Abstract
Background
Dogs with spinal cord injury are at increased risk of developing bacteriuria due to increased residual urine volume. Cranberry extract inhibits binding of E. coli to uroepithelial cells, potentially reducing risk of bacteriuria.
Hypothesis
Cranberry extract reduces risk of bacteriuria in dogs after acute TL‐IVDH.
Animals
Client‐owned dogs with acute onset TL‐IVDH causing nonambulatory status.
Methods
Randomized, placebo‐controlled, blinded, prospective clinical trial. Dogs with acute TL‐IVDH were recruited 48 hours postoperatively and randomized to receive cranberry extract or placebo in a masked fashion. Urine cultures and neurological examinations were performed 2, 4, and 6 weeks postoperatively. The number of dogs with bacteriuria (all bacterial species) and bacteriuria (E. coli) were primary and secondary outcome measures and were evaluated using chi‐squared test. Urine antiadhesion activity (AAA) was measured in a subset (N = 47) and examined in a secondary analysis evaluating additional risk factors for bacteriuria.
Results
Bacteriuria was detected 17 times in 94 dogs (6 placebo, 11 cranberry, P = .12). There were 7 E. coli. positive cultures (1 placebo, 6 cranberry, P = .09). Dogs in both groups had positive urine AAA (14/21: placebo, 16/26: cranberry), and dogs with urine AAA had significantly fewer E. coli positive cultures (n = 1) than dogs without it (n = 4) (P = .047).
Conclusions and Clinical Importance
This clinical trial did not show a benefit of oral cranberry extract but had low power. Cranberry extract supplementation did not impact urine AAA, but a possible association between urine AAA and lower risk of E. coli bacteriuria was identified. Other doses could be investigated.
Keywords: Escherichia coli, Incontinence, Proanthocyanidins, Spinal cord injury
Abbreviations
- AAA
antiadhesion activity
- CFU
colony‐forming unit
- E. coli
Escherichia coli
- HPF
high powered field
- PACs
proanthocyanidins
- TL‐IVDH
thoracolumbar intervertebral disk herniation
- USG
urine specific gravity
- UTI
urinary tract infection
Acute thoracolumbar intervertebral disk herniation (TL‐IVDH) is common in chondrodystrophoid breeds of dog such as the Dachshund.1 Herniated disk material injures the overlying spinal cord, and while the resulting paralysis receives much attention, the effect on bladder function is also critical to both dog and owner. Injury to the thoracolumbar spinal cord causes failure of micturition, resulting in urine retention and overflow incontinence, and a predisposition to the development of bacteriuria.2, 3, 4
After spinal cord injury, recovery of hindlimb function and micturition occur gradually.5 Bladder dysfunction secondary to myelopathy is a risk factor for UTI in dogs in both the perioperative period6, 7 and the subsequent 3 months despite recovery of motor function and voluntary urination,8 with E. coli accounting for 58% of the infections. Current definitions of urinary tract infection (UTI) require the presence of clinical signs such as pain and pollakiuria,9, 10 and the frequency with which bacteriuria in this population develops into UTI has not been investigated. This is compounded by the difficulty in detecting signs in paraparetic dogs. As such, prior reports can be interpreted as describing bacteriuria. However, given the higher risk of bacteriuria and its potential to cause UTI in the period after surgery for TL‐IVDH, use of an effective preventative treatment during this period could be beneficial. Historically, cranberries are widely used to improve the health of the lower urinary tract in humans.11, 12, 13 They contain a mixture of organic acids as well as fructose and glucose and phytochemicals such as proanthocyanidins (PACs).12, 14 In vitro, A‐type PACs inhibit the adhesion of type I and P‐fimbriated uropathogenic bacteria, in particular Escherichia coli (E. coli), to uroepithelial cells reducing the risk of UTIs.15, 16
There are numerous clinical trials evaluating the efficacy of cranberry juice and extract in humans with different causes of recurrent UTI. The results of these trials often appear contradictory, reflecting the use of a wide range of different cranberry products and the evaluation in different clinical scenarios. Recent meta‐analyses conclude that there is no statistical evidence that cranberry extract reduces the recurrence of UTIs across different populations,17 with the exception of young women with recurrent UTIs.13 There are several studies in humans with spinal cord injury, also with conflicting results.17, 18, 19, 20 However, it is difficult to extrapolate these findings to dogs recovering from SCI, because these studies were performed in permanently paralyzed patients with no voluntary voiding. We hypothesized that oral cranberry extract would reduce the frequency of bacteriuria in the 6‐week postoperative period after surgery for acute TL‐IVDH. The aim of this randomized, double‐blinded, placebo‐controlled clinical trial was to evaluate the effect of a product containing cranberry‐derived PACs on the frequency of bacteriuria, and E. coli bacteriuria in dogs recovering from acute TL‐IVDH.
Materials and Methods
Study Design
This study was a prospective, randomized, double‐blinded, placebo‐controlled multicenter clinical trial performed in dogs with acute TL‐IVDH. The study was designed and reported according to the CONSORT guidelines.18 All protocols were reviewed and approved by the NCSU Institutional Animal Use and Care Committee (protocol number: 11‐009‐O). Previously reported data on the frequency of bacteriuria in this population of dogs8 were used to calculate the group size for this 2 arm trial with an online power estimator.a An estimate of the size of anticipated treatment effect was based on human clinical trials in which cranberry extract reduced the incidence of UTIs in women by approximately 50% when compared with placebo or lactobacillus.19 Fifty‐two animals would be needed to produce a 50% reduction in bacteriuria with an 80% power and a 5% significance level; increasing the number of animals to 75 per treatment arm would increase power to 91.3%, allow detection of a smaller therapeutic effect, and allow for case attrition. The study aimed to recruit 75 animals per treatment group. The primary outcome measure was the total number of urine samples with bacteriuria that reached specific thresholds in terms of numbers of colony‐forming units (CFU) recorded over a 6‐week period; the number of E. coli positive cultures was defined as the secondary outcome measure.
Inclusion criteria were acute onset (less than 2 days) of nonambulatory paraparesis or paraplegia due to an acute TL‐IVDH, surgical decompression, and ability to return for rechecks at 2, 4, and 6 weeks after surgery. Dogs with an active urine sediment on urinalysis (defined as >5 wbc/high power field (HPF) ± bacteriuria) that could indicate bacteriuria at time of presentation, a history of recurrent UTIs (more than 2 a year), or a disease or disease treatment that can predispose to bacteriuria (diabetes mellitus, hyperadrenocorticism, immunosuppression) were excluded.
Three centers recruited cases to the trial from April 2011 to October 2013. Owners of dogs that met the inclusion criteria were informed of the trial at time of or the day after surgery by a study coordinator in each center, and participating owners signed an informed consent. A urinalysis was performed to exclude dogs with evidence of bacteriuria at admission based on the presence of an active urine sediment (as defined above). Urine cultures were not used to exclude dogs at onset of the trial because treatment was initiated at the 48‐hour postoperative time point. Once entered into the trial, dogs were randomized to 1 of 2 treatment groups in a 1 : 1 ratio by the NC State Veterinary Hospital pharmacy. The randomization sequence was determined for each center with a random number generator.b Oral administration of either cranberry extractc or placebo was started 48 hours after presentation and continued daily for 6 weeks using the manufacturer's dose rate (Table 1). The cranberry extract and placebo tablets were compounded by the manufacturer to look identical and were supplied to the NC State Veterinary Hospital pharmacy in matching containers labeled A or B. The pharmacists, investigators, and owners were blinded to group identity. Dogs’ routine postoperative management was determined by the attending clinician. While in the hospital, inability to urinate was managed by manual bladder expression when possible, aided by the addition of oral diazepam (0.25–0.5 mg/kg po q8h) and phenoxybenzamine (0.5 mg/kg po q12h) at the discretion of the attending clinician. If manual expression was not possible, intermittent sterile catheterization was performed; an indwelling catheter was placed in female dogs. Dogs were discharged to their owners when their postoperative pain was adequately controlled and they could urinate on their own, or their owners were able to manually express them.
Table 1.
Dose rate of cranberry extract based on weight. The same dosing schedule was used for placebo and cranberry extract
| Body Weight | Tablets | Dose of PCAs |
|---|---|---|
| <10 lbs | ¼ tablet | 4 mg |
| 10–19 lbs | ½ tablet | 8 mg |
| 20–39 lbs | 1 tablet | 16 mg |
| 40–59 lbs | 1 ½ tablets | 24 mg |
| 60–79 lbs | 2 tablets | 32 mg |
PCA, proanthocyanidins; lbs, pounds.
Data Collection
At entry into the trial 48 hours postoperatively, the neurological function of dogs was graded on a scale of 0 to 5 (Table 2), and the method of urine voiding during hospitalization was recorded as voluntary or involuntary; catheterization was noted. Dogs were reevaluated 2, 4, and 6 weeks postoperatively. At each reexamination, owners completed a short questionnaire detailing changes in urination and hindlimb motor function over the preceding 2 weeks (Data S1). Their neurological function was graded from 0 to 5, and their method of urination was classified as voluntary or involuntary (manual expression). A urine sample was taken by cystocentesis (or sterile catheterization if the bladder was small), and urinalysis and urine culture were performed. If urine volume was limited, a urine culture alone was performed. If there was excess urine, a sample was frozen at −80°C within an hour of sampling for quantification of antiadhesion activity (AAA).
Table 2.
Scoring of dogs’ neurological grade using the modified Frankel scale
| Grade | Description |
|---|---|
| 0 | Normal |
| 1 | Back pain |
| 2 | Ataxia/ambulatory paraparesis |
| 3 | Nonambulatory paraparesis |
| 4 | Paraplegic with pain perception |
| 5 | Paraplegic with absent pain perception |
Urinalysis included testing for protein with sulfosalicylic acid turbidimetry, urine sediment analysis, and a colorimetric urinalysis reagent test strip evaluating urine protein, blood/hemoglobin, pH, ketones, and glucose. For urine culture, a 1‐μL calibrated loop was used to streak well‐mixed urine samples onto plates containing Columbia agar with 5% sheep blood (CBA) and MacConkey agar (MAC). The CBA plate was incubated at 36°C for 18–24 hours in 5% CO2. The MAC plate was incubated for the same period at 36°C in ambient air conditions. If there was growth, it was quantified (colony‐forming units [CFU]/mL) and bacterial species were determined with an automated system.d Antimicrobial susceptibility patterns for bacterial isolates were determined using a standard extended susceptibility panel appropriate for the bacterial isolate.d The number and species of bacterial isolates were recorded along with an approximate concentration (colony‐forming units [CFU]/mL) of each isolate in the sample. Samples were recorded as positive for bacteriuria when there were more than 1,000 CFU/mL in cystocentesis samples and 10,000 CFU/mL in catheterized samples. Lack of bacterial growth after 48 hours was considered a negative urine culture. Dogs with positive cultures were treated with a 14‐day course of antibiotics selected based on the antimicrobial susceptibility pattern. Dogs with Enterococcus faecium infections with inactive urine sediment that were classed as multidrug resistant were not treated.
An assay for antiadhesion activity (AAA) was performed in a subset of samples that were frozen at the time of collection. The assay was performed at the Rutgers University Blueberry and Cranberry Research Center using a human red blood cell (HRBC) hemagglutination assay that is specific for uropathogenic P‐fimbriated E. coli.20 This assay quantifies the ability to suppress HRBC agglutination induced by E. coli, providing a surrogate method to assay AAA of urine. Briefly, 30 μLs of each urine sample were incubated with 10 μL of a uropathogenic P‐fimbriated E. coli suspension for 10 minutes on a rotary shaker at room temperature. Freshly drawn HRBCs (A1, Rh positive) were suspended in PBS, and 10 μL were added to the urine test suspensions. These were incubated for a further 20 minutes at room temperature on a rotary shaker and then evaluated with microscopy. Antiadhesion activity was scored based on a visual estimate of percent agglutination with 0 = no AAA; 1 = 50% AAA (interpreted as moderate urine AAA); 1.5 = 75% AAA; 2 = 100% AAA (interpreted as significant urine AAA). Antiadhesion assays were repeated 4 times per sample, and the results were averaged. Controls included wells containing bacteria and PBS, HRBCs and PBS, bacteria and test material, HRBC and test material, and bacteria and HRBC. The group identity was masked from the laboratory performing the testing. Based on the presence of AAA in the urine of dogs in the placebo group, urine was sampled from 30 additional dogs that presented to the NC State Neurology Service with acute onset of myelopathy. Wherever possible, up to 3 samples were taken over a period of 1 month with a minimum of 1 day between sampling. The samples were assayed for AAA by the Rutgers University Blueberry and Cranberry Research Center as described above.
As a quality control measure, the identity of the placebo and cranberry extract tablets was investigated using the AAA assay. Twenty bottles of placebo (10) and cranberry extract (10) from 2 separate centers (half from each center) were sent to the Rutgers Laboratory. As for the urine testing, the testing center was blinded to the identity (placebo vs cranberry extract) of the tablets. Tablets from each bottle were suspended at a concentration of 60 mg/mL in phosphate‐buffered saline, neutralized with 1 M sodium hydroxide, and diluted 2‐fold before using the same hemagglutination inhibition assay as was used for the urine samples, but the results were expressed as MIC value (in mg/mL).
Statistical Analysis
Statistical analysis was to be performed when 150 cases had been enrolled and completed the protocol or at the end of the enrollment period, whichever was sooner. It was performed with group assignments as A or B. The identity of the treatment group receiving cranberry extract was only revealed once all statistical analyses were complete. The age, breed, sex, and neurological grade at time of entry into the study were summarized for each group and compared using Kruskal‐Wallis test for continuous data and by constructing contingency tables and performing a chi‐square test for categorical data with a Fisher's Exact test performed when appropriate due to small sample size.
The presence of bacteriuria in each individual was categorized as yes or no. The risk of bacteriuria and, more specifically, E. coli bacteriuria was compared between groups by construction of a contingency table and performing a chi‐square or Fisher's Exact test. A conditional power analysis was performed using data collected at the end of the case enrollment period.21 This analysis provides a prediction of the likelihood of detecting a significant difference between treatment groups if all the planned cases are recruited based on the study data to inform the investigator on the potential futility of continued case recruitment.22, 23 As a secondary analysis, the effect of a variety of variables on the risk of bacteriuria was investigated by logistic regression. These included sex, ability to urinate, catheterization, gait score, and crystalluria. As several of these variables were correlated (for example, gait score, and ability to urinate), each set of variables was fit individually. The relationship between AAA (expressed both as present yes or no, and score for each animal (maximum score if more than 1 sample was tested)) and treatment group, risk of bacteriuria and risk of E. coli bacteriuria were investigated as a secondary analysis in the subset of animals for which these data were available. Contingency tables were constructed, and a Fisher's Exact test was performed. P values < .05 were recognized as statistically significant with Bonferroni corrections for multiple comparisons where appropriate. All analyses were performed using SAS.e
Results
Study Population
One hundred and fifteen dogs were recruited and randomized during the planned recruitment period (Fig 1). An analysis of the data was performed, and recruitment was halted based on the lack of trend for efficacy of treatment and a lower rate of bacteriuria than anticipated during study design (see statistical analysis).
Figure 1.
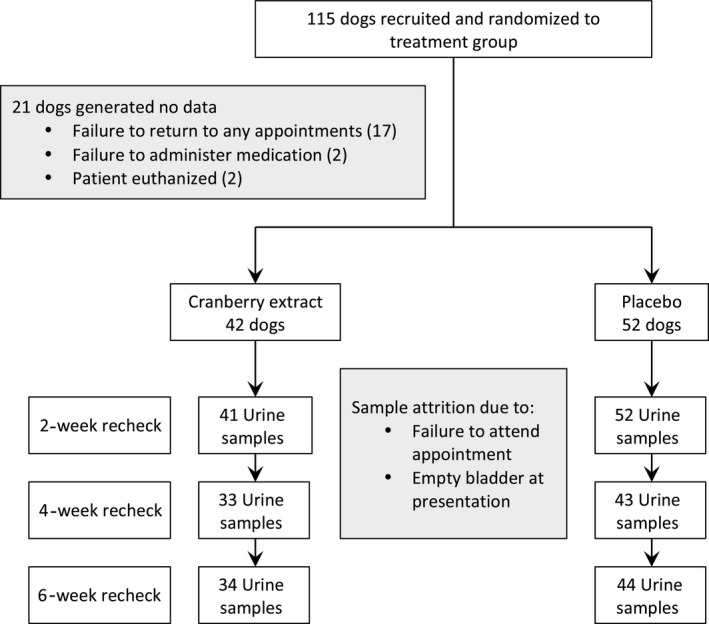
Flowchart documenting study design and numbers of animals and samples taken at each time point.
Seventeen dogs were withdrawn by their owners (due to difficulty of attending rechecks), 2 dogs were euthanized before the 2‐week recheck for failure to improve neurologically, and 2 owners failed to administer the tablets. Thus, data were generated from 94 dogs, 42 randomized to the cranberry extract group and 52 to the placebo group. Urine samples were not obtained at every recheck for some dogs either because of a lack of urine in the bladder or a failure to attend the appointment (Fig 1). All 94 dogs had at least 1 urine culture on a recheck, and the mean number of urine samples was similar in both groups with 2.8 per dog in the cranberry group and 2.7 per dog in the placebo group.
Dachshunds accounted for 62% (58 of 94) of the dogs; other breeds represented included mixed breed (13), Pekingese (6), Shih Tzu (5), Chihuahua (3) Beagle (2), Bassett hound (2), and 1 each of Cocker spaniel, Border collie, Viszla, Pomeranian, and Miniature poodle. Details of the signalment and neurological grade at trial entry are provided in Table 3. There were no significant differences between groups. The neurological recovery of dogs is summarized in Figure 2; there were no significant differences between the 2 groups at any of the time points (2 weeks: P = .73; 4 weeks: P = .56; 6 weeks: P = .48). The majority of dogs recovered the ability to urinate voluntarily over the 6‐week course of the study with only 3 dogs in the cranberry extract group and 4 dogs in the placebo group remaining paralyzed and unable to urinate (Table 4). While in hospital, 8 of the 22 dogs in the cranberry extract group that were unable to urinate voluntarily were managed by sterile intermittent catheterization and 14 were manually expressed. Twelve of the 26 dogs unable to urinate in the placebo group were catheterized, and the remaining 14 were manually expressed. Once discharged, all dogs that were unable to urinate voluntarily were managed by manual expression.
Table 3.
Composition of treatment groups at entry into the trial
| Cranberry Extract n = 42 | Placebo n = 52 | ||
|---|---|---|---|
| Dachshunds: n (%) | 23 (55%) | 35 (67%) | |
| Sex | 18 FS, 1M, 23 MC | 2 F, 16 FS, 6 M, 28 MC, | |
| Age years: mean (SD) | 5.35 (2.26) | 5.91 (2.78) | |
| Neurological grade at presentation/trial entry | 2 | 0/12 dogs | 0/13 dogs |
| 3 | 16/13 dogs | 17/18 dogs | |
| 4 | 13/9 dogs | 24/15 dogs | |
| 5 | 13/8 dogs | 11/6 dogs | |
F, females; FS, female spayed; M, male; MC, male castrated (F vs M: P = .41; grade at pre: P = .7; grade at trial entry: P = .85, Dachshund Y or N P = .21; age: P = .29).
Figure 2.
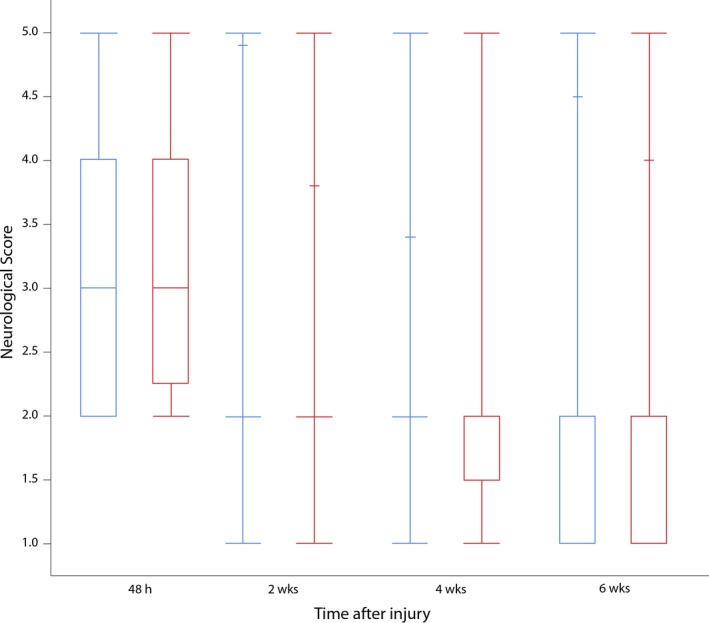
Neurological grades of cranberry (blue) and placebo (red) groups at each postoperative evaluation. Data are expressed as the median (mid horizontal line), quartiles (box), and maximum and minimum. h: hours, wks: weeks.
Table 4.
Number of dogs unable to urinate voluntarily at each evaluation
| Time from Surgery | Cranberry Extract | Placebo |
|---|---|---|
| 48 hours | 22 | 26 |
| 2 weeks | 5 | 8 |
| 4 weeks | 4 | 6 |
| 6 weeks | 3 | 4 |
Urine Culture Results
A total of 247 cultures were performed, 108 in the cranberry extract group and 139 in the placebo group. There were 21 positive cultures in 17 dogs, 1 of which (Staphylococcus epidermidis, <1,000 CFU/mL, with no evidence of inflammation in the urine sediment) was believed to represent contamination. In the remaining 16 dogs with 20 positive cultures, 3 of the cultures were the same organism as the previous culture, the owners having failed to start the antibiotics or started them just before the follow‐up culture. One of the dogs had a positive culture at 2 and 6 weeks, but was negative at 4 weeks. This dog was classified as having 2 positive cultures; thus, 16 dogs were classified as having 17 positive cultures (Data S2). This gave an overall frequency of bacteriuria in this group of 94 dogs of 18.09% and a frequency of dogs with bacteriuria of 17.02%. Nine positive cultures occurred at the 2‐week evaluation, 3 at 4 weeks, and 5 at 6 weeks. Twelve of the dogs were female, and 4 were male. Escherichia coli was the most common cause of bacteriuria, with 7 infections in 6 dogs (5 females, 1 male). One infection was classified as multidrug resistant, 1 dog had positive cultures at 4 and 6 weeks (this was counted as the same organism due to late initiation of antibiotic treatment). There were 3 Klebsiella pneumoniae infections, 3 Enterococcus faecium infections, 2 of which were multidrug resistant, 2 Staphylococcus pseudintermedius infections, 1 Enterobacter, and 1 Streptococcus infection. Of these positive cultures, urine cytology was not available in 2 due to inadequate urine sample volume, and there were no white blood cells in 3 samples (1 Enterobacter, 1 Streptococcus, and 1 E. coli), rare white blood cells in 8 samples, fewer than 10/HPF in 2 samples, and >30/HPF in the remaining 2 samples (Data S2).
Completion of questionnaires for each 2‐week period by the owners was erratic. Data on clinical signs related to positive urine cultures are provided in Data S2.
Statistical Analysis
Ten of the affected dogs were in the cranberry extract group (10/42; 24%) and 6 in the placebo group (6/52; 12%). There was no significant difference in frequency of bacteriuria between the 2 groups (P = .12). As cranberry extract targets adhesion of E. coli specifically, the presence of this organism between groups was then compared. Six of the 7 (86%) E. coli positive cultures were in the group receiving cranberry extract, and 1/7 (14%) was in the placebo group, with no significant difference between groups (P = .09) thus disproving the working hypothesis. To determine whether recruitment of the planned number of animals should be completed, a conditional power analysis was performed. This analysis revealed a 0.6% chance (futility index of 0.994) of detecting a significant difference between treatment groups if 75 animals per group were recruited. Case recruitment was therefore terminated.
A secondary analysis was performed to examine factors associated with the presence of bacteriuria. A logistic regression was performed to fit sex, initial neurological status, and urination status (voluntary versus involuntary), catheterization, neurological grade, and crystalluria at time of urine sampling to the presence of bacteriuria. Of the factors examined, female sex was highly associated with bacteriuria (P = .0023), as was involuntary urination status at 48 hours and 2 weeks (P = .029 and .025, respectively). When corrected for multiple testing, female sex remained significant whereas involuntary urination did not (Data S3). The other variables were not significant risk factors (Data S3).
Urine Antiadhesion Activity
The presence of AAA was measured in 47 dogs during the trial, 26 in the cranberry extract group, and 21 in the placebo group. Eight dogs had 2 samples evaluated, 4 in each treatment group. Sixteen of 26 dogs (62%) in the cranberry group had AAA (5 with a score of 1 and 11 with a score of 2) and 10 did not. Fourteen of 21 dogs (67%) in the placebo group had activity (4 with a score of 1 and 7 with a score of 2) and 7 did not. Of the dogs with 2 samples evaluated, 3 of the 4 dogs in each group were positive on both samples, and 1 was negative on one and positive on the other. There was no significant difference in AAA scores between the groups (Wilcoxon Rank Sum test: P = .9). The effect of the presence of AAA (categorized as Yes if positive in any of the measurements, or No) was compared with the risk of bacteriuria and more specifically of E. coli by construction of a contingency table and performing a Fisher's Exact test. There were 9 dogs with bacteriuria, 4 were negative for urine AAA, and 5 were positive. Of these, there were 5 dogs with E. coli positive cultures, 4 were negative for urine AAA, and 1 was positive. There was no association between bacteriuria and presence of AAA (P = .7), but the presence of AAA was negatively associated with E. coli bacteriuria (P = .047).
The group of 30 dogs from which urine samples were taken to determine endogenous urine AAA prevalence in dogs with myelopathy was comparable to the dogs in the clinical trial with 9 mix breeds, 8 Dachshunds, 3 Shih Tzus, 3 Cocker spaniels, 2 French bulldogs, and 1 each of poodle, Pekingese, Labrador retriever, Yorkshire terrier, and Mastiff. Twenty‐eight of the dogs presented for surgery for acute TL‐IVDH, 2 for chronic TL‐IVDH, and 1 for cervical stenotic myelopathy. Their mean age was 5.3 years (SD: 2.45) with 15 FS, 13 MC, and 2 M. A total of 71 samples were taken, 19 dogs were sampled twice, and the remaining 11 dogs were sampled 3 times. Fifteen of these samples showed positive AAA in 9 dogs; thus, 30% of dogs tested positive for AAA at least once. Of these 9 dogs, 3 had 2 urine samples tested and 6 had 3 urine samples tested. Four dogs were positive in all the samples (2 dogs had 2 samples and 2 dogs had 3 samples), 4 were positive in 1 of 3 samples, and 1 was positive in 1 of 2 samples.
None of the placebo tablets showed any AAA, and all the cranberry extract tablets had 0.23‐0.47 mg/mL AAA thus confirming the correct identity of the tablets and appropriate AAA in the cranberry extract tablets.
Discussion
This placebo‐controlled, randomized, prospective clinical trial failed to show a reduction in the risk of bacteriuria or E. coli bacteriuria in dogs receiving oral cranberry extract in the 6 weeks after decompressive surgery for TL‐IVDH. While the planned number of cases was not recruited in the designated recruitment period, reducing study power, a conditional power analysis demonstrated the low likelihood of detecting a significant difference between groups if all cases were recruited and the study was terminated. Secondary analysis demonstrated a significant reduction in the number of E. coli positive cultures in dogs with at least 1 positive test for urine AAA independent of treatment group. However, this analysis involved only a subset of dogs and this exploratory dataset should be interpreted with caution. We also demonstrated that over 60% of the dogs tested in both the placebo and cranberry extract groups had detectable urine AAA. A reevaluation of the placebo and cranberry extract tablets with blinded external testing confirmed their identity was correct. An additional group of 30 dogs from a similar demographic was tested for urine AAA; 30% had detectable activity in at least 1 sample.
This study reported the frequency of bacteriuria in the 6‐week period after surgery for acute TL‐IVDH. During this critical period, dogs are recovering neurological function and can experience suboptimal urine voiding, putting them at increased risk of persistent bacteriuria and possibly UTI. The definition of an active UTI has evolved over time to include the presence of clinical signs (stranguria, pollakiuria, incontinence). However, clinical signs of a UTI might not be evident in dogs with myelopathy due to inability to void and changes in bladder sensation; requiring the presence of clinical signs could introduce unwanted variability in interpretation. In addition, while owners were given questionnaires at each recheck to document signs of a UTI at home, their return rate was poor. Cytological analysis of the urine was performed if there was adequate urine, but urine culture took precedence if urine volume was limited. As a result of these factors, we reported the presence of bacteriuria based on the number of bacterial colonies in culture only using the same thresholds as reported in a prior study (data from which were used for purposes of study design).
The overall frequency of bacteriuria at 18% was lower than a previous study in which 38% of dogs developed bacteriuria during this period.8 This decreased frequency impacted the original study design, thus reducing the power of the planned study. Potential explanations for the lower rate include a slightly less severe mean grade of injury, and stringent exclusion of dogs that have other risk factors for developing a UTI. The other factor that influenced study power was the failure to recruit 150 cases as originally planned. Power analyses performed during design of the clinical trial demonstrated 52 dogs per treatment arm would achieve a study with 80% power, while increasing to 75 dogs would result in a study with a power of 91%. By the end of the recruitment period, we had recruited 117 dogs, reaching the number required for a study with an 80% power but not the planned 91% power. However, attrition for a variety of reasons resulted in data suitable for analysis in only 94 dogs. It is standard practice to perform interim analyses during a clinical trial with the aim of identifying positive or negative outcomes as soon as possible. If there is no evidence of treatment trend, a conditional analysis should be performed using the data collected.22, 23 This analysis gives an estimate for the likelihood of observing a statistically significant difference when the planned statistical analysis is performed with the data collected thus far. This analysis revealed that there was a 0.6% chance of detecting a significant result if all dogs were recruited, supporting the decision to terminate the study.
The biggest risk factor for developing bacteriuria in this study was being female. This is consistent with the findings of other studies24 and relates to the anatomy of the female lower urinary tract. In addition, as demonstrated previously, inability to urinate voluntarily was also a risk factor.8 The influence of catheterization was also considered in this study but did not increase the risk of developing bacteriuria. Catheterization was intermittent and of short duration during initial hospitalization only, and as such, was considered unlikely to influence bacteriuria frequency at the 2‐, 4‐, and 6‐week time points evaluated in this study.
The results of this trial did not indicate a reduction in the overall or E. coli‐specific bacteriuria rate in treated dogs even though E. coli was the most common finding in positive cultures, accounting for 41% of the infections. Proanthocyanidins found in cranberry extract have been shown to reduce E. coli infections, in particular P‐fimbriated E. coli, by inhibiting adhesion to uroepithelial cells, a critical step in the generation of a UTI.25 Many cranberry products are available, varying from purified PACs to cranberry extract to cranberry juice, and some cranberry products might actually be devoid of PACs.26 For this trial, we selected a product that provided purified PACs at a dose rate established by the manufacturer based on testing in normal dogs, and quality controlled in terms of quantification of AAA. The hemagglutination inhibition test used to measure urine and tablet AAA is validated as a means of detecting E. coli adhesion to human red blood cells and provides a surrogate marker of prevention of bacterial adhesion to uroepithelial cells.20 While not routinely used in human clinical trials, it has been shown to be of benefit in assessing dosing of products containing PACs in both humans and dogs.15, 27 However, there is a paucity of data available for dogs, and the relevance of this test in dogs remains to be confirmed. In spite of confirmation of the AAA or lack thereof in batches of tablets used in the trial, there was no difference in urine AAA between treated and untreated dogs and this might account for the lack of treatment effect. The failure to demonstrate efficacy could also be related to other limitations including lack of full study power due to failure to recruit all 150 dogs, and lower bacteriuria frequency when compared to the population used to design the trial. However, the data generated in this trial do not demonstrate any trend toward a therapeutic effect; indeed, there were 6 E.coli‐positive cultures in the cranberry extract group compared to 1 in the placebo group.
Potential reasons for the lack in difference of urine AAA between the groups include client noncompliance, inappropriate dose rate, and poor bioavailability. Indeed, noncompliance is cited as the most important cause of failure of human clinical trials due to the palatability and volume of products that need to be consumed.17 Owners reported that their dogs consumed the tablets, and they obtained refills at the appropriate time, but it is difficult to confirm this vital step when performed at home. Dosing of PACs is important in humans, and the recommended dose of 36 mg of PACs per person per day has recently been shown to be inadequate at achieving protective levels of AAA in the urine.15 Moreover, while once daily dosing has been used traditionally, twice daily dosing achieves nyctohemeral protection, especially important when considering that urine voiding is critical to expulsion of bacteria and does not occur overnight.25 The dogs in this study were dosed once daily according to the manufacturer's instructions which were based on demonstration of altered AAA in dogs’ urine after dosing,27 but it is possible that dose rates, frequency, and timing might need to be optimized for specific groups of dogs.
We performed a secondary analysis to explore the possible relationship between urine AAA and E. coli bacteriuria rate independent of treatment group. We discovered a significant reduction of E coli positive cultures in dogs with at least 1 positive urine AAA test. Urine was not evaluated in every dog, decreasing the strength of conclusions we can draw, but this exploratory finding is intriguing and further evaluation of this possible therapeutic effect is needed.
In the current study, over 60% of dogs getting placebo had urine AAA on at least 1 measurement. This high rate led us to confirm the AAA activity in blinded samples of tablets from both treatment arms and to investigate a similar population of dogs after the trial. The number of dogs with AAA in this control population was lower at 30% but still surprisingly high. Humans have been reported to have AAA as a result of production of endogenous inhibitors such as uromodulin (Tamm‐Horsfall glycoprotein).15 Diet has been recognized to have an influence, potentially explaining different rates in different populations,15 and genetic factors also play a role.28 Urine AAA is also affected by parameters such as urine specific gravity, salt content of diet, and time of day,15 and these results raise many questions about urine AAA and the validity of this assay in dogs. Further examination of the source of urine AAA was beyond the remit of this trial, but reveals an important area of future research as there are no other reports published on canine urine AAA at this time.
Conclusions
We evaluated a population of dogs prone to bacteriuria and sometimes UTI to evaluate the effects of cranberry extract. Finding an inexpensive and low‐risk strategy to reduce the risk of E. coli UTI in groups of dogs with recurrent UTIs would be of benefit. This clinical trial failed to show a benefit in the population evaluated. However, given the association identified between E. coli positive cultures and urine AAA, further examination of the optimal dose rate, frequency, and timing of PACs in dogs is indicated.
Supporting information
Data S1. Effect of Cranberry Extract on Myelopathy‐Associated Urinary Tract Infections Client Take‐ Home Questionnaire.
Data S2. Investigation of different parameters potentially associated with urinary tract infections
Data S3. Investigation of different parameters potentially associated with bacteriuria.
Acknowledgments
We acknowledge the efforts of clinicians who contributed cases to the clinical trial: Drs. Bray, Campbell, Crook, Korani, Krebs, Kwan, Lewis, McDonald‐Lynch, Rowe, Schwartz, and Waldron.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Cases were recruited for this trial at 3 centers: North Carolina State University College of Veterinary Medicine, Gulf Coast Veterinary Neurology, and University of Georgia, Athens College of Veterinary Medicine. Trial coordination and data analysis and manuscript preparation were performed at North Carolina State University College of Veterinary Medicine.
This clinical trial was supported by Morris Animal Foundation. Crananadin was donated by Nutramax at no cost; Nutramax provided no input on study design, data analysis, or presentation of the results.
These data were presented at the 2014 ACVIM Forum, Nashville, Tennessee, as a part of a larger presentation on UTIs in paralyzed dogs. An abstract describing the findings has not been published.
Footnotes
Random Integer Generator, v2.0; O'Reilly Institute, Trinity College, Dublin 2, Ireland
Crananadin, Nutramax Laboratories Veterinary Sciences Inc., Lancaster, SC
Sensititre, Trek Diagnostic Systems, Thermo‐Fisher, Oakwood Village, OH
SAS Institute, Cary, NC. Software version: 9.3
References
- 1. Brisson BA. Intervertebral disc disease in dogs. Vet Clin North Am Small Anim Pract 2010;40:829–858. [DOI] [PubMed] [Google Scholar]
- 2. Oliver JE, Bradley WE, Fletcher TF. Spinal cord representation of the micturition reflex. J Comp Neurol 1969;137:329–346. [DOI] [PubMed] [Google Scholar]
- 3. Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002;113(Suppl 1A):67S–79S. [DOI] [PubMed] [Google Scholar]
- 4. Esclarín De Ruz A, García Leoni E, Herruzo Cabrera R. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 2000;164:1285–1289. [PubMed] [Google Scholar]
- 5. Atalan G, Parkinson TJ, Barr FJ, et al. Urine volume estimations in dogs recovering from intervertebral disc prolapse surgery. Berl Munch Tierarztl Wochenschr 2002;115:303–305. [PubMed] [Google Scholar]
- 6. Bubenik L, Hosgood G. Urinary tract infection in dogs with thoracolumbar intervertebral disc herniation and urinary bladder dysfunction managed by manual expression, indwelling catheterization or intermittent catheterization. Vet Surg 2008;37:791–800. [DOI] [PubMed] [Google Scholar]
- 7. Stiffler KS, Stevenson MAM, Sanchez S, et al. Prevalence and characterization of urinary tract infections in dogs with surgically treated type 1 thoracolumbar intervertebral disc extrusion. Vet Surg 2006;35:330–336. [DOI] [PubMed] [Google Scholar]
- 8. Olby NJ, Mackillop E, Cerda‐Gonzalez S, et al. Prevalence of urinary tract infection in dogs after surgery for thoracolumbar intervertebral disc extrusion. J Vet Intern Med 2010;24:1106–1111. [DOI] [PubMed] [Google Scholar]
- 9. Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: Antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weese JS. Decision making in urinary tract infection and bacteriuria. Proceedings ACVIM Forum. In: Indianapolis; 2015.
- 11. Guay DRP. Cranberry and urinary tract infections. Drugs 2009;69:775–807. [DOI] [PubMed] [Google Scholar]
- 12. Nowack R. Cranberry juice– a well‐characterized folk‐remedy against bacterial urinary tract infection. Wien Med Wochenschr 2007;157:325–330. [DOI] [PubMed] [Google Scholar]
- 13. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst 2012;10:CD001321. doi: 10.1002/14651858.CD001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howell AB, Reed JD, Krueger CG, et al. A‐type cranberry proanthocyanidins and uropathogenic bacterial anti‐adhesion activity. Phytochem 2005;66:2281–2291. [DOI] [PubMed] [Google Scholar]
- 15. Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti‐adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. BMC Infect Dis 2010;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta K, Chou MY, Howell A, et al. Cranberry products inhibit adherence of P‐Fimbriated Escherichia Coli to primary cultured bladder and vaginal epithelial cells. J Urol 2007;177:2357–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jepson R, Craig J, Williams G. Cranberry products and prevention of urinary tract infections. J Am Med Assoc 2013;310:1395–1396. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191–1194. [PubMed] [Google Scholar]
- 19. Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry‐lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. Brit Med J 2001;322:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P‐fimbriated Escherichia coli in vitro. Phytochem 2000;54:173–181. [DOI] [PubMed] [Google Scholar]
- 21. Jennison C, Turnbull BW. Group Sequential Methods with Applications to Clinical Trials. Boca Raton, FL: Chapman, Hall CRC; 2000. [Google Scholar]
- 22. Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med 2005;24:2747–2764. [DOI] [PubMed] [Google Scholar]
- 23. Proschan MA, Lan K, Wittes JT. Power: Conditional, unconditional, and predictive In: Proschan MA, Lan K, Wittes JT, eds. Statistical Monitoring of Clinical Trials. Statistics for Biology and Health. New York, NY: Springer New York; 2006:43–66. [Google Scholar]
- 24. Ling GV, Norris CR, Franti CE, et al. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995). J Vet Intern Med 2001;15:341–347. [PubMed] [Google Scholar]
- 25. Smee N, Loyd K, Grauer G. UTIs in small animal patients: Part 1: Etiology and pathogenesis. J Am Anim Hosp Assoc 2013;49:1–7. [DOI] [PubMed] [Google Scholar]
- 26. Prior RL, Lazarus SA, Cao G, et al. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium Spp.) using high‐performance liquid chromatography/mass spectrometry. J Agric Food Chem 2001;49:1270–1276. [DOI] [PubMed] [Google Scholar]
- 27. Howell AB, Griffin DW, Whalen MO. Inhibition of P‐fimbriated Eschericial Coli adhesion in an innovational ex‐vivo model in dogs receiving a bioactive cranberry tablet (crananidin). J Vet Int Med Abstract 2010;24:678. [Google Scholar]
- 28. Olden M, Corre T, Hayward C, et al. Common variants in UMOD associate with urinary uromodulin levels: A meta‐analysis. J Am Soc Nephrol 2014;25:1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Effect of Cranberry Extract on Myelopathy‐Associated Urinary Tract Infections Client Take‐ Home Questionnaire.
Data S2. Investigation of different parameters potentially associated with urinary tract infections
Data S3. Investigation of different parameters potentially associated with bacteriuria.


