of PET/MRI and navigator scans to precisely monitor the position of the subject over time will likely overcome these problems in the future.
The major limitation of the work here is intrinsic to the FDG technique itself. Since BAT can metabolize both glucose and lipids; FDG uptake does not measure the combined metabolism of both substrates. Therefore, the FDG PET/CT technique even with absolute quantitation, dynamic imaging, and arterial input functions, necessarily underestimates BAT metabolic activity.
Under conditions similar to those used in the present study, upon exposure to cold there is an initial rapid metabolism of intracellular triglycerides via oxidation with subsequent increasing contributions by circulating glucose and FFA [21]. Quantitative estimates in healthy humans of whole-body cold-induced BAT uptake of glucose and FFA are 10.8 ± 4.5 μmol/min and 2.3 ± 0.8 μmol/min respectively, or approximately 5:1 [21]. Similar values have been reported for cold-induced BAT FDG uptake by other groups [22,25]. After 5 hours of cooling without shivering, healthy subjects with BAT deposits had a 15% increase in resting energy expenditure; 30% of this was accounted by glucose and 70% by FFA from plasma indicating most of the additional energy at this time point does not come from stored intracellular triglycerides but rather from the circulation [26]. Therefore, FDG PET/CT does not clearly measure total BAT metabolic activation highlighting the need for new techniques. These limitations spur development of novel methods as alternatives to FDG PET/CT such as new tracers, supraclavicular skin temperature, dual energy CT, magnetic resonance spectroscopy, and magnetic resonance imaging [27-29].
Several additional issues are worth noting. 1) Absolute quantitation of glucose metabolism with kinetic modeling was not performed in the present study--only glucose uptake relative to whole brain uptake. The brain reference was selected for normalization for several reasons: a) whole brain glucose uptake is relatively constant across subjects, b) is independent of insulin levels, c) uptake is less subject to variance arising from differences in dose, lean body weight, body surface area, and tracer delivery; and d) BAT FDG uptake during a fast/cold exposure or after a high-calorie meal during normal ambient conditions follows brain uptake [18]. Normalization avoids invasiveness, minimizes complexity, and decreases expensive scanner time. 2) The PET/CT scans from each of the two sessions on different days were not merged which would have allowed direct voxel-wise comparisons [24]. Subtle differences in positioning (neck and shoulder angles, rotation, etc.; arrows in Figure 1) made registration across sessions difficult without resorting to diffeomorphic methods that were not used. 3) There was no direct correlation between the glucose uptake measures with invasive FDG autoradiography or biochemical assay of the same tissues. For example, the histology of the various ranges of glucose uptake could in principle separate beige/brite fat from BAT. This must await future preclinical studies. 4) Partial volume effects in the PET/CT underestimate FDG uptake in small fat deposits. 5) Cooling was performed with room air; specialized cooling vests may offer better control. In this regard, it is worth noting that the entire FDG uptake period occurred at the stated room temperature (i.e., 17°C or 21°C). Moving the patient to the scanner or having body temperature change subsequently will not affect the measurements for an extended period thereafter. 6) Possible shivering was not measured (e.g., EMG) or controlled. 7) Whole body BAT uptake was not measured, just from below the clavicles superiorly. 8) Sample sizes were small and will require larger numbers for definitive results regarding BAT recruitment in FMS as well as to generalize to other groups of subjects. Of note, all subjects were female because fibromyalgia affects predominantly women. Also, future studies might recruit only subjects with abundant BAT to decrease the variance; however, this is not possible if BAT recruitment is impaired in patients. 9) Although the protocol is simple and efficient to implement clinically, cost remains a significant barrier to widespread implementation of FDG PET/CT. 10) Many studies use SUV thresholds for BAT uptake in the 1-2 range [14,21,27,30,31]. The thresholds using the present approach (SUV 2 using whole body weight) appear slightly more conservative. 11) Importantly, past approaches to quantitate BAT have not specifically attempted to minimize BAT FDG uptake. 12) As mentioned above, the characteristics of BAT metabolism are highly dependent on time; the FDG method is largely sensitive to the first 15 minutes of FDG uptake. Additional controls could include monitoring or making constant levels of metabolites (e.g., glucose, fatty acids, etc.) that all can impact uptake. Finally, this approach must be applied with caution especially in pathologic or nonphysiologic conditions (e.g., pharmacologic challenge). Under pathological (diabetes) or non-physiologic conditions (pharmacologic challenge), changes in biochemistry (e.g., hyperglycemia) impact the FDG technique.
The protocol described herein appears reliable and sensitive to the effects of cold on BAT activity. The automation improves efficiency for analysis and minimizes operator bias. It can be readily implemented using the software that is publically available and will improve the measurement of ci-BAT activity beyond what is currently available. For the first time, the protocol provided preliminary evidence for dysregulation in cold-inducible BAT activation in fibromyalgia syndrome.
Acknowledgements
This work was supported by NIAMS 3RO1AR056092, VA 5I01CX000501, the Department of Veterans Affairs, and the University of Minnesota. We thank our subjects for their generosity. The scripts for these analyses are available at https://github.com/CNUHub or upon request to Joel T. Lee (leexx235@gmail.com).
Disclosure of conflict of interest
None.
References
- 1.Kimball JW, editor. Kimball’s biology pages. Creative Commons Attribution 3.0 Unported (CC BY 3.0) License. 2016. http://www.biology-pages.info/A/AdiposeTissue.html (accessed September 1, 2016)
- 2.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 4.Calvani R, Leeuwenburgh C, Marzetti E. Brown adipose tissue and the cold war against obesity. Diabetes. 2014;63:3998–4000. doi: 10.2337/db14-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 6.Borga M, Virtanen KA, Romu T, Leinhard OD, Persson A, Nuutila P, Enerback S. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography) Methods Enzymol. 2014;537:141–159. doi: 10.1016/B978-0-12-411619-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, Brans B. Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging. 2014;41:776–791. doi: 10.1007/s00259-013-2611-8. [DOI] [PubMed] [Google Scholar]
- 9.van der Lans AA, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol. 2014;307:R103–113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 10.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 11.Skillen A, Currie GM, Wheat JM. Thermal control of brown adipose tissue in 18F-FDG PET. J Nucl Med Technol. 2012;40:99–103. doi: 10.2967/jnmt.111.098780. [DOI] [PubMed] [Google Scholar]
- 12.Williams G, Kolodny GM. Method for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. AJR Am J Roentgenol. 2008;190:1406–1409. doi: 10.2214/AJR.07.3205. [DOI] [PubMed] [Google Scholar]
- 13.Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, Kolodny G, Laham R. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 14.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 15.Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, Govendir MA, Emmett L, Greenfield JR. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 2016;23:602–609. doi: 10.1016/j.cmet.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Balink H, Hut E, Pol T, Flokstra FJ, Roef M. Suppression of 18F-FDG myocardial uptake using a fat-allowed, carbohydrate-restricted diet. J Nucl Med Technol. 2011;39:185–189. doi: 10.2967/jnmt.110.076489. [DOI] [PubMed] [Google Scholar]
- 17.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. 2013;98:57–64. doi: 10.3945/ajcn.113.059022. [DOI] [PubMed] [Google Scholar]
- 19.Larson AA, Pardo JV, Pasley JD. Review of overlap between thermoregulation and pain modulation in fibromyalgia. Clin J Pain. 2014;30:544–555. doi: 10.1097/AJP.0b013e3182a0e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrieze A, Schopman JE, Admiraal WM, Soeters MR, Nieuwdorp M, Verberne HJ, Holleman F. Fasting and postprandial activity of brown adipose tissue in healthy men. J Nucl Med. 2012;53:1407–1410. doi: 10.2967/jnumed.111.100701. [DOI] [PubMed] [Google Scholar]
- 21.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Ruth MR, Wellman T, Mercier G, Szabo T, Apovian CM. An automated algorithm to identify and quantify brown adipose tissue in human 18F-FDG-PET/CT scans. Obesity (Silver Spring) 2013;21:1554–1560. doi: 10.1002/oby.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB. Human brown adipose tissue depots automatically segmented by positron emission tomography/computed tomography and registered magnetic resonance images. J Vis Exp. 2015 doi: 10.3791/52415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 26.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne) 2012;3:15. doi: 10.3389/fendo.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu HH, Chung SA, Nayak KS, Jackson HA, Gilsanz V. Differential computed tomographic attenuation of metabolically active and inactive adipose tissues: preliminary findings. J Comput Assist Tomogr. 2011;35:65–71. doi: 10.1097/RCT.0b013e3181fc2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu HH. Magnetic resonance of brown adipose tissue: a review of current techniques. Crit Rev Biomed Eng. 2015;43:161–181. doi: 10.1615/CritRevBiomedEng.2015014377. [DOI] [PubMed] [Google Scholar]
- 30.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boon MR, Bakker LE, van der Linden RA, Pereira Arias-Bouda L, Smit F, Verberne HJ, van Marken Lichtenbelt WD, Jazet IM, Rensen PC. Supraclavicular skin temperature as a measure of 18F-FDG uptake by BAT in human subjects. PLoS One. 2014;9:e98822. doi: 10.1371/journal.pone.0098822. [DOI] [PMC free article] [PubMed] [Google Scholar]


