Abstract
Background
Mean systolic blood pressure in apparently healthy cats has been reported as approximately 125 mmHg using direct assessment, but there is greater variability in reported values using indirect assessment. Increasing age and the white‐coat effect are associated with increased systolic blood pressure.
Hypothesis/Objectives
To report Doppler‐derived blood pressure measurements from a large population of apparently healthy cats and to assess epidemiologic factors associated with recorded blood pressures.
Animals
A total of 780 cats in rehoming centers enrolled in a screening program for heart murmurs and cardiac disease.
Methods
Cats were considered healthy based on history and physical examination. Cats with known hypertension, hyperthyroidism, or clinical signs of systemic disease and pregnant or nursing queens were excluded. After an acclimatization period, systolic blood pressure was measured using the Doppler sphygmomanometry method following the recommendations of the ACVIM Consensus Statement. General linear model analysis was performed to identify factors associated with variation in systolic blood pressure.
Results
Median (interquartile range, IQR) systolic blood pressure for the group was 120.6 (110.4–132.4) mmHg. Factors significantly associated with higher systolic blood pressure in a general linear model were increased age, increased nervousness, male sex, neutering, or history of being a stray. The model explained 29.2% of the variation in systolic blood pressure.
Conclusions and Clinical Importance
The age, demeanor, sex, neuter status and history of being a stray should be taken into account when assessing systolic blood pressure in apparently healthy cats.
Keywords: Feline, Hypertension, Screening, Shelter
Abbreviations
- IQR
interquartile range—25th percentile to 75th percentile
- SBP
systolic blood pressure
Systemic blood pressure is routinely measured in cats in clinical settings. Measurement of blood pressure is indicated when the cat has evidence of target organ damage, or when diseases associated with secondary systemic hypertension are diagnosed.1 Target organ damage in the cat includes left ventricular hypertrophy,2, 3 retinopathy or choroidopathy,4, 5 progression of chronic kidney disease,6, 7 and encephalopathy or stroke.8 Diseases associated with secondary systemic hypertension in the cat include chronic kidney disease,9, 10 hyperthyroidism,9 primary hyperaldosteronism,11 and pheochromocytoma.12 If none of these diseases are present, and stress‐related effects on blood pressure (“white‐coat hypertension”)13 have been excluded, then cats with persistent hypertension are considered to have idiopathic hypertension. However, it can be difficult to definitively rule out subclinical renal disease or primary hyperaldosteronism, which can make an unconditional diagnosis of idiopathic hypertension difficult.
Measurement of systemic blood pressure can be carried out directly using intra‐arterial assessment,13, 14 although this generally requires anesthesia or sedation, or indirectly using oscillometric15, 16 or Doppler sphygmomanometry9, 17, 18, 19 techniques. Indirect methods are more commonly used in clinical practice.1 Normal values for systolic blood pressure (SBP) in apparently healthy cats have been reported as 125.1 ± 10.6 mmHg and 126.0 ± 4.4 mmHg using intra‐arterial radiotelemetric implants for direct assessment.13, 14 Normal SBP measured in cats using indirect methods has shown more variability. Using Doppler techniques, normal SBP values of 118 ± 10.6 mmHg, 162 ± 38 mmHg, 131.9 ± 17.6 mmHg, and 131.6 (115–143.7) mmHg have been reported.9, 17, 18, 19 With oscillometric techniques, normal values of 139.4 ± 26.9 mmHg and 115.4 ± 10.1 mmHg have been reported.15, 16 However, the majority of these studies contained fewer than 100 cats. Increasing age15, 19 and the white‐coat effect13 are associated with increased SBP in cats.
The aim of our study was to report blood pressure measurements using the Doppler method from a large population of apparently healthy cats and to assess factors associated with the variation in SBP.
Materials and Methods
This study was approved by the Royal Veterinary College ethics and welfare committee (Unique Reference Number 2010 1004).
The cats enrolled in this study originally were enrolled as part of a study of apparently healthy cats to assess the prevalence of heart murmurs on auscultation and myocardial disease on echocardiography, and the methods for this study have been described previously.20 In brief, cats from 2 rehoming centers in the South East of the United Kingdom1 were included in a cross‐sectional study (the “CatScan” study). All cats aged 6 months or older that were available for rehoming were enrolled, provided they were considered healthy based on known history and routine physical examination as performed by the veterinary staff at the rehoming center. Cats were excluded if they were hypertensive (SBP ≥180 mmHg); if they were receiving any medical treatment reported to affect blood pressure; if they were hyperthyroid or on medical management for hyperthyroidism; if they had systemic disease causing clinical signs (e.g, cats with azotemia identified on routine screening blood testings were included as long as the cats were not polyuric or polydipsic); or if they were pregnant or nursing queens. Cats were not excluded if they had a murmur auscultated or if they had echocardiographic evidence of cardiac disease. Serum biochemistry and total thyroxine concentrations only were measured if the rehoming center staff thought it to be clinically necessary on the basis of known history or physical examination.
Data collected included age (often as an estimated age range), date of birth (if known), sex, neuter status (if known), breed (based on appearance rather than using pedigrees), and whether the cat was a stray or had been relinquished to the home by a previous owner. Cats then were assessed as to their temperament, with cats rejected at this stage if they were deemed aggressive or too nervous to be handled. Cats that were initially classified as too aggressive or too nervous to handle such that it was impossible to measure blood pressure could be reassessed at a later date. These cats were included if they were no longer too aggressive or nervous to be handled at the subsequent examination.
While cats were habituating to the examination room, their medical records were assessed and any concurrent or historical medical problems were recorded, as was the date of neutering, if known. Cats would be habituated for at least 5 minutes routinely, and for up to 20 minutes if the cat appeared anxious. Once settled, cats were weighed and their body condition score (BCS) was assessed on a scale of 1–9 based on the Purina scale.21 Cats underwent indirect systolic blood pressure (SBP) measurement, performed as recommended by the ACVIM Consensus Statement1 using a Parks Doppler unit2 in a quiet room. All measurements were performed by a single observer (JRP) who had 3 years of experience using Doppler sphygmomanometry. Blood pressure cuff size was selected so that the cuff width was 30–40% of the circumference of the limb at the cuff site. Cats were allowed to settle into a position of their choosing once the cuff had been attached. For convenience, the default limb used was the right forelimb and this was only altered if that limb was missing or injured, if the cat resented the right forelimb being touched, or if variable or unexpectedly low or high results were generated from the right forelimb. If the right forelimb was not used, the left forelimb was used. After at least 30 seconds of acclimatization (time dependent on the level of nervousness of the cat), the sphygmomanometer was attached, the hair over the artery was moistened with ultrasound gel, and the probe was placed over the region of the first palmar common digital artery. Hair was not removed in any cat for the purposes of blood pressure assessment. Again, a small period of acclimatization was allowed before the machine was turned on, the strongest signal located, and measurements were made. Throughout the assessment, the cuff was kept as level as possible with the level of the right atrium. For cats in lateral recumbency, the sternum was used as an estimate of the right atrial level. For cats that were sitting or were in sternal recumbency, a point 40% up an imaginary line from the sternum to the dorsal spinous processes at the level of the caudal border of the scapula was used as an estimate of the right atrium. At least 6 measurements were recorded, with the first measurement being automatically discarded and any discordant results also being excluded. If discordant results were obtained, additional measurements were taken until there were 5 similar values to average. In addition to recording the cuff size and site, the position of the cat was recorded (standing, sitting, lying in sternal recumbency, lying in left lateral recumbency, or lying in right lateral recumbency) as was the cat's demeanor22 during the blood pressure assessment (Table 1). Cats may have been considered aggressive during assessment of SBP but not excluded from the overall study if the aggression was not sustained and did not result in injury to the people involved.
Table 1.
Assessment of cat demeanor during blood pressure measurement
| Demeanor | Assessment |
|---|---|
| Calm | Relaxed throughout procedure, looking around, relaxed body position, eye contact accepted with eye blinking, ears forward, tail held in vertical position, purring, trilling, and/or kneading |
| Cooperative but anxious | Generally calm and still looking around, but showing some signs of nervousness (usually tail between legs or slightly crouched body position) |
| Nervous | Hiding (either hiding head into the person restraining or whole body under a blanket) or crouched, shivering, avoiding eye contact, ears folded sideways and downwards, tail between legs, may be purring/kneading |
| Aggressive | Hissing, growling, or swiping with claws |
| Excited | Attempting to play with the blood pressure equipment, difficult to keep still as more interested in interacting with people and/or the environment |
At the completion of the study, medical records kept by the rehoming center were reassessed to make sure that no cat had been subsequently diagnosed with a condition that would exclude it from the project after its screening.
Statistical Analysis
Statistical analysis was performed using commercially available software,3 normality of continuous data was assessed graphically, and non‐normally distributed data are presented as median (interquartile range (IQR): 25th percentile to 75th percentile). Data are graphically represented using box and whisker plots with outliers defined using the Tukey method in which outliers are values > “75th percentile plus one and half times the interquartile range.” Outliers were not excluded from any statistical analysis.
Univariable analysis was performed using the Mann‐Whitney U‐test or Kruskal‐Wallis as appropriate to compare continuous, non‐normally distributed data. Correlations were assessed using Spearman's rank correlation coefficients. General linear model analysis was performed to identify factors associated with variation in SBP. All factors significant at P < 0.2 were taken forward in a manual backwards stepwise elimination model construction approach. First‐order interactions were assessed for final model variables, and the model residuals were checked graphically for normality.
Results
This population has been described previously,20 and only a summary of the population characteristics is presented. Data collection occurred between October 2011 and February 2013. During this time period, 1,007 cats were considered eligible for the project. After excluding cats for aggression (n = 93) or being too nervous to handle (n = 134), 780 underwent the full screening process (the “CatScan population”).
The median age group in the CatScan population was 1–<3 years, with the oldest age group being 17–<19 years and the youngest age group (as defined by the study design) being 6–<12 months. Three hundred and thirty‐eight (43.3%) cats of the whole CatScan population were male, and 623 (79.9%) cats of the whole CatScan population were neutered. Four (0.5%) were of unknown neuter status. Fifteen different breeds were observed, of which 758 (97.2%) cats were nonpedigree. The majority (n = 535, 68.6%) of the cats in the CatScan population were relinquished to the rehoming centers, with 245 (31.4%) being found as strays. Median body weight was 3.65 (IQR, 3.11–4.46) kg, and median BCS was 5/9 (IQR, 4–5/9).
Median (IQR) SBP for the group was 120.6 (110.4–132.4) mmHg. During assessment of SBP, the majority of cats (n = 647, 82.9%) required cuff size 2, 7.7% (n = 60) required cuff size 1, and 9.4% (n = 73) required cuff size 3. Systolic blood pressure was measured using the right forelimb in 98.2% (n = 766) of cats and using the left forelimb in 1.8% (n = 14). Most cats (n = 370, 47.4%) adopted a sitting position for SBP assessment. Other cats preferred sternal recumbency (n = 253, 32.4%), standing (n = 121, 15.5%), lying in left lateral recumbency (n = 34, 4.4%), or lying in right lateral recumbency (n = 2, 0.3%). Most cats were considered reasonably calm at the time of SBP measurement with 43.1% (n = 336) being classified as calm and 39.6% (n = 309) being classified as cooperative but anxious. Cats that were considered nervous made up 15.3% (n = 119) of the population, whereas 1.8% (n = 14) were considered excited and 0.3% (n = 2) showed some signs of aggression.
To compare SBP and age, the number of age categories was decreased so that sufficient numbers of cats were in each group. The revised age categories were juvenile (6–<12 months), young adult (1–<3 years), adult (3–<9 years), and senior (≥9 years). There was a significant increase in SBP with increasing age category (overall P < 0.001, all posthoc comparisons significant at P < 0.001 except juvenile vs young adult which was not significant at P = 0.012 using a Bonferroni correction; Figure 1).
Figure 1.
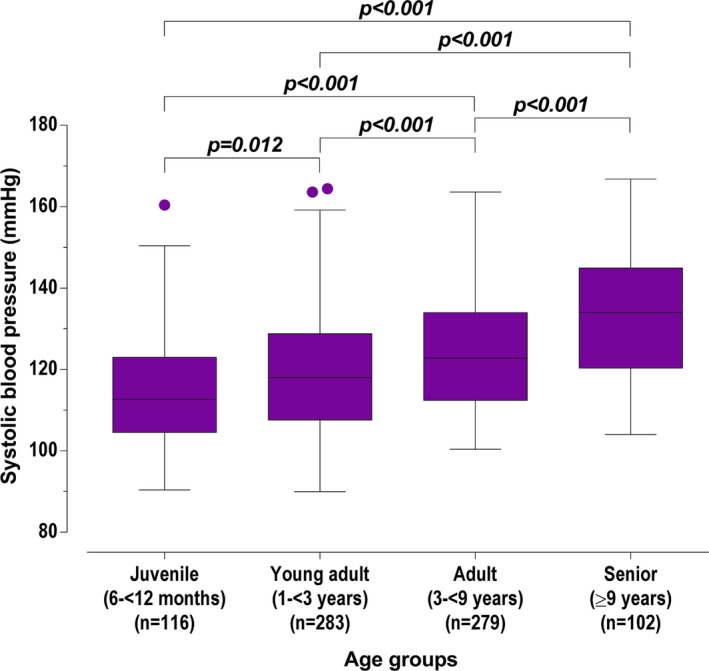
Box and whisker plots for systolic blood pressure of screened cats, grouped by age categories. Box representing median and IQR, whiskers using the Tukey method (values up to 75th percentile + 1.5 IQR), and dots representing outliers (values greater than 75th percentile + 1.5 IQR).
Median SBP in males (122.2 [112.0–137.6] mmHg) was higher (P < 0.001) than in females (119.6 [109.5–129.6] mmHg), and SBP in neutered cats (121.6 [111.6–133.2] mmHg) was higher (P < 0.001) than in unneutered cats (114.0 [105.2–127.4] mmHg). There was no difference in the SBP when comparing pedigree cats and nonpedigree cats (P = 0.911) or when comparing strays and relinquished cats (P = 0.194).
Body condition score had a significant association with SBP (P = 0.001). Based on BCS, cats that were underweight (BCS ≤ 4/9; 117.6 [106.4–130.8] mmHg) had significantly lower SBP than cats with ideal weight (BCS 5/9; 121.2 [111.2–132.4] mmHg, P = 0.003) and significantly lower SBP than cats that were overweight (BCS ≥ 6/9; 122.8 [114.7–138.6] mmHg, P = 0.002). There was no significant difference in the SBP of cats that were ideal weight or overweight (P = 0.118; Figure 2). There was a weak positive correlation between weight and blood pressure (rho = 0.231, P < 0.001).
Figure 2.
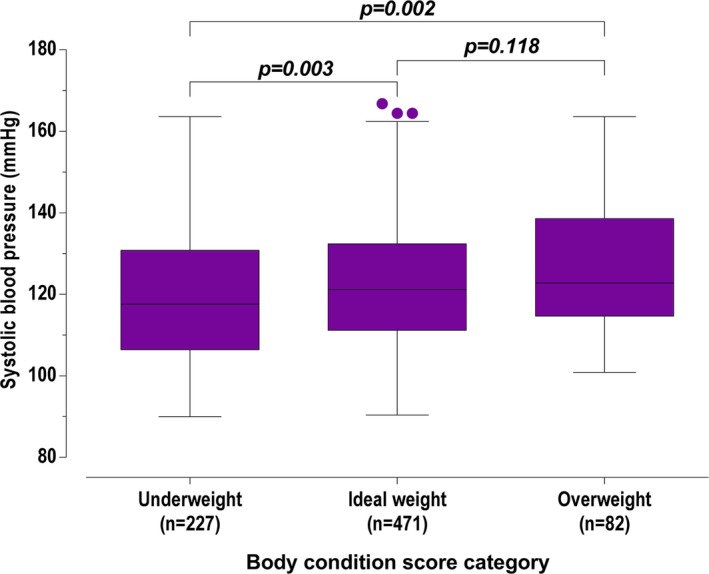
Systolic blood pressure of screened cats, grouped by underweight, ideal weight, and overweight. Box representing median and IQR, whiskers using the Tukey method (values up to 75th percentile + 1.5 IQR), and dots representing outliers (values greater than 75th percentile + 1.5 IQR).
Systolic blood pressure was unaffected by the position the cat adopted during assessment of blood pressure (P = 0.051) but was lower (P = 0.004) if the left forelimb was used (107.0 [99.2–115.7] mmHg) than if the right forelimb was used (120.8 [110.8–132.4] mmHg).
Because of low numbers of cats classified as excited or aggressive during assessment of demeanor during blood pressure assessment, these cats were grouped with the nervous cats for analysis. Systolic blood pressure was significantly different (P < 0.001) when comparing cats that were “calm,” cats that were “cooperative but anxious,” and “nervous, excited, or aggressive” cats. Systolic blood pressure in cats that were considered “calm” (112.8 [105.2–123.5] mmHg) was lower than SBP in cats that were considered “cooperative but anxious” (123.2 [115.6–133.4] mmHg, P < 0.001) and lower than that in cats that were considered “nervous, excited, or aggressive” (131.6 (118.8–142.4) mmHg, P < 0.001). Systolic blood pressure was lower (P = 0.001) in cats that were “cooperative but anxious” than in cats that were “nervous, excited, or aggressive” (Figure 3).
Figure 3.
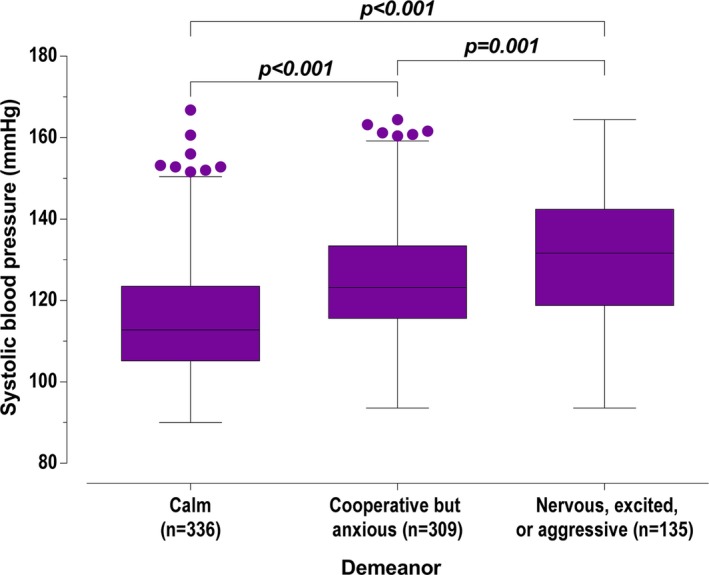
Systolic blood pressure of screened cats, grouped by demeanor during measurement. Box representing median and IQR, whiskers using the Tukey method (values up to 75th percentile + 1.5 IQR), and dots representing outliers (values greater than 75th percentile + 1.5 IQR).
Serum total thyroxine concentrations were measured in 216 (27.7%) cats and were within the reference interval for the laboratory used in all cases. Serum creatinine concentration was measured in 174 (22.3%) cats, with a median value of 1.46 mg/dL (128.8 μmol/L) with an IQR of 1.28–1.66 mg/dL (113.4–147.1 μmol/L). Serum creatinine concentration was <1.60 mg/dL (140 μmol/L) in 116 cats; 57 cats had serum creatinine concentrations of 1.60–2.80 mg/dL (140–250 μmol/L) and 1 cat had a serum creatinine concentration of 3.24 mg/dL (286.7 μmol/L). There was no significant difference (P = 0.07) between the SBP of cats with measured serum creatinine concentration (122.4 [111.9–135.1] mmHg) and SBP of cats without serum creatinine concentration measured (120.0 [110.0–132.0] mmHg).
In the general linear model, factors statistically significantly associated with higher SBP were increasing age category, being more nervous during assessment, being male, being neutered, and being stray (Table 2). Cats without any of these factors (i.e, a calm, 6–<12 months old, unneutered, female that had been relinquished to the rehoming center) would be expected to have a SBP of 104.3 (101.0–107.6) mmHg (Intercept value in the general linear model – baseline). Based on the regression coefficients (B), increasing levels of nervousness would increase the expected SBP by 9.4 (7.4–11.5) mmHg (cooperative but anxious) or by 16.2 (13.5–18.8) mmHg (nervous, excited, or aggressive) from the baseline. Increasing age to 1–<3 years would increase the expected SBP by 3.1 (0.2–6.0) mmHg, increasing to 3–<9 years would increase the expected SBP by 8.6 (5.7–11.6) mmHg, and increasing to ≥9 years would increase the expected SBP by 17.5 (13.8–21.2) mmHg from the baseline. Male cats would be expected to have a SBP 3.9 (2.0–5.8) mmHg higher than the baseline, whereas neutered cats would be expected to have a SBP 3.3 (0.8–5.8) mmHg higher than the baseline. Being a stray increased the SBP 2.5 (0.3–4.6) mmHg from the baseline. The adjusted R‐squared for this model was 0.292, and the residuals were normally distributed. Posthoc analysis of age and demeanor showed significant differences between almost all groups (P < 0.001 for all comparisons of “calm,” “cooperative but anxious,” and “nervous, excited, or aggressive”; P < 0.001 for all comparisons of “juvenile,” “young adult,” “adult,” and “senior,” except “juvenile” vs. “young adult” which was not significant at P = 0.010 using a Bonferroni correction).
Table 2.
General linear model of systolic blood pressure, n = 776. Intercept is the baseline value without the effect of any of the factors. B is the regression coefficient, representing the independent effect for each factor. See text for further explanation
| B | 95% CI of B | P value | |
|---|---|---|---|
| Intercept | 104.3 | 101.0–107.6 | <0.001 |
| Demeanor | |||
| Calm | Reference | ||
| Cooperative but anxious | 9.4 | 7.4–11.5 | <0.001 |
| Nervous, excited, or aggressive | 16.2 | 13.5–18.8 | <0.001 |
| Age | |||
| Juvenile (6–<12 months) | Reference | ||
| Young adult (1–<3 years) | 3.1 | 0.2–6.0 | 0.034 |
| Adult (3–<9 years) | 8.6 | 5.7–11.6 | <0.001 |
| Senior (≥9 years) | 17.5 | 13.8–21.2 | <0.001 |
| Sex | |||
| Female | Reference | ||
| Male | 3.9 | 2.0–5.8 | <0.001 |
| Neuter status | |||
| Unneutered | Reference | ||
| Neutered | 3.3 | 0.8–5.8 | 0.011 |
| Origin | |||
| Relinquished | Reference | ||
| Stray | 2.5 | 0.3–4.6 | 0.023 |
Discussion
Median SBP in this large population of apparently healthy cats was 120.6 (110.4–132.4) mmHg. Factors independently associated with a higher SBP were increasing age category, being more nervous during assessment, being male, being neutered, and being a stray. This model explained 29.2% of the variation in SBP in this population of apparently healthy cats, and therefore, a large proportion of the variation is still unaccounted for. To the authors’ knowledge, this is the largest reported study of SBP in apparently healthy cats to date,9, 13, 14, 15, 16, 17, 18, 19 and as such has several strengths. The wide spread of ages of the cat population studied and the large number of cats included allowed for the identification of several important factors affecting SBP that can be applied to the wider population. Because all cats were assessed by a single observer using the same technique, it was possible to avoid variation caused by multiple observers and multiple techniques. We used an indirect Doppler method to measure SBP. Doppler measures have been reported to be have good correlation with direct arterial measures (although with a large negative bias) in 1 study,23 whereas another study reported good correlation and agreement.24 A more recent study has suggested poor agreement between Doppler values and direct measurements in anesthetized cats.25 However, the median value obtained in our study was similar to values obtained in studies of healthy cats using intra‐arterial radiotelemetric implants for direct assessment (125.1 ± 10.6 mmHg and 126.0 ± 4.4 mmHg).13, 14
Increasing levels of nervousness at the point of assessment of SBP (assessed at the time based upon body position, tail position, ear position, eye contact, and general demeanor) was found to be associated with increasing SBP in our population of cats. A similar finding has been reported in dogs,26 although a previous study of cats did not find an association.15 This increase in SBP with increasing levels of nervousness is likely to be because of varying degrees of the “white‐coat effect,” which has been documented in cats13 and is an important consideration in making a diagnosis of hypertension in people.27
Age was found to explain some of the variation in SBP within our population of cats, with increasing age groups having increased SBP. An association is recognized between increasing age and increased SBP in people,28 possibly because of a progressive decline in endothelial function.29 Similar effects of age on SBP have been reported in dogs26 and cats,15 although some smaller studies of cats have failed to show an effect.9, 17 A recent longitudinal study demonstrated increasing SBP over time in both healthy cats and cats with chronic kidney disease.19
In people, males have higher SBP than females,28 and this also has been reported in dogs.26 As yet, no study has identified an effect of sex on SBP in cats,17 and our study is the first to report higher SBP in male than female cats.
Neutered cats had higher SBP than unneutered cats. A similar finding, although reported to be nonsignificant, was found in 1 other study of cats.15 Conversely, unneutered male dogs were found to have higher SBP than neutered dogs and unneutered female dogs were found to have lower SBP than neutered dogs,26 and therefore, the role of neuter status in SBP seems unclear across species.
Cats that had been relinquished to the rehoming centers by their owners had lower SBP in the multivariable model than those that were found as strays. It could be hypothesized that cats that are found as strays are less accustomed to human contact than cats relinquished by their owners and thus are more nervous, even if this may not be obvious to an observer.
Our study did not identify an effect of breed on SBP, although the majority of cats were nonpedigree and the numbers for each pedigree breed were small, limiting our ability to assess the effect of individual breeds. Other studies also have not found an effect of breed on SBP in cats.15, 18 Breed accounts for an important proportion of the variability in SBP in dogs, with Beagles and sight hounds having high SBP and retrievers and setters having low SBP,26 but there is more variation among dog breeds than there is among cat breeds.
At the univariable level, there appeared to be an increase in SBP with increasing BCS, although there was no significant difference between ideal‐weight cats and overweight cats. This observation may in part be a reflection of the low numbers of overweight cats in our study. Obesity is associated with an increase in SBP in people, thought to be partially caused by sympathetic and renin‐angiotensin‐aldosterone activation, although the precise relationship currently is not fully elucidated.30 Similar associations have not been reported previously in cats,15 but have been reported in dogs.26
Although cuff size and cuff site both were univariably associated with SBP, this finding did not hold true in the multivariable model. Cats that required cuff size 1 had lower SBP than the other cats, but these cats would be expected to be smaller than cats that required cuff size 2 or 3, and therefore probably would be younger. Therefore, this finding is probably explained by the effect of age on SBP, particularly because cuff size was not included in the final multivariable model. Cats that had the left forelimb used for assessment of SBP had lower values than cats that had the right forelimb used. The protocol for this portion of the study was to use the right forelimb unless the cat resented the right forelimb being touched or if inconsistent or unexpected results were generated from the right forelimb. As such, only a small number of cats had the left forelimb used, several of which were used to confirm unexpectedly low values obtained in the right forelimb, and thus, this result is not surprising. It should not be assumed from these data that true differences between limbs used were found, because the limb used was not randomized. No difference in SBP was found when using either a forelimb or the tail in cats in 1 study.16
Limitations
Cats were considered apparently healthy on the basis of history and physical examination, but routine serum biochemistry and total thyroxine concentrations were not measured in every cat, and thus, cats with clinically relevant azotemia or hyperthyroidism masking azotemia could have inadvertently been included. Nevertheless, because there was no statistical difference between the SBP of the cats that did and did not have serum creatinine concentration measured, it is less likely that cats with occult azotemia resulting in systemic hypertension were included. Retinal examinations were not performed, and it is possible that cats with retinopathies secondary to underlying systemic hypertension were not detected. Because cats spent variable amounts of time in the rehoming centers, it is possible that subtle clinical signs of disease were not detected, particularly in cats that only spent short periods of time in the rehoming center. It is therefore conceivable that cats with systemic diseases (including systemic hypertension) that were not showing obvious clinical signs were included in our population. This is particularly true because clinical signs associated with mild hypertension can be very subtle. If cats with mild systemic hypertension were included in this population, then the upper end of our reported range for SBP might be higher than for a truly normal population. Also, if cats with systemic disease causing hypotension were included in the population, this would have the opposite effect of artifactually decreasing the lower end of the range for SBP.
Our study used an auscultatory Doppler SBP measurement rather than an automated instrument. Repeated measurements collected using auscultation can be subject to a repeated‐measurement bias31 in which the operator is either consciously or subconsciously influenced by the first value obtained. This can introduce a subjective effect on the recorded values. However, our study replicated the methodology used in routine clinical practice, and thus, the results still should be applicable for clinicians using this method.
The age for many cats in our study, especially those found as strays, often was unknown, and thus, age ranges were estimated, which may not have been accurate in all cats. This factor could have affected the strength of the association between increasing age and increasing SBP. However, collapsing the age groups of cats into juvenile, young adult, adult, and senior should limit the potential source of error for this factor. For the safety of both the cats and the investigator, cats that showed signs of aggression or that were too nervous to handle were not included, and thus, it was not possible to include all apparently healthy cats. Because demeanor was shown to have an effect on SBP, excluding extremely nervous or aggressive cats may have influenced the impact of this factor on the multivariable model. However, it still was possible to include many nervous cats and some aggressive cats. Our study therefore also provides information on blood pressure in nervous and aggressive cats that might help prevent inappropriate labeling of these cats as hypertensive. Demeanor was assessed subjectively rather than using any objective measures and thus could be a source of error.
Because our population was collected from a rehoming center, the baseline characteristics of these cats are more likely to reflect those of the general cat population than cats in referral centers. However, the prevalence of pedigree cats in our population is much lower than has been reported in the general population,32 and as such, it may not be possible to extrapolate these findings to pedigree cats. Only 82/780 (10.5%) of the cats in our population were considered overweight, whereas at least 20% of the general cat population are reported to be overweight or obese.33 Our study did not simulate a veterinary practice visit, which has been shown to increase SBP in cats,13 and thus, our results are more applicable to cats that have been allowed an acclimation period before SBP measurement.
Although the effects of demeanor, age, sex, neuter status, and a history of being a stray independently have a statistically significant effect on SBP, it is only likely that combinations of these factors will have a clinically relevant effect. This should therefore be considered when interpreting clinical SBP data.
Conclusion
Systolic blood pressure in our population of apparently healthy cats measured using the Doppler technique was 120.6 (110.4–132.4) mmHg. Variations in SBP are associated with the demeanor, age, sex, and neuter status of the cat as well as whether the cat was relinquished to the rehoming center or was found as a stray. These results should be applicable to the general population of nonpedigree cats in the United Kingdom.
Acknowledgments
The authors gratefully acknowledge the assistance of all of the staff at Battersea Dogs & Cats Home and Cats Protection's National Cat Adoption Centre. They also thank the individuals who adopted cats seen as part of the screening program.
Conflict of Interest Declaration: Professor Virginia Luis Fuentes has performed consultancy work for CEVA, Boehringer Ingelheim, and Aratana. Professor Virginia Luis Fuentes has received research funding from Winn Feline Foundation. Doctor Jessie Payne's PhD was funded by the Evetts Luff Foundation and IDEXX Laboratories. None of these companies had any involvement in the design of the study or of the interpretation of the results.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Where the work was done: Royal Veterinary College, Hawkshead Lane, North Mymms, Hatfield, Hertfordshire AL9 7TA, United Kingdom; Battersea Dogs & Cats Home, 4 Battersea Park Road, London SW8 4AA, United Kingdom; Cats Protection, National Cat Adoption Centre, Chelwood Gate, Haywards Heath, East Sussex RH17 7TT, United Kingdom.
Meeting, if any, at which the paper was presented: Veterinary Cardiovascular Society Autumn meeting 2013.
Footnotes
Battersea Dogs & Cats Home's central London branch, UK, and Cats Protection's National Cat Adoption Centre, East Sussex, UK
Model 811‐B Doppler Ultrasonic Flow Detector, Parks Medical Electronics Inc., Aloha, Oregon
GraphPad Prism 5, GraphPad Software, 2007, and PASW Statistics 20, 2011
References
- 1. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 2. Nelson OL, Reidesel E, Ware WA, et al. Echocardiographic and radiographic changes associated with systemic hypertension in cats. J Vet Intern Med 2002;16:418–425. [DOI] [PubMed] [Google Scholar]
- 3. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: Clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003;17:89–95. [DOI] [PubMed] [Google Scholar]
- 4. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000;217:695–702. [DOI] [PubMed] [Google Scholar]
- 5. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004;65:245–252. [DOI] [PubMed] [Google Scholar]
- 6. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003;222:322–329. [DOI] [PubMed] [Google Scholar]
- 7. Reynolds BS, Lefebvre HP. Feline CKD: Pathophysiology and risk factors — what do we know? J Feline Med Surg 2013;15:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown CA, Munday JS, Mathur S, et al. Hypertensive encephalopathy in cats with reduced renal function. Vet Pathol 2005;42:642–649. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990;4:58–62. [DOI] [PubMed] [Google Scholar]
- 10. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002;220:1799–1804. [DOI] [PubMed] [Google Scholar]
- 11. Ash RA, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: A series of 13 cases. J Feline Med Surg 2005;7:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wimpole JA, Adagra CFM, Billson MF, et al. Plasma free metanephrines in healthy cats, cats with non‐adrenal disease and a cat with suspected phaeochromocytoma. J Feline Med Surg 2010;12:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belew AM, Barlett T, Brown SA. Evaluation of the white‐coat effect in cats. J Vet Intern Med 1999;13:134–142. [DOI] [PubMed] [Google Scholar]
- 14. Brown SA, Langford K, Tarver S. Effects of certain vasoactive agents on the long‐term pattern of blood pressure, heart rate, and motor activity in cats. Am J Vet Res 1997;58:647–652. [PubMed] [Google Scholar]
- 15. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998;39:567–573. [DOI] [PubMed] [Google Scholar]
- 16. Mishina M, Watanabe T, Fujii K, et al. Non‐invasive blood pressure measurements in cats: Clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci 1998;60:805–808. [DOI] [PubMed] [Google Scholar]
- 17. Sparkes AH, Caney SM, King MC, et al. Inter‐ and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999;13:314–318. [DOI] [PubMed] [Google Scholar]
- 18. Lin CH, Yan CJ, Lien YH, et al. Systolic blood pressure of clinically normal and conscious cats determined by an indirect Doppler method in a clinical setting. J Vet Med Sci 2006;68:827–832. [DOI] [PubMed] [Google Scholar]
- 19. Bijsmans ES, Jepson RE, Chang YM, et al. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med 2015;29:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015;1:S244–S257. [DOI] [PubMed] [Google Scholar]
- 21. Laflamme D. Development and validation of a body condition score system for cats: A clinical tool. Feline Pr 1997;25:13–18. [Google Scholar]
- 22. Bradshaw JWS, Casey RA, Brown SL. The Behaviour of the Domestic Cat, 2nd ed Wallingford, Oxfordshire: CABI Publishing; 2012. [Google Scholar]
- 23. Caulkett NA, Cantwell SL, Houston DM. A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Vet Surg 1998;27:370–377. [DOI] [PubMed] [Google Scholar]
- 24. Binns SH, Sisson DD, Buoscio DA, et al. Doppler ultrasonographic, oscillometric sphygmomanometric, and photoplethysmographic techniques for noninvasive blood pressure measurement in anesthetized cats. J Vet Intern Med 1995;9:405–414. [DOI] [PubMed] [Google Scholar]
- 25. da Cunha AF, Saile K, Beaufrère H, et al. Measuring level of agreement between values obtained by directly measured blood pressure and ultrasonic Doppler flow detector in cats. J Vet Emerg Crit Care (San Antonio) 2014;24:272–278. [DOI] [PubMed] [Google Scholar]
- 26. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract 1996;37:116–125. [DOI] [PubMed] [Google Scholar]
- 27. Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: Blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 2012;28:270–287. [DOI] [PubMed] [Google Scholar]
- 28. Smulyan H, Asmar RG, Rudnicki A, et al. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001;37:1374–1380. [DOI] [PubMed] [Google Scholar]
- 29. Dolores Herrera M, Mingorance C, Rodriguez‐Rodriguez R, et al. Endothelial dysfunction and aging: An update. Ageing Res Rev 2010;9:142–152. [DOI] [PubMed] [Google Scholar]
- 30. Davy KP, Hall JE. Obesity and hypertension: Two epidemics or one? Am J Physiol Regul, Integr Comp Physiol 2004;286:R803–R813. [DOI] [PubMed] [Google Scholar]
- 31. Gupta P, Mittal L, Rizzo RA, et al. In‐use comparison of blood pressure measurements from an automated blood pressure instrument with those from a mercury sphygmomanometer. Biomed Instrum Technol/Assoc Advance Med Instrum 2009;43:158–163. [DOI] [PubMed] [Google Scholar]
- 32. O'Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015;17:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. German AJ. The growing problem of obesity in dogs and cats. J Nutr 2006;136:1940S–1946S. [DOI] [PubMed] [Google Scholar]


