Abstract
Background
Up to 25% of elderly humans have proteinuria, often associated with underlying lesions. Data concerning the presence of proteinuria in elderly dogs is scarce.
Objectives
To describe the presence and persistence of proteinuria and to compare urinary protein : creatinine ratio (UPC) between free catch and cystocentesis urine samples in apparently healthy elderly dogs.
Animals
Hundred apparently healthy elderly dogs.
Methods
Prospective study. Owners of 100 elderly dogs were asked to collect 2 free catch urine samples. Dogs were considered healthy based on owner's perception and an age chart, based on ideal bodyweight, was used to define dogs as senior or geriatric. UPC of urine collected by free catch and cystocentesis were compared. Overt proteinuria and borderline proteinuria were defined as UPC >0.5 and between 0.2 and 0.5, respectively, if examination of sediment did not explain proteinuria. Proteinuria was considered persistent if present at both sampling times.
Results
At baseline, 71 owners succeeded in collecting urine. Eleven percent of dogs had overt proteinuria, 14% were borderline proteinuric, and 75% nonproteinuric. Thirty‐seven repeated urine samples, with a median time interval of 31 days (range 10–90), were available. Nineteen percent of dogs had a persistently increased UPC (>0.2), with persistent overt proteinuria present in 8%. A strong correlation (ρ = 0.88) was found between UPC of urine collected by free catch and cystocentesis.
Conclusions and Clinical Importance
As 19% of study dogs had persistent proteinuria, our findings emphasize that measurement of proteinuria should be part of geriatric health screening. For UPC in dogs, free catch urine provides a good alternative to cystocentesis.
Keywords: Canine, Health screening, Hypertension, Urinary protein : creatinine ratio
Abbreviations
- BCS
body condition score
- CKD
chronic kidney disease
- IRIS
International Renal Interest Society
- SBP
systolic blood pressure
- SSA
sulfosalicylic acid
- UPC
urinary protein : creatinine ratio
- USG
urine specific gravity
Life expectancy of dogs and cats is increasing, and senior pets represent 30–40% of patients in veterinary practice.1 Aging pets and their associated health concerns, together with an increasing demand for higher standard of care by owners, favor routine health screening in this population.1, 2 The goal of health screening is to detect subclinical abnormalities at a time when therapeutic intervention might have most benefit.1
Aging in humans evokes structural and functional renal alterations.3 Renal structural changes occur in clinically healthy geriatric beagle dogs.4 Proteinuria, that affects up to 25% of elderly humans, is often associated with an underlying disease such as systemic hypertension, chronic kidney disease (CKD), diabetes mellitus, amyloidosis, or multiple myeloma. Proteinuria is not considered a physiologic age‐related change in human medicine.5, 6 Twenty‐five percent of middle‐aged to old, apparently healthy cats have borderline proteinuria.7 Data concerning incidence of proteinuria in elderly dogs are limited to studies assessing microalbuminuria in aging dogs.8, 9
A distinction between prerenal (abnormal plasma content of proteins), renal, and postrenal (entry of protein into the urine after it enters the renal pelvis) causes of proteinuria is made and between functional (transient) and abnormal renal proteinuria.10 Besides localization of proteinuria, its persistence is important as only persistent renal proteinuria indicates the presence of ongoing renal dysfunction. Indeed, the American College of Veterinary Internal Medicine (ACVIM) consensus statement advises repeated measurements of urinary protein : creatinine ratio (UPC).10 In cases with persistent renal proteinuria, further monitoring, diagnostic workup, and therapeutic intervention are advised.10, 11 Persistent proteinuria is associated with increased risk of death and progression of CKD in humans.12 Similarly, persistent overt proteinuria in cats and dogs with CKD is associated with an increased risk of developing uremic crisis and death.13, 14 According to the 2005 ACVIM consensus statement and the International Renal Interest Society (IRIS) guidelines, the decision threshold for UPC in cases with persistent proteinuria is 0.5 in dogs and 0.4 in cats.10, 11 There is a distinction between borderline (dogs: UPC 0.2–0.5; cats: UPC 0.2–0.4) and overt proteinuria (dogs: UPC >0.5; cats: >0.4).10, 11, 15 Borderline proteinuria is a negative prognostic factor in cats with CKD.16 The clinical importance of borderline proteinuria in dogs still needs to be elucidated. In the authors' and others' experience, cystocentesis samples are mentioned to be more reliable for UPC measurement, but this is not well documented.17 However, because of possible drawbacks such as appropriate immobilization of dog and localization of bladder, risk for laceration, hematuria, or owner noncompliance, cystocentesis is often not performed in clinical practice. Collecting urine by free catch has a minimal influence on UPC, in both dogs and cats.17, 18 This could promote the performance of urinalysis in clinical practice and can encourage practitioners to add urinalysis to routine health screening of elderly dogs.
Hence, this study's major aim was to describe the presence and persistence of borderline or overt renal proteinuria in a population of elderly dogs that were apparently healthy according to their owners. An additional aim was to compare results of UPC between free catch and cystocentesis urine samples.
Materials and Methods
Animals
This prospective study was performed at the Faculty of Veterinary Medicine, Ghent University. Participating dogs were recruited for a study evaluating extensive health screening in 100 senior and geriatric dogs, declared healthy based on owners' perception.1 Using their ideal bodyweight, dogs were selected based on the “Age Analogy Chart” by Fortney and Goldston (0 to <10 kg: from 8 years; 10 to <25 kg: from 7 years; 25 to <50 kg: from 6 years; and >50 kg: from 4 years). The distinction between senior and geriatric was also based on this chart.1 Exclusion criteria were use of medication within 2 months before consultation and during the study period. Preventive medication (such as vaccination or deworming) was allowed until 2 weeks before consultation.
The health screening consisted of a comprehensive history, blood pressure measurement, physical examination including neurological, ophthalmological, and orthopedic examination, complete blood examination, and urinalysis. In all cases, all of these procedures were performed in the same order by the same author (AW) without sedation or anesthesia. Blood pressure measurement was performed, before other examinations, at a single time point by indirect Doppler ultrasonography2 according to the ACVIM guidelines.19 Systolic hypertension was defined as a systolic blood pressure (SBP) >160 mmHg.19 Blood examination (complete blood count, serum biochemistry profile, and serum total thyroxine) and urinalysis on a sample obtained by ultrasound‐guided cystocentesis were performed after completing an extensive physical examination. For the results of the health screening, the reader is referred to Willems et al.1 This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the local and national ethical committees (EC 2012/181). All owners were informed about the study and gave their written informed consent.
Sampling Methods
Owners of dogs participating to the health screening study were asked to collect 2 free catch urine samples from their dog: one on the morning of the health screening consultation and a second morning sample after a time interval of approximately 2 weeks. The owners were instructed to use clean (not sterile) containers and to attempt to minimize contact of the caught urine with the animal's body. If sediment analysis was not possible within 60 minutes, owners were asked to store the urine in a cool environment (preferably at 4°C).
As part of the health screening study, ultrasound‐guided cystocentesis was performed (10 mL, 22 G needle) at baseline. During this procedure, the urinary bladder was examined for the presence of urolithiasis.
Urinalysis
Urinalysis of urine samples collected by cystocentesis consisted of measurement of urine specific gravity (USG) with a manual refractometer; UPC3; urinary pH4; urinary dipstick test5; sediment analysis; and bacterial culture6. Urinalysis of free catch urine consisted of measurement of UPC and sediment analysis.
Sediment of cystocentesis samples was prepared and examined within 30–60 minutes after collection, by centrifuging 5 mL of urine in a conical‐tipped tube for 3 minutes at 447 × g, followed by removal of 4 mL of the supernatant. The sediment was resuspended by flicking the tube several times.20 One unstained drop of the resuspended sediment was microscopically examined (40× magnification) to count the mean number of RBCs, WBCs, and epithelial cells per high‐power field (HPF), as previously described.20, 21 Bacteriuria, spermaturia, and lipiduria were noted, and casts and crystals were evaluated according to a semiquantitative scale (absent, mild [1/HPF], moderate [1–3/HPF], or severe [≥3/HPF]).21 Sediments were classified as active on the basis of the presence of one or more of the following findings: bacteriuria, a moderate number of casts, and >5 RBCs, WBCs, or epithelial cells/hpf. Samples with none of these findings were classified as inactive.21 Sediment of free catch urine samples was similarly prepared and examined, preferably within 60 minutes after collection. Otherwise, these samples were stored at a cool environment until analysis.
The dogs were considered nonproteinuric when their UPC was <0.2. Borderline proteinuria and overt proteinuria were defined as UPC between 0.2 and 0.5 and UPC >0.5, respectively, if urine sediment was categorized as inactive.10 Cases with borderline proteinuria or overt proteinuria were included for further statistical analysis, only if sediment did not explain proteinuria. Persistence of borderline or overt proteinuria was evaluated using a second urine sample after 2 weeks.
Statistics
Analyses were performed with SAS.7 Level of significance was set at 5% (P < .05). Persistence of borderline proteinuria or overt proteinuria was calculated with a frequency procedure. To assess the correlation between UPC of urine collected by free catch versus cystocentesis, the nonparametric spearman correlation coefficient (ρ) was used. The Wilcoxon signed rank‐sum test was used to compare the UPC of urine collected by free catch versus cystocentesis at baseline. Further, the Bland–Altman method was used to assess the level of agreement between the 2 sampling methods.
Results
Animals
Initial urine samples collected by cystocentesis were available in 97 dogs and free catch urine samples were available in 71 dogs. Owners of the remaining 29 dogs did not manage to collect free catch urine, with dogs not being cooperative as the main reason. Of the 71 included dogs, 27 were senior and 44 geriatric, based on the “Age Analogy Chart”.1 Thirty‐seven dogs were female (8 intact, 29 neutered), and 34 were male (22 intact, 12 neutered). When considering the weight classes, 13 dogs were small breed, 21 medium sized, 27 large breed, and 10 giant breed. The majority (44 of 71, 62%) had an ideal body condition (BCS 4/9–5/9), 3% (2 of 71) were mildly underweight (BCS 3/9), and 35% (25 of 71) were overweight or obese (BCS >5/9) with 4% (3 of 71) having overt obesity (BCS ≥8/9).22 Forty‐six percent (33 of 71) of dogs had an increased blood pressure, which was moderate (SBP 160–180 mmHg) in 21% (15/71) and severe (SBP >180 mmHg) in 25% (18/71). One dog was diagnosed with hypothyroidism. Five dogs had renal azotemia based on blood and urine results, and follow‐up in 1 dog confirmed CKD IRIS stage 2 (normotensive, borderline proteinuria). No follow‐up was available in the other 4 azotemic dogs.
In 42 of 71 dogs (60%), a follow‐up free catch urine sample was available within a time interval of 10 days to 3 months (median time interval 31 days). Of these 42 dogs, 17 were senior and 25 geriatric. Twenty‐one dogs were female (5 intact, 16 neutered), and 21 were male (15 intact, 6 neutered). There were 7 small breeds, 12 medium‐sized dogs, 18 large breeds, and 5 giant breeds. Fifty‐seven percent (24/42) had an ideal BCS at baseline, and 40% (17/42) had BCS >5/9 with overt obesity present in 7% (BCS ≥8/9; 3/42). At baseline, 24% (10/42) and 24% (10/42) had a moderate or severely increased blood pressure, respectively.
Urinalysis at Baseline
Results of urinalysis at baseline and during follow‐up are summarized in a flowchart (Fig 1). Urine specific gravity was measured in 69 of 71 cystocentesis samples at baseline. Of 2 remaining dogs, no USG was available. Urine specific gravity ranged from 1.007 to 1.012 in 8% (6 of 69), from 1.013 to 1.030 in 38% (26 of 69), and was >1.030 in 54% (37 of 69) of dogs. Forty‐eight percent (33 of 69) had a positive dipstick result, 29% (20 of 69) showed trace of proteins, and 22% (15 of 69) had a negative dipstick result. In one remaining dog, no dipstick analysis was available. Of the positive results, 24% (8 of 33) had a USG ranging from 1.013 to 1.030 and 76% (25 of 33) had a USG >1.030. None of the samples with USG ranging from 1.007 to 1.012 had a positive result. In 5 of the 71 free catch urine samples at baseline, no sediment analysis was available, because of insufficient sample volume. Macroscopically, these samples had a bright, light to dark yellow appearance. Because of the absence of sediment analysis, postrenal origin of proteinuria could not be assessed; therefore, these samples were excluded for further statistical analysis. Of the 66 remaining samples, the majority had an inactive sediment (94%, 62 of 66). Four dogs with an active sediment (presence of pyuria and bacteriuria, in 1 dog combined with microscopic hematuria) were diagnosed with an E. coli cystitis on the cystocentesis sample. Of those, 2 neutered female dogs were administered amoxicillin–clavulanic acid (12.5 mg/kg PO q12h)8 for 10 days. Examination 14 days later revealed an inactive sediment without evidence of bacteriuria. One intact male dog was retested after 14 days and had resolution of bacteriuria without treatment. In 1 other intact male dog, the same advice was given, but this dog was lost to follow‐up. Of these 4 dogs with an active sediment at baseline, 1 was nonproteinuric on both consecutive samples, 1 had persistent borderline proteinuria despite showing an inactive sediment at follow‐up, 1 had transient borderline proteinuria, and 1 had overt proteinuria at baseline but was lost to follow‐up. The latter 2 dogs were excluded for further statistics, because a postrenal origin for the observed proteinuria could not be excluded. In one other dog, a urolith was detected while performing ultrasound‐guided cystocentesis, and sediment analysis revealed calcium‐oxalate crystals. The dog was nonproteinuric at baseline and during follow‐up. The urolith was later removed, and analysis confirmed calcium‐oxalate urolithiasis.
Figure 1.
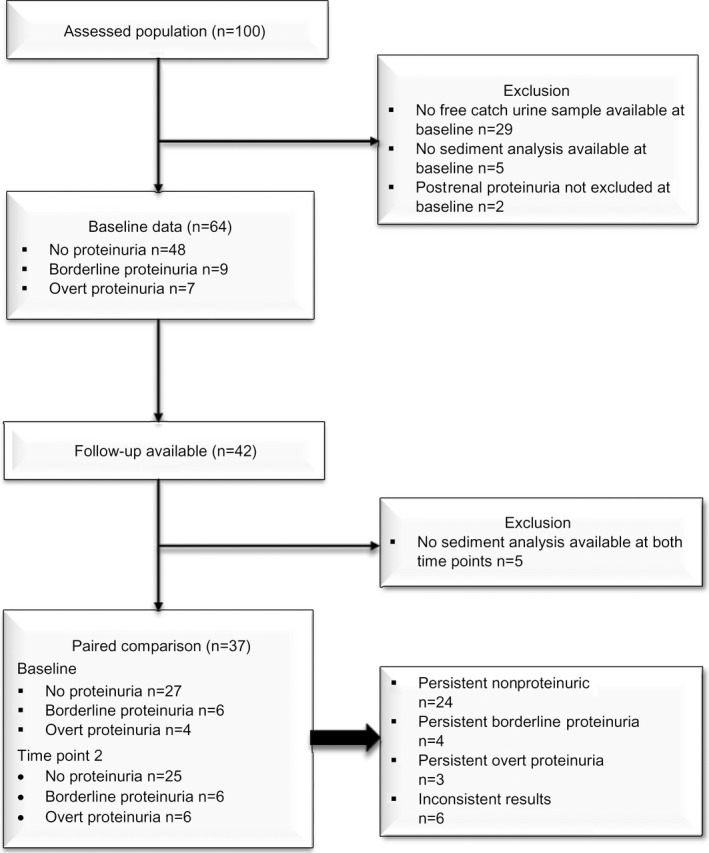
Flow diagram presenting further classification of enrolled dogs.
After exclusion of dogs without sediment analysis (n = 5) and the dogs in which postrenal origin of proteinuria was suspected (n = 2), 64 dogs remained. Of these remaining dogs, 48 (75%) were nonproteinuric, 9 (14%) had borderline proteinuria, and another 7 (11%) had overt proteinuria (range 0.53–4.56, median 1.0), which could not be explained by urinary sediment. The 5 dogs with renal azotemia were all nonproteinuric and normotensive at baseline. The dog with hypothyroidism showed overt proteinuria (UPC 0.66) at baseline. None had severe skin inflammation, and 12 (23%) dogs had moderate to severe gingivitis, with associated dental calculus.1 Of these, 9 were nonproteinuric, 1 had borderline, and 2 overt proteinuria. No other underlying cause for proteinuria in the latter 2 cases was found. Severe hypertension was present in one.
Urinalysis of Repeated Urine Samples
Of the 42 repeated free catch urine samples, sediment analysis was available at both time points only in 37 of dogs. In the other dogs, sediment analysis was not performed because of insufficient sample volume. Hence, 37 paired samples remained for statistical analysis. At baseline, the majority (34 of 37, 92%) had an inactive sediment and only 3 dogs (8%) had an active sediment. At the second time point, similar findings were present (inactive 35 of 37 [95%], active 2 of 37 [5%]). The 2 dogs with an active sediment at the second time point both had moderate bacteriuria, without the presence of pyuria or hematuria. Urine bacterial culture was not performed (free catch urine). Both dogs were nonproteinuric at that time point.
Of these 37 dogs, 27 (73%) were nonproteinuric, 6 (16%) had borderline proteinuria, and 4 (11%) overt proteinuria at baseline. At the second time point, 25 dogs (68%) were nonproteinuric, 6 (16%) had borderline proteinuria, and 6 (16%) overt proteinuria, not explained by sediment analysis. From 3 of 5 dogs with renal azotemia at baseline, repeated urine samples were available. The dog later diagnosed with CKD IRIS stage 2 developed proteinuria (UPC 0.5), and the remaining 2 remained nonproteinuric. Of the 12 dogs with moderate to severe gingivitis at baseline, follow‐up data were available in 5. Three remained nonproteinuric, 1 switched from no proteinuria to overt proteinuria, and 1 from overt proteinuria to borderline proteinuria.
Persistent overt proteinuria was present in 3 of 37 (8%) and persistent borderline proteinuria in 4 of 37 (11%). Of the 3 dogs with persistent overt proteinuria, 2 had a UPC determination between 0.5 and 1.0 and 1 between 1.0 and 2.0. Twenty‐four (65%) never had proteinuria. Characteristics of the dogs with persistent borderline or overt proteinuria are presented in Table 1. Inconsistent results were present in 6 dogs, which could not be attributed to sediment analysis. One dog had transient borderline proteinuria, 1 switched from borderline to overt proteinuria, 1 dog switched from overt to borderline proteinuria, and 3 nonproteinuric dogs at baseline showed borderline (n = 2) or overt (n = 1, dog with CKD IRIS stage 2) proteinuria on the consecutive urine sample. One additional dog had severe persistent proteinuria (UPC >3.5), but was excluded from statistical analysis because of missing sediment analysis at time point 2. One of the dogs with persistent overt proteinuria (UPC 0.6) was diagnosed with hypothyroidism at baseline.
Table 1.
Descriptive characteristics (number, percentage; 95% confidence interval) of dogs with persistent overt proteinuria and borderline renal proteinuria in repeated free catch urine samples
| Characteristics | Total (N = 37) | Proteinuric (N = 3) | Borderline Proteinuric (N = 4) |
|---|---|---|---|
| Age–class | |||
| Senior | 16 | 2 (12.5%; 1.6–38.3%) | 1 (6.3%; 0.2–30.2%) |
| Geriatric | 21 | 1 (4.8%; 0.1–23.8%) | 3 (14.3%; 3.1–36.3%) |
| Breed size | |||
| Small | 7 | 0 (0%; 0.0–40.6%) | 1 (14.3%; 0.4–57.9%) |
| Medium | 9 | 2 (22.2%; 2.8–60%) | 2 (22.2%; 2.8–60%) |
| Large | 17 | 0 (0%; 0.0–19.5%) | 0 (0%; 0.0–19.5%) |
| Giant | 4 | 1 (25%; 0.6–80.6%) | 1 (25%; 0.6–80.6%) |
| SBP (mmHg) | |||
| <160 | 13 | 1 (7.7%; 0.2–36%) | 2 (15.4%; 1.9–45.5%) |
| 160–180 | 16 | 1 (6.3%; 0.2–30.2%) | 1 (6.3%; 0.2–30.2%) |
| >180 | 8 | 1 (12.5%; 0.3–52.7%) | 1 (12.5%; 0.3–52.7%) |
| BCS | |||
| ≤3/9 | 1 | 0 (0%; 0.0–97.5%) | 0 (0%; 0.0–97.5%) |
| 4–5/9 | 21 | 2 (9.5%; 1.2–30.4%) | 2 (9.5%; 1.2–30.4%) |
| >5/9 | 15 | 1 (6.7%; 0.2–32%) | 2 (13.3%; 1.7–40.5%) |
SBP, systolic blood pressure; BCS, body condition score on a 9‐point scale.
Free Catch and Cystocentesis Sample at Baseline
Comparison of UPC from free catch urine samples (median 0.12, range 0.04–4.56) and cystocentesis urine samples (median 0.13, range 0.04–3.26) at baseline revealed a significant and strong correlation (ρ = 0.88) between the 2 sampling methods. The mean absolute difference in UPC between both sampling methods was 0.02, with a standard deviation of 0.22 and a maximal absolute difference of 1.3. There was no significant difference between the 2 methods (P = .44). However, the variability did seem to increase with increasing values of the UPC, as shown by the Bland–Altman plot (Fig 2). In 13 of 71 (18%) cases, another ACVIM classification would be assigned to the UPC result obtained by free catch or cystocentesis.
Figure 2.
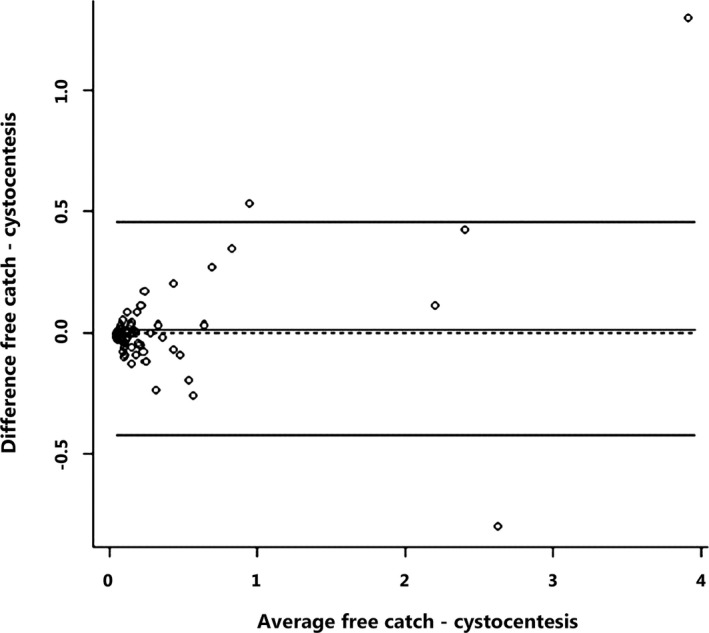
Bland–Altman plot to assess the level of agreement for urinary protein : creatinine ratio (UPC) measurements between free catch and cystocentesis urine samples (N = 71). Free catch, UPC measurements on free catch urine; cytocentesis, UPC measurements on cystocentesis urine.
Discussion
Twenty‐five percent of apparently healthy senior and geriatric dogs had renal proteinuria (14% borderline, 11% overt) at baseline on free catch urine. Eight percent of the dogs with repeated free catch urine samples had persistent overt renal proteinuria and another 11% persistent borderline renal proteinuria. Despite the low numbers of repeated samples available, these findings suggest that approximately 1 of 5 apparently healthy elderly dogs might have a persistently increased UPC. Our findings at baseline are lower compared to a previous study,8 which reports microalbuminuria in spot urine samples in 36–49% of elderly dogs. This is a consequence of the fact that microalbuminuria is a more sensitive screening test than the conventional UPC measurement in dogs.
Several methods exist to evaluate whether dogs are proteinuric. Measuring UPC is among the most commonly used methods to quantify and monitor proteinuria in veterinary medicine. In practice, dipstick test (common) or sulfosalicylic acid (SSA) turbidimetric test (les common) is used as a semiquantitative measure of urinary protein.23 Dipstick analysis provides an easy, rapid, in‐house test, although a previous study only found moderate specificity and poor positive predictive value in dogs.24 If a false‐negative dipstick result is suspected, a SSA test or microalbuminuria test can be employed.23 Microalbuminuria is defined as the presence of a small amount (1–30 mg/dL) of albumin in the urine, below the limit of detection of urinary dipstick tests.23 Microalbuminuria can also remain undetected by UPC determination.24 Higher urinary albumin concentrations (>30 mg/dL) are termed overt albuminuria and are usually detected by urine dipstick tests or UPC.23
Prerenal (eg, hemoglobinuria) and postrenal (eg, cystitis, prostatitis, urolithiasis, neoplasia) causes of proteinuria were considered unlikely based on the physical examination, blood examination, ultrasound of the bladder, and urinalysis (sediment). Therefore, we can conclude that the observed persistent proteinuria is likely renal in origin.10 Proteinuria is observed in up to 25% of human geriatric patients and is not considered a normal age‐related decline in renal function.5, 6 In only 1 of the persistently borderline or overt proteinuric dogs, an underlying disease, for example, hypothyroidism, could be detected based on routine health screening. In that dog, treatment with l‐thyroxine9 was initiated, after the second free catch sample was collected. Hypertension was recorded in the dog at initial examination. No follow‐up of UPC or SBP was available. Hypothyroidism is associated with decreased Glomerular filtration rate (GFR) in dogs25 and both in humans and rats with mild proteinuria.26 Other diseases such as CKD, glomerulopathy, diabetes mellitus, or multiple myeloma, associated with persistent proteinuria, were not detected in dogs with persistent borderline or overt proteinuria. In human medicine, associations between periodontal disease and glomerulonephritis exist.27 Because of the limited sample size, the effect of gingivitis on proteinuria in dogs could not be evaluated. In 1 dog, CKD IRIS stage 2 was confirmed. This dog was nonproteinuric at baseline and developed proteinuria (UPC 0.5) at the second time point. Of the remaining 4 azotemic dogs, CKD could not be excluded because of missing follow‐up data, although no polydipsia was present. None of the other azotemic dogs had proteinuria at initial (n = 4) or follow‐up (n = 2) examination.
Hypertension is considered an important cause of proteinuria in human medicine, and an association with proteinuria is described in dogs.13, 19 Although 44% (7 of 16) of borderline or overt proteinuric dogs at baseline had a moderate to severe increased blood pressure (data not shown), increased blood pressure was frequently (45%, 29/64) encountered in the present study population. The high percentage of hypertension in our study is in contrast with studies on dogs in general and on healthy geriatric dogs.28, 29 However, comparison between studies is difficult because various definitions for hypertension are used and because dogs with laboratory abnormalities, heart murmur, or obesity were not excluded in the current study.29 Based on the examinations that are part of general health screening, an obvious underlying cause for hypertension could not be defined, except for 1 dog with hypothyroidism. An influence of age (and breed) on blood pressure has been previously suggested, warranting an age‐dependent reference interval.30 Despite precautions taken, white coat hypertension must be considered as a cause of the increased blood pressure, because repeated blood pressure measurements, preferably in the house environment of the dogs, were not performed. A study in 3‐year‐old beagles revealed a gradual decrease in SBP and normalization of hypertension in all animals after 4–5 measurements, each on a different day.31 This illustrates importance of stress, but also the fact that an acclimatization period of 5–10 minutes might not be sufficient in dogs.
Obesity is another known risk factor for the development of proteinuria and CKD in humans.32 Typical glomerular changes observed in these patients, called obesity‐related glomerulopathy, are associated with hemodynamic changes and lipotoxicity.32 Similar glomerular lesions are described in dogs with experimental‐induced obesity.33 In our study population, 41% (15 of 37) had a BCS compatible with overweight/obesity and a similar percentage (43%; 3 of 7) of dogs with persistent borderline or overt proteinuria were obese.22 Because of the small sample size, the effect of BCS on proteinuria in dogs could not be evaluated. A recent study on 20 overweight/obese dogs and 22 ideal bodyweight control dogs found no effect of obesity on the level of proteinuria/microalbuminuria, concluding that clinicopathological abnormalities consistent with obesity‐related glomerulopathy are absent.34 However, this contrasts with the significant reduction in proteinuria seen after weight loss in dogs with naturally occurring obesity.35 Larger scale studies, including renal biopsies, are necessary to further assess the effect of canine obesity on proteinuria.
The small number of repeated urine samples makes it difficult to draw conclusions on the effect of age, breed/size, and BCS on UPC. To the authors' knowledge, no breed or size effect has been previously described, and further studies are warranted. The effect of sediment analysis on UPC was not evaluated, because of the low number of samples with an active sediment (5%, 4 of 71). A previous study already emphasized the importance to interpret UPC in light of urinary sediment findings.21
The majority (84%, 31 of 37) of dogs remained in the same UPC category on both consecutive samples. In none of the cases, overt proteinuria normalized. In 1 case, transient borderline proteinuria was present; in another, overt proteinuria switched to borderline proteinuria. In 4 cases (10%), dogs switched to a higher classification on the consecutive sample, not explained by the sediment. There is daily variation in UPC values.36 To indicate disease progression or adequate treatment response, differences in UPC have to be >80% at a UPC near 0.5.36 In the dogs with inconsistent consecutive UPC categories, a variance of >80% was only seen in 1 sample (switching from no proteinuria to overt proteinuria). The other observed changes might be just a consequence of daily UPC variation. The daily UPC variation may also lead to incorrect classification of dogs in UPC categories, for example, classification of a borderline proteinuric dog as normal or vice versa. To overcome the effect of daily UPC variation, UPC determination on pooled urine samples can be considered.37 Within the group of persistent overt proteinuria, severity of proteinuria remained similar at the consecutive sample. These findings suggest that a single UPC measurement, in the absence of an active sediment, might be adequate to advise the owners to investigate for an underlying cause, follow up, and initiate treatment for proteinuria, in agreement with recent guidelines.11, 15
A significant strong correlation was found for UPC from free catch and cystocentesis urine samples. The mean absolute difference in UPC values was low, strengthening the hypothesis that both techniques give a similar value in most dogs. However, the mean absolute difference was may be low because the majority of dogs did not have severe proteinuria. Possibly, with higher UPC values, a larger mean absolute difference could have been observed. These findings are consistent with previous studies in 81 dogs and 43 cats, which revealed that UPC may be reliably measured in free catch urine samples.17, 18 The largest absolute differences occurred in dogs with overt proteinuria and were not associated with a change in classification across proteinuria categories. In 13 of 71 cases, a difference (median 0.11, range 0.04–0.24) in UPC between both techniques led to an altered classification. Of these 13 cases, 9 showed an increase in UPC on the cystocentesis sample. Possibly, stress associated with the prior health examination or performance of cystocentesis could have caused this increase. Based on our data, this might have led to a change in classification in 12.7% (9 of 71) dogs. However, when examining the 13 cases with different classification, all UPC values were close to the decision threshold values between no proteinuria, borderline proteinuria, and overt proteinuria. In agreement with our findings, a previous study indicated that UPC values close to the decision thresholds must be interpreted cautiously as variation in UPC may result in misclassification.21 Based on the limited data, we cannot conclude that stress has caused these changes. Influence of setting of urine collection on UPC measurement in dogs exists as significantly higher UPC in samples (both free catch and cystocentesis) obtained in the hospital environment were found compared to home samples.38 In the present study, several owners collected urine just before entering the clinic instead of in the home environment, so conclusions on influence of environment on UPC cannot be drawn based on our data. Overall, a significant strong correlation was found between UPC measured on free catch and cystocentesis samples.
This study has some limitations. First, the small sample size makes it difficult to draw conclusions on the effect of breed/size and BCS on UPC. Although collecting free catch urine is often thought to be convenient for the owner,17 this was not supported by our results. At baseline, 29% (29 of 100) of owners failed to catch urine. The majority of them tried, but did not succeed because of a noncooperative dog. Development of practical and harmless urine collecting kits for dogs and cats is a positive evolution that can facilitate and promote the performance of urinalysis in small animal practice. Second, no further diagnostics (thoracic radiographs, abdominal ultrasonography, serology for vector borne diseases, renal biopsies) were performed in dogs with persistent overt renal proteinuria. This did not allow to exclude underlying pathologies, often described in human medicine.6 Third, no follow‐up of blood pressure measurement was performed. Therefore, stress‐related hypertension could not be excluded. Fourth, no determination of microalbuminuria, considered a more sensitive screenings tool, was performed.39 Microalbuminuria is a good indicator of early renal disease in dogs, although it is not specific.15 Considering the only limited evidence of clinical benefit of measuring microalbuminuria over UPC in dogs and cats and the fact that the analysis is not widely commercially available, it was decided not to perform this analysis.15 Similarly with microalbuminuria, previous studies have addressed the usability of dipstick analysis to screen for proteinuria.24, 40 A combination of USG >1.012 and only +1 or less protein on the dipstick analysis has a good specificity for the absence of proteinuria, but only a moderate sensitivity.40 Therefore, it can be concluded that although dipstick analysis can be seen as a rapid, easy, and cheap test for assessment of proteinuria, the sensitivity is clearly a limitation. In addition, a positive dipstick result needs to be followed by quantification of proteinuria to determine whether treatment might be indicated for the patient. In our data, 3 dogs with a negative dipstick test showed borderline (N = 2) or overt (N = 1) proteinuria and 4 dogs with a USG >1.012 and only +1 or less protein on dipstick analysis did have borderline (N = 2) or overt (N = 2) proteinuria. Considering that 16 of 64 dogs had borderline or overt proteinuria at initial presentation, careful conclusions on the usability of dipstick analysis as screenings test for proteinuria should be made. This, combined with the fact that UPC is among the most commonly used methods to quantify and monitor proteinuria in veterinary medicine, led to the decision to use UPC as the screening tool for proteinuria in this study. Fifth, no standardized containers were used for collection of free catch urine samples. Protein binding to hydrophilic surfaces is mainly believed to be important with very low concentrations of albumin (microalbuminuria).38 Therefore, it seems unlikely to have significantly affected our UPC measurements, although falsely decreased UPC cannot be excluded.38 Sixth, urinalysis could not be performed for all free catch samples within 60 minutes after collection. However, in such cases, samples were stored at 4°C and analyzed within 12 hours (for the majority within 4 hours). A significant increase in UPC of dogs only occurs after 12 hours of storage at 4°C.21 Finally, the majority (69/71) of cystocentesis samples were collected within a time frame of 4 hours after collection of free catch urine samples. In 2 cases, a time frame of 4–12 hours was maintained. Urinary protein : creatinine measurements from spot urine samples have been shown to accurately reflect the quantity of proteins excreted in the urine over a 24‐hour period.41 Furthermore, there is no significant variability in UPC over 8‐hour collection periods.42 Therefore, within‐day variation of UPC can be considered minimal, and the larger time frame in the latter 2 dogs unlikely affected our results.
In conclusion, 25% of apparently healthy elderly dogs had renal proteinuria at baseline on free catch urine samples, of which 14 and 11% had borderline proteinuria and overt proteinuria, respectively. Of the dogs with repeated urine samples, 19% had a persistently increased UPC with 8% having persistent overt renal proteinuria. Our findings emphasize that measurement of proteinuria should be part of routine health screening of the elderly dog. The strong correlation between UPC values of urine collected by free catch or cystocentesis suggests that both collection methods can be appropriate to assess UPC in veterinary practice, but careful interpretation is warranted for values that are close to the decision threshold.
Acknowledgments
Conflict of Interest Declaration: For this work, a grant of Hill's Pet Nutrition was received. None of the authors has any financial or personal relationship that could inappropriately influence or bias the content of the manuscript.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Faculty of Veterinary Medicine, Ghent University, Belgium.
This work was presented as an oral abstract at the European Veterinary Conference Voorjaarsdagen, April 9–11, 2015.
Footnotes
Willems A, Paepe D, Marynissen S, et al. Screening of apparently healthy senior and geriatric dogs. J Vet Intern Med, [accepted].
Parks Medical Electronics Inc, Aloha, OR
Iricell IQ, Instrumentation Laboratory, Zaventem, Belgium
iChem velocity urine Chemistry System, Beckman Coulter, Suarlée, Belgium
iCHEM velocity stick, Beckman Coulter
Wask copan, BioMerieux Media Square, Brussels, Belgium
SAS version 9.3, SAS Institute Inc., Cary, NC
Clavubactin, Lelypharma B.V., Lelystad, the Netherlands
Forthyron, Eurovet Animal Health BV, Bladel, the Netherlands
References
- 1. Fortney WD. Implementing a successful senior/geriatric health care program for veterinarians, veterinary technicians, and office managers. Vet Clin North Am Small Anim Pract 2012;42:823–834. [DOI] [PubMed] [Google Scholar]
- 2. Diez M, Picavet P, Ricci R, et al. Health screening to identify opportunities to improve preventive medicine in cats and dogs. J Small Anim Pract 2015;56:463–469. [DOI] [PubMed] [Google Scholar]
- 3. Silva FG. The aging kidney: A review – part I. Int Urol Nephrol 2005;37:185–205. [DOI] [PubMed] [Google Scholar]
- 4. Smets PMY, Lefebvre HP, Aresu L, et al. Renal function and morphology in aged beagle dogs before and after hydrocortisone administration. Plos ONE 2012;7:e31702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heidenreich S, Zierden E, Zidek W. Microalbuminuria in very old patients. Geriatr Nephrol Urol 1995;5:9–13. [Google Scholar]
- 6. Verma V, Kant R, Sunnogrot N, Gambert SR. Proteinuria in the elderly: Evaluation and management. Int Urol Nephrol 2012;44:1745–1751. [DOI] [PubMed] [Google Scholar]
- 7. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: Findings in apparently healthy middle‐aged and old cats. J Feline Med Surg 2013;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prevalence of microalbuminuria in veterinary clinic staff‐owned dogs, 2003. In: Littman MP, ed. Protein‐Losing Nephropathy in Small Animals. In: Acierno MJ, Labato MA, eds. Kidney disease and renal replacement therapies. Vet Clin North Am Small Anim Pract 2011;41:31–62.Available at: http://www.heska.com/Documents/RenalHealthScreen/erd_data.aspx. Accessed July 24, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Radecki S, Donnely R, Jensen WA, et al. Effect of age and breed on the prevalence of microalbuminuria in dogs. J Vet Intern Med 2003;17:406 [abstract]. [Google Scholar]
- 10. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (Small Animal). J Vet Intern Med 2005;19:377–385. [DOI] [PubMed] [Google Scholar]
- 11. Treatment recommendations for CKD in dogs 2015. Available at: http://www.iris-kidney.com/guidelines/guidelines_updates_2014-2015.aspx. Accessed December 8, 2015.
- 12. Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. J Am Med Assoc 2010;303:423–429. [DOI] [PubMed] [Google Scholar]
- 13. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 14. King JN, Tasker S, Gunn‐Moore DA, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007;21:906–916. [PubMed] [Google Scholar]
- 15. IRIS Canine GN Study Group Diagnosis Subgroup , Littman MP, Daminet S, et al. Consensus recommendations for the diagnostic investigation of dogs with suspected glomerular disease. J Vet Intern Med 2013;27:S19–S26. [DOI] [PubMed] [Google Scholar]
- 16. Syme HM, Markwell PJ, Pfeiffer D, Elliot J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006;20:528–535. [DOI] [PubMed] [Google Scholar]
- 17. Beatrice L, Nizi F, Callegari D, et al. Comparison of urine protein‐to‐creatinine ratio in urine samples collected by cystocentesis versus free catch in dogs. J Am Vet Med Assoc 2010;236:1221–1224. [DOI] [PubMed] [Google Scholar]
- 18. Vilheda HCR, Santos RR, Sargo TJ, et al. Urine protein‐to‐creatinine concentration ratio in samples collected by means of cystocentesis versus manual compression in cats. J Am Vet Med Assoc 2015;246:862–867. [DOI] [PubMed] [Google Scholar]
- 19. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 20. Wamsley H, Alleman R. Complete urinalysis In: Elliott J, Grauer GF, eds. BSAVA manual of Canine and Feline Nephrology and Urology, 2nd ed Quedgeley, Gloucs: British Small Animal Veterinary Association; 2007:87–116. [Google Scholar]
- 21. Rossi G, Giori L, Campagnola S, et al. Evaluation of factors that affect analytic variability of urine protein‐to‐creatinine ratio determination in dogs. Am J Vet Res 2012;73:779–788. [DOI] [PubMed] [Google Scholar]
- 22. WSAVA Nutritional Assessment Guidelines Task Force Members , Freeman L, Becvarova I, Cave N, et al. Nutritional assessment guidelines. J Small Anim Pract 2011;52:385–396. [DOI] [PubMed] [Google Scholar]
- 23. Grauer GF. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet Clin North Am Small Anim Pract 2007;37:283–295. [DOI] [PubMed] [Google Scholar]
- 24. Lyon SD, Sanderson MW, Vaden SL, et al. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio, and species‐specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc 2010;236:874–879. [DOI] [PubMed] [Google Scholar]
- 25. Gommeren K, van Hoek I, Lefebvre HP, et al. Effect of thyroxine supplementation on glomerular filtration rate in hypothyroid dogs. J Vet Intern Med 2009;23:844–849. [DOI] [PubMed] [Google Scholar]
- 26. Van hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review. Gen Comp Endocrinol 2009;160:205–215. [DOI] [PubMed] [Google Scholar]
- 27. Ardalan MR, Ghabili K, Pourabbas R, Shoja MM. A causative link between periodontal disease and glomerulonephritis: A preliminary study. Ther Clin Risk Manag 2011;7:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remillard RL, Ross JN, Eddy JB. Variance of indirect blood‐pressure measurement and prevalence of hypertension in clinically normal dogs. Am J Vet Res 1991;52:561–565. [PubMed] [Google Scholar]
- 29. Meurs KM, Miller MW, Slater MR, et al. Arterial blood pressure measurement in a population of healthy geriatric dogs. J Am Anim Hosp Assoc 2000;36:497–500. [DOI] [PubMed] [Google Scholar]
- 30. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 31. Schellenberg S, Glaus TM, Reusch CE. Effect of long‐term adaptation on indirect measurements of systolic blood pressure in conscious untrained beagles. Vet Rec 2007;161:418–421. [DOI] [PubMed] [Google Scholar]
- 32. Wahba IM, Mak RH. Obesity and obesity‐initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2007;2:550–562. [DOI] [PubMed] [Google Scholar]
- 33. Henegar JR, Bigler SA, Henegar LK, et al. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 2001;12:1211–1217. [DOI] [PubMed] [Google Scholar]
- 34. Tefft KM, Shaw DH, Ihle SL, et al. Association between excess body weight and urine protein concentration in healthy dogs. Vet Clin Pathol 2014;43:255–260. [DOI] [PubMed] [Google Scholar]
- 35. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effect of weight loss in obese dogs on indicators of renal function or disease. J Vet Intern Med 2013;27:31–38. [DOI] [PubMed] [Google Scholar]
- 36. Nabity MB, Boggess MM, Kashtan CE, et al. Day‐to‐day variation of the urine protein: Creatinine ratio in female dogs with stable glomerular proteinuria caused by X‐linked hereditary nephropathy. J Vet Intern Med 2007;21:425–430. [DOI] [PubMed] [Google Scholar]
- 37. LeVine DN, Zhang DW, Harris T, et al. The use of pooled vs serial urine samples to measure urine protein:creatinine ratios. Vet Clin Pathol 2010;39:53–56. [DOI] [PubMed] [Google Scholar]
- 38. Duffy ME, Specht A, Hill RC. Comparison between urine protein: creatinine ratios of samples obtained from dogs in home and hospital settings. J Vet Intern Med 2015;29:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whittemore JC, Gill VL, Jensen WA, et al. Evaluation of the association between microalbuminuria and the urine albumin‐creatinine ratio and systemic disease in dogs. J Am Vet Med Assoc 2006;229:958–963. [DOI] [PubMed] [Google Scholar]
- 40. Zatelli A, Paltrinieri S, Nizi F, et al. Evaluation of a urine dipstick test for confirmation or exclusion of proteinuria in dogs. Am J Vet Res 2010;71:235–240. [DOI] [PubMed] [Google Scholar]
- 41. Grauer GF, Thomas CB, Eicker SW. Estimation of quantitative proteinuria in the dog, using the urine protein‐to‐creatinine ratio from a random, voided sample. Am J Vet Res 1985;46:2116–2119. [PubMed] [Google Scholar]
- 42. Jergens AE, McCaw DL, Hewett JE. Effects of collection time and food consumption on the urine protein/creatinine ratio in the dog. Am J Vet Res 1987;48:1106–1109. [PubMed] [Google Scholar]


