Abstract
Background
Cavalier King Charles spaniels (CKCSs) are predisposed to degenerative mitral valve disease (DMVD) and studies have indicated a genetic cause.
Animals
A total of 8,860 CKCSs were examined at shows or private clinics from 1991 to 2010.
Objectives
To analyze the effects of a breed scheme for CKCS on the age at which a murmur consistent with DMVD was first detected.
Methods
The presence or absence of a murmur consistent with mitral regurgitation was noted and age a murmur was first detected recorded.
Results
A total of 16,887 examinations were performed on 8,860 dogs. The median age dogs developed a murmur were slightly younger in male than female dogs (7.8 versus 8.3 years, 95% confidence interval [CI] 7.4–8.1 versus 8.0–8.4, P < .001) and cardiologists detected murmurs in younger dogs than did general practitioner (GP) veterinary surgeons (7.2 versus 8.6 years, 95% CI 7.0–7.4 versus 8.3–8.7 P < .001). In bitches examined by GP vets during the study, there was a significantly increased age of detection of murmurs over time (8.6–9.2 years, 95% CI 8.3–9.1 and 8.5–10.9, P = .001) but not for male dogs examined by GP vets or dogs of either sex examined by cardiologists.
Conclusions and Clinical Importance
This study suggests that the age incidence of murmurs associated with DMVD might be increased by application of breeding guidelines based on auscultation alone. This benefit was only seen in a subgroup and compliance of breeders with this voluntary scheme was poor.
Keywords: Dog, Genetic, Inheritance, Mitral
Abbreviations
- CI
confidence interval
- CKCS
Cavalier King Charles spaniel
- DMVD
degenerative mitral valve disease
- DVD
degenerative valve disease
- GP
general practitioner
Degenerative valve disease (DVD) usually affecting the mitral valve is the most common cardiac disease in dogs being responsible for 75–80% of all cardiac disease.1, 2 Progressive DVD with deposition of mucopolysaccharides and collagen degeneration of the mitral valve alone or in combination with the tricuspid valve leads to incompetence. This is characterized by a left apical systolic murmur, the loudness of which is related to the severity of the disease.3, 4 Initially, the regurgitant volume is small and hemodynamically insignificant. However, as the disease progresses, the regurgitant volume increases, systolic function deteriorates, and both ventricular dilatation and atrial dilatation are seen. Eventually, in some dogs, heart failure or sudden death occurs. Analysis of a Swedish insurance database revealed that the mortality curve from congestive heart failure follows the incidence of murmur curve but with a lag of 3–4 years.5 Mitral valve prolapse and murmur intensity are predictors of progression6, 7 and LA : Ao ratio over 1.7 and mitral E wave inflow velocities over 1.2 m/s are predictors of cardiac‐related death.8
Small breeds are predisposed, but many studies have shown a particularly high incidence and an early onset in the Cavalier King Charles spaniel (CKCS) in many different countries6, 9, 10, 11 and a survey of UK CKCS indicated that 42.8% die from heart disease12 which is similar to the figure from Sweden with a mortality of 37% for CKCS under 10 years. Many studies have looked at the inherited basis for the disease.13, 14, 15, 16 A polygenic dominant trait has been suggested and 2 loci have now been identified.17 The disease is highly heritable and selection against the disease should be successful.16 A study in Denmark showed that a mandatory breeding scheme significantly decreased the prevalence of DVD over an 8‐ to 10‐year period.18
The aim of this study was to analyze the results of the UK CKCS database developed during breed screening to examine whether the screening was having an impact on the age at which murmurs were first detected.
Materials and Methods
In the United Kingdom, the Cavalier King Charles Spaniel Club began collecting data in 1991 and held a seminar in 199619 from which breeding guidelines were developed.20 These suggested that any dog used for breeding should be at least 5 years old and free from an audible murmur consistent with degenerative mitral valve (DMVD) disease. Dogs over 2½ years could be used for breeding if their parents were over 5 years before they developed a murmur. The scheme was based on the presence or absence of a murmur suggestive of DMVD. Participation by breeders was entirely voluntary. The results of this testing were recorded on a database. A list of dogs that were over 5 years of age when they first developed a murmur was published by the Cavalier Club. This was refined in 2006 so that only dogs tested by a cardiologist would be included in the “over 5 list” and breeders were encouraged to have their dogs tested once over 5 years by a cardiologist.21 It was hoped that by breeding from dogs who develop murmurs later in life, or whose parents do, the average age at which a CKCS develops DMVD would increase.
Animals
Dogs were voluntarily presented by breeders to a veterinary surgeon (general practitioner, GP) at their local clinic or to a cardiologist at a Club show or “Health Day.” Cardiologist was defined as a veterinary surgeon with a further qualification in cardiology (RCVS Certificate or Diploma [RCVS, ACVIM, or ECVIM] in veterinary cardiology). Age, color, sex, and pedigree data from the Kennel Club were recorded. Cardiac auscultation was performed in a quiet room with the dog gently restrained. The presence or absence of a murmur consistent with DMVD was recorded and the grade of murmur scored out of 6.3 The experience of the auscultator was also recorded, indicating whether they were a GP or cardiologist. Results were submitted to a central database and the results recorded on a software package.1
Dogs were excluded if they were too lively or respiratory noises were such that identification of quiet murmurs was not possible.
Statistical Methods
Data were entered into a Microsoft Excel Spreadsheet and analyzed by commercially available statistical software programs.2 Basic descriptive statistics were followed by time to event analysis. The event of interest was being diagnosed for the first time with a heart murmur, and survival time was the age at which this occurred. Dogs who were murmur‐free at their last examination were right‐censored (classed as “lost to follow‐up”) at the age when this examination occurred. Initial univariable survival analysis used the Kaplan–Meier method and the Peto–Peto–Prentice test for equality of survivor functions. This was followed by a number of parametric survival models and by Cox's proportional hazards regression, with explanatory variables being year quartiles, sex, and whether the tester was a cardiologist. Graphical plots and examination of Schoenfeld residuals indicated that the data did not fully fit the assumptions of proportional hazards, but the results from the other survival models were very similar and here we report those of the Cox regression. Initial explorations considered the effect of each year separately, but this approach was discarded in favor of treating time periods as quartiles: 1991–1995, 1996–2000, 2001–2005, and 2006–2010. Survival analyses were run twice, firstly including all examinations and secondly excluding first examinations. The findings for both analyses were similar and this paper reports the results when all examinations were included. The statistical significance of terms in the Cox regression was assessed with the Wald statistic and/or changes in the log‐likelihood statistic as appropriate. Chi‐square tests were used for categorical data and ages were compared with analysis of variance. Statistical significance was taken as P < .05 on a 2‐sided null hypothesis.
Results
The forms submitted from 1991 to May 2010 were analyzed. A total of 16,887 examinations were performed on 8,860 dogs. A total of 4,856 were performed in the first time quartile, 5,417 in the second, 3,860 in the third, and 2,854 in the last quartile. A total of 10,915 examinations (65%) were performed on female dogs and 4,048 of those by a cardiologist (37%). A total of 5,972 examinations (35%) were performed on male dogs and 2,365 by a cardiologist (40%). Overall, 10,474 examinations (62%) were performed by GP vets and 6,413 (38%) by cardiologists (Fig 1). It was apparent that over the course of the study, the ratio of dogs examined by cardiologists had increased from 24.9% examined by a cardiologist in the first quartile, and 23.6% in the second, to 41.4% in the third and 77.6% in the fourth. The ratio of male to female dog examinations did not change over the quartiles.
Figure 1.
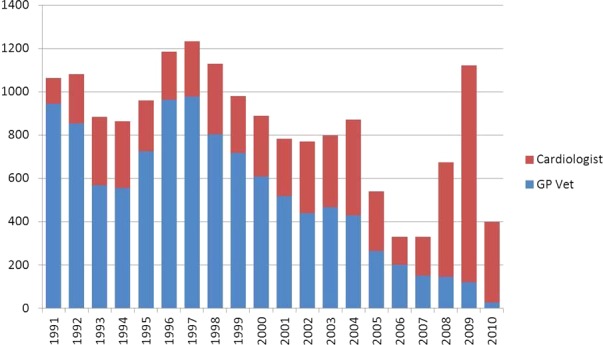
Number of dogs examined per year showing the relative numbers of dogs examined by general practitioner veterinary surgeons (blue) and cardiologists (red).
The mean age of dogs in this study was 3.9 years with a median of 3.2 years. There was a significant difference between the ages of dogs seen by GP vets and cardiologists (P < .001) with a mean of 4.2 and 3.4 years and a median of 3.5 and 2.6 years, respectively. The age profile of dogs presented is shown in Fig 2.
Figure 2.
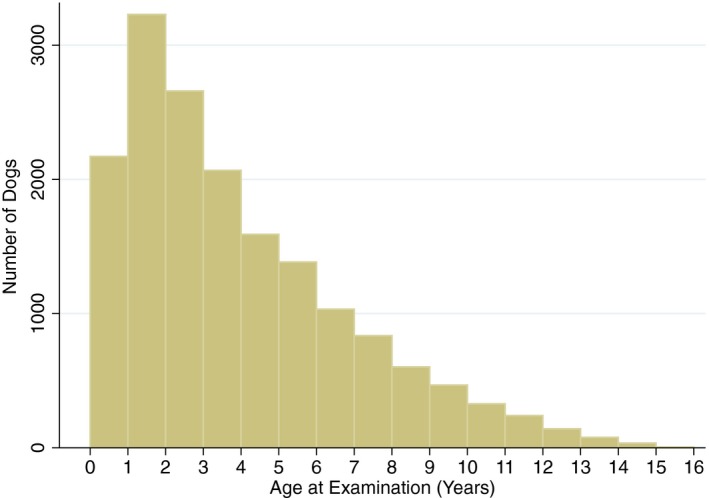
The age profile of dogs examined during the study. 1‐ to 2‐year‐old dogs represented the highest numbers of dogs examined and this number gradually declined with age.
When comparing GP vets and cardiologists, cardiologists detected murmurs in younger dogs than GP vets in all time quartiles with a hazard ratio (HR) between 1.8 and 2.3 (P < .001). The estimated median age at which a murmur was first detected for CKCS examined by GP vets was 8.6 years whereas that for cardiologists was 7.2 years. Between 1991–1995 and 2005–2010, there was a significant increase in the age of detection of murmurs in female dogs by GP vets from 8.6 to 9.2 years (HR = 0.64, P = .001, Fig 3), but this change over time was not apparent for male dogs and for neither male nor female dogs examined by cardiologists (P > .3). Univariable analysis indicated that males developed murmurs at a younger age than females (HR 1.14, 95% confidence interval [CI] 1.04–1.25), with the predicted median age (at which 50% of CKCS will be murmur‐free) being 7.8 years for males and 8.3 years for females (Fig 4). However, when a multivariable model was fitted with sex, cardiologist, and time quartile, the HR for sex reduced to 1.10 (95% CI 0.99–1.21) and was no longer statistically significant (P = .053).
Figure 3.
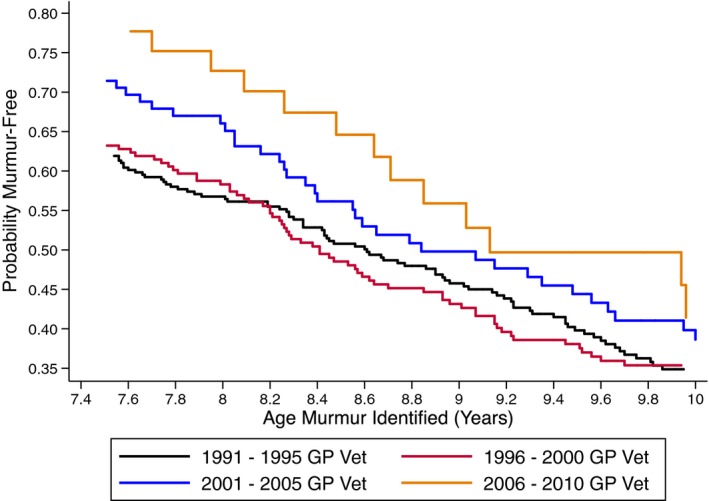
Probability of remaining murmur‐free for female dogs examined by general practitioner veterinary surgeons in each of the time period quartiles. The age for the median probability increases with increasing time periods.
Figure 4.
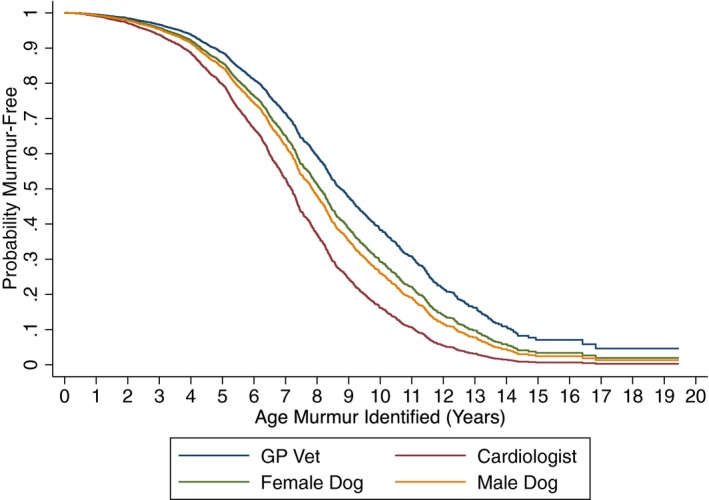
Probability of remaining murmur‐free by age. Male dogs compared against female dogs and dogs of either sex examined by either general practitioner (GP) veterinary surgeons compared against cardiologists. There were differences between male and female dogs and also between dogs examined by GP veterinary surgeons and cardiologists.
There was little change in the age profile of dogs presented during the scheme. Divided by quartiles, 30.1% of dogs were over 5 years in the first quartile, 29.5% in the second, 31.7% in the third, and 30.6% in the fourth.
In a further analysis, pedigrees were examined for dogs over 3 years from the 2006 to 2010 quartile. The dogs were classified according to whether they, their parents, and their grandparents had followed the breeding advice. A total of 790 dogs were identified in this quartile of which 31 (4%) had followed the breeding advice. No clear difference for remaining free of a murmur was evident between the 2 groups (P > .45, Fig 5).
Figure 5.
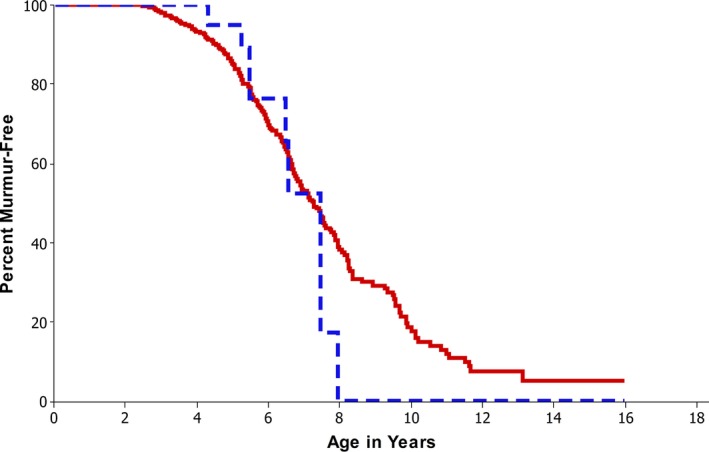
Probability of remaining murmur‐free for dogs over 3 years in the last quartile (2006–2010) comparing dogs whose parents and grandparents were in the scheme (red) with those that were not part of the scheme (blue). From the database, 794 dogs were in this group, and 763 (red) had not followed the breeding criteria, whereas 31 (4%) had.
Discussion
The present study is the first analysis of the UK CKCS database and suggests that the advice given to breeders is having an important effect to decrease the incidence of the disease. In bitches examined by GP veterinary surgeons, the age at which 50% of CKCS develop a murmur had increased by 0.6 years from 8.6 to 9.2 years comparing the quartiles 1991–1995 with 2006–2010 and this was statistically significant (P < .001). The 2 interquartile values (1996–2000 and 2001–2005) showed a similar trend in increasing age. The breeding advice was introduced after it was shown that parental chronic valvular disease status was an important factor in influencing the probability and intensity of heart murmurs in their offspring when these were 5 years old.13 More recently, this has been supported by a mixed model analysis of 4‐ to 5‐year‐old CKCS which showed that the presence and severity of chronic valvular disease was highly heritable and that selection against the disease should be successful.16 This benefit was not detected in male dogs examined by GP vets or in CKCS of either sex examined by cardiologists. Although the age did increase, it was not statistically significant. This is likely to be due to the low numbers of male dogs examined by cardiologists until 2008 and the fact that many more female than male dogs were examined by GP vets during the entire study (Fig 1). If there was an effect, we conclude that it must have been small. The numbers of dogs declined during the study period probably due to waning enthusiasm among breeders. A TV show in 2008 highlighted the problem of heart disease in CKCS and this resulted in increasing compliance with the scheme.
More female dogs are presented for screening than male dogs and this ratio has been similar throughout this study. It may be the reason that statistical significance was only reached in female dogs as the study may have been underpowered to detect the difference in male dogs. It is likely that as a stud dog can sire multiple litters, fewer male dogs need to be screened than female dogs. In recent years, the top 3 stud dogs have produced 145, 125, and 85 litters.22 In addition, in any championship show, more female dogs tend to be entered than male dogs.
The data showed that cardiologists tend to detect murmurs in younger dogs compared to GP veterinary surgeons with a difference in the median age of 1.4 years. The reason for this may be training as it has been shown that medical personnel appropriately trained detect quieter murmurs than those not trained.23, 24 Younger dogs tend to be presented to cardiologists (0.8 years younger, Table 1) so it is important that testing of young dogs and the “over 5” test is performed by cardiologists in order that quiet murmurs will be detected. The presentation of younger dogs to cardiologists is likely to be because younger dogs tend to be brought to dog shows. The number of dogs examined by cardiologists increased throughout the scheme due to the increasing numbers of cardiologists in the United Kingdom and the introduction of the breeding advice with the “over 5” test which could only be certified by cardiologists.
Table 1.
Mean and median ages for dogs examined by GP vets and cardiologists
| Age of dogs/years | ||
|---|---|---|
| Mean | Median | |
| Cardiologist | 3.5 | 2.6 |
| GP Vet | 4.2 | 3.4 |
Echocardiography has been used as an early predictor of DMVD as mitral valve prolapse is an early link to the subsequent development of mitral regurgitation.6, 25 However, when the scheme was started in 1991, there were few veterinary surgeons with access to echocardiography and so it was decided to use auscultation for the scheme. Furthermore, few vets in the United Kingdom had further qualifications in cardiology, so the scheme allowed GP vets to certify the presence or absence of a murmur to make the scheme as widely available as possible initially.
The age at which a murmur was detected in CKCS in the present study is older than has been previously reported with 50% of CKCS affected by 6–7 years of age.6, 9, 11, 26 However, this scheme uses dogs presented voluntarily by breeders at shows or to their local practices and relies a completed form being returned to the central database. Hence, some younger dogs with murmurs detected by their local veterinary surgeon may not be presented at shows or their forms not returned if a murmur was detected.
It was disappointing that the age profile of the dogs presented during the scheme did not change significantly. This is despite the breeding advice introduced in 1996 stressing the importance of examining dogs over 5 years.13 There was a slight increase in the numbers of dogs in 2006 and 2007 and this coincided with increased activity by the Club advertising the amended breeding guidelines.20
The results of the present study conflict with the analysis of the Swedish scheme.26 However, the 2 schemes are different. The Swedish scheme had different criteria for assessment and was compulsory although it was also based on auscultation. It was introduced in 2001 and that study compared 6‐year‐old dogs in 2007 with those in 2009. The prevalence of heart murmurs in 6‐year‐old CKCS changed from 52 to 55% with no significant difference. It is likely that the lack of progress reflects the short time span of the scheme in relation to the generational interval compared to the UK scheme. Also, many more dogs were examined in the UK scheme (8,860 versus 353 dogs). The results of a Danish scheme have recently been reported.18 This scheme was based on auscultation and echocardiography and was compulsory. It studied the effect of the guidelines over the period 2001 to 2011, and by 2010–2011, dogs that were part of the scheme showed a 73% risk reduction of having a murmur due to DMVD compared to those dogs that were not part of the scheme. The increased effect compared to our study may be a reflection that it was compulsory rather than voluntary although it is difficult to quantify the additive effects of the echocardiogram. In the UK scheme, in the quartile 2006–2010, only 4% of CKCS followed the breeding advice based on an auscultation scheme. It was felt that the addition of an echocardiogram would have eroded compliance further as so few were available in the United Kingdom at the start of the scheme.
As the disease is highly inherited,13, 16 it was disappointing that an initial genomewide study did not reveal that development of early onset mitral murmurs was due to a single gene effect.15 However, this is consistent with the earlier suggestion of a polygenic threshold trait.13 In contrast, a recent study17 found that 2 loci (CFA‐13 and CFA‐14) were associated with mitral valve disease in CKCS and 20 and 11 genes, respectively, are associated with these 2 regions. Further studies are warranted into promising candidate genes. The apparent differences in these 2 studies may reflect the number of dogs enrolled (36 versus 142 CKCS, respectively). However, because DMVD is genetically transmitted, a breeding program should improve the incidence of the disease, as shown by the results of this study and the Danish result.
Several studies4, 5, 8, 27, 28, 29 have shown that some dogs with DMVD go on to develop heart failure and ultimately die. Hence, if the age they develop a murmur becomes later, the age of death due to DMVD should also move to an older age and the dogs may have died of intercurrent disease. The mean time for asymptomatic CKCS with DMVD to develop heart failure was 27.2 ± 13.5 months after diagnosis.27 However, dogs with ISACHC class 2 had a median survival time of 33 months and ISACHC class 3, 9 months,8 which is similar to results reported by the QUEST study28 with a mean time to cardiac death or treatment failure of 267 days, if treated with pimobendan and furosemide. Unfortunately, to date, no drugs have been shown to increase the asymptomatic period27, 29, 30, 31 although the results of the EPIC study are eagerly anticipated and are expected to show that the use of pimobendan delays the onset of congestive heart failure by 15 months in dogs with cardiomegaly due to DMVD.3 A breeding scheme, which would delay onset and reduce incidence of disease, would therefore be helpful to improve quality and quantity of life and would reduce the emotional and financial burden an owner suffers during the disease of their pets.
The present study suggests that the UK breeding scheme is having an impact on the age incidence of murmurs associated with DVD. Overall, during this study, the age at which 50% of CKCS female dogs examined by GP veterinary surgeons developed a murmur has increased by 0.6 years.
Study Limitations
Very few of these dogs had further investigations to confirm that the murmurs detected were attributable to DVD. However, other studies have indicated that in a CKCS, a left‐sided apical systolic murmur is highly likely to be caused by DVD6, 7 and as the diagnostic criteria were not changed during the study, any effects caused by other murmurs such as flow murmurs would not have affected the age incidence of murmurs. This study used the age when a murmur was first recorded as an event. However, there was no compulsion for regular visits so it is impossible to say exactly when the dogs developed murmurs. This could have introduced errors into the study, but as this requirement did not change throughout the study, it is likely that any potential error was nullified. The database used a simple form including age, sex, and dog identification so in further analysis it was not possible to include any other factors in the models. Breed schemes are only effective if breeders use them. In the United Kingdom, only the Cavalier King Charles Spaniel Club is active in CKCS breed screening and figures indicate that their members breed only 21% of CKCS puppies registered with the Kennel Club. Only 2 of the top 12 stud dogs in 2009/2010 are owned by a member of the Club and 1 of the 12 sired 73 puppies before he was 2 years old.32 Hence, many dogs are bred by breeders who are not members of the Club or did not take part in the breed scheme. Wider participation or compulsion is likely to result in a greater effect on the age incidence.
Acknowledgments
Conflict of Interest Declaration: Simon Swift serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Foxpro database, Microsoft Corporation, One Microsoft Way, Redmond, WA, USA
Stata 12 and Minitab 16, StataCorp LP, College Station, TX, USA
Boswood A, Gordon S and Haggstrom J EPIC Trial Results 2016 ACVIM Forum
References
- 1. Buchanan J. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 2. Mattin MJ, Boswood A, Church DB, et al. Prevalence of and risk factors for degenerative mitral valve disease in dogs attending primary‐care veterinary practices in England. J Vet Intern Med 2015;29:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Häggstrom J, Kvart C, Hansson S. Heart sounds and murmurs: Changes related to severity of chronic valvular disease in Cavalier King Charles spaniels. J Vet Intern Med 1995;9:75–85. [DOI] [PubMed] [Google Scholar]
- 4. Ljungvall I, Rishniw M, Porciello F, et al. Murmur intensity in small‐breed dogs with myxomatous mitral valve disease reflects disease severity. J Small Anim Pract 2014;55:545–550. [DOI] [PubMed] [Google Scholar]
- 5. Häggstrom J. Chronic Valvular Disease in Cavalier King Charles Spaniels – Epidemiology, Inheritance and Pathophysiology. Uppsala Sweden: Swedish University of Agricultural Sciences; 1996. PhD Thesis. [Google Scholar]
- 6. Häggstrom J, Hanson K, Kvart C, Swenson L. Chronic valvular disease in the Cavalier King Charles spaniel in Sweden. Vet Rec 1992;131:549–553. [PubMed] [Google Scholar]
- 7. Pedersen HD, Lorentzen KA, Kristensen O. Echocardiographic mitral valve prolapsed in Cavalier King Charles spaniels: Epidemiology and prognostic significance for regurgitation. Vet Rec 1999;144:315–320. [DOI] [PubMed] [Google Scholar]
- 8. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and the prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 9. Darke PGG. Valvular incompetence in Cavalier King Charles spaniels. Vet Rec 1987;120:365–366. [DOI] [PubMed] [Google Scholar]
- 10. Malik R, Hunt GB, Allen GS. Prevalence of mitral valve insufficiency in Cavalier King Charles spaniels. Vet Rec 1992;130:302. [DOI] [PubMed] [Google Scholar]
- 11. Beardow AW, Buchanan JW. Chronic mitral valve disease in Cavalier King Charles spaniels: 95 cases. J Am Vet Med Assoc 1993;203:1023–1029. [PubMed] [Google Scholar]
- 12. The Kennel Club. Available at: http://www.thekennelclub.org.uk/download/1533/hscavalierkingcharlesspaniel.pdf. Accessed January 20, 2013.
- 13. Swenson L, Häggstrom J, Kvart C, Juneja K. Relationship between parenteral cardiac status in Cavalier King Charles spaniels and prevalence and severity of chronic valvular disease in offspring. J Am Vet Med Assoc 1996;208:2009–2012. [PubMed] [Google Scholar]
- 14. Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med 1999;13:448–456. [DOI] [PubMed] [Google Scholar]
- 15. French AT, Ogden R, Eland C, et al. Genome‐wide analysis of mitral valve disease in Cavalier King Charles spaniels. Vet J 2012;193:283–286; doi: 10.1016/j.tvjl.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 16. Lewis T, Swift S, Woolliams JA, Blott S. Hereditability of premature mitral valve disease in Cavalier King Charles spaniels. Vet J 2011;188:73–76. [DOI] [PubMed] [Google Scholar]
- 17. Madsen MB, Olsen LH, Häggstrom J, et al. Identification of 2 loci associated with the development of myxomatous mitral valve disease in Cavalier King Charles spaniels. J Hered 2011;102(S1):S62–S67. [DOI] [PubMed] [Google Scholar]
- 18. Birkegard AC, Reimann MJ, Martinussen T, et al. Breeding restrictions decrease the prevalence of myxomatous mitral valve disease in Cavalier King Charles spaniels over an 8–10 year period. J Vet Intern Med 2016;30:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darke PGG, Fuentes VL, Kvart C, Swenson L. Mitral Valve Disease in Cavalier King Charles Spaniels Seminar Proceedings. Published by Intervet UK, 1997.
- 20. The Cavalier Club. Available at: http://www.thecavalierclub.co.uk/health/hearts/mvd.html. Accessed January 28, 2016.
- 21. The Cavalier Club. Available at: http://www.thecavalierclub.co.uk/health/hearts/scheme2006.html. Accessed January 28, 2015.
- 22. The Kennel Club. Available at: http://www.the-kennel-club.org.uk/services/public/mateselect/test/Default.aspx, then individual dogs. Accessed January 28, 2016.
- 23. Pedersen HD, Häggstrom J, Falk T, et al. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers and agreement with color Doppler echocardiography and phonocardiography. J Vet Intern Med 1999;13:56–64. [PubMed] [Google Scholar]
- 24. Dhupar S, Vashist S, Shah N, Sokal M. Improvement of cardiac auscultation skills in pediatric residents with training. Clin Pediatr 2007;46:236–240. [DOI] [PubMed] [Google Scholar]
- 25. Olsen LH, Martinussen T, Pedersen HD. Early echocardiographic predictors of myxomatous mitral valve disease in dachshunds. Vet Rec 2003;152:293–297. [DOI] [PubMed] [Google Scholar]
- 26. Lundin T, Kvart C. Evaluation of the Swedish breeding program for Cavalier King Charles spaniels. Acta Vet Scand 2010;52:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kvart C, Häggstrom J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med 2002;16:80–88. [PubMed] [Google Scholar]
- 28. Häggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 29. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral insufficiency. J Am Vet Med Assoc 2007;231:1061–1069. [DOI] [PubMed] [Google Scholar]
- 30. Ouellet M, Belanger MC, DiFruscia R, Beauchamp G. Effect of pimobendan on echocardiographic values in dogs with asymptomatic mitral valve disease. J Vet Intern Med 2009;23:258–263. [DOI] [PubMed] [Google Scholar]
- 31. Keene BW, Fox PR, Hamlin R, et al. Efficacy of Bay 41‐9202 (bisoprolol) Oral Solution for the Treatment of Chronic Valvular Heart Disease (CVHD) in Dogs. Proceedings of the ACVIM forum, 2012.
- 32. Ford G. “Cavaliers at Championship Shows – 2011” Published by the Cavalier King Charles Spaniel Club, 2011.


