Abstract
Background
Leptin and its receptor play a role in several disease processes such as pancreatitis and heart disease. However, their association with gallbladder mucocele (GBM) in dogs has not been reported.
Hypothesis/Objectives
To evaluate differences in the expression of leptin and leptin receptor between dogs with and without GBM.
Animals
Twenty‐five healthy dogs, including 9 laboratory beagle dogs, and 22 client‐owned dogs with GBM.
Methods
Serum leptin concentration was determined in blood samples of all dogs by ELISA. Canine gallbladder samples were collected from 9 dogs with GBM that underwent surgery for therapeutic purposes and from 9 healthy laboratory beagle dogs as a normal control group. Samples were analyzed for leptin and leptin receptor mRNA by real‐time polymerase chain reaction.
Results
Serum leptin concentration was significantly higher in dogs with GBM than in healthy dogs (medians of 7.03 and 2.18 ng/mL, respectively; P < .001). Patients with GBM that had undergone surgery had significantly higher serum leptin concentrations than those that had not (medians of 12.2 and 4.09 ng/mL, respectively; P = .001). However, no difference in serum leptin concentration was found between dogs with GBM with or without endocrinopathies. The mRNA expression levels of leptin and its receptor were significantly increased in the gallbladder tissues of dogs with GBM.
Conclusions and Clinical Importance
Dysregulation of leptin might be involved in the pathophysiology of GBM, and leptin concentrations might be associated with GBM severity.
Keywords: Canine, Gallbladder mucocele, Leptin, Leptin receptor
Abbreviations
- BCS
body condition score
- CCK
cholecystokinin
- GBM
gallbladder mucocele
- PCR
polymerase chain reaction
- VAN
vagal afferent neurons
Gallbladder mucocele (GBM) in dogs is defined as a distended gallbladder with inappropriate accumulation of inspissated bile and mucus.1 Although clinical signs vary and some dogs with GBM have no clinical signs, GBM is a common cause of life‐threatening biliary tract diseases such as extrahepatic biliary obstruction and bile peritonitis. The etiology of GBM is unclear but disordered gallbladder motility, inflammation of the gallbladder wall associated with disturbances in the protective mechanism of the gallbladder epithelium against bile acids, and endocrinopathies such as hyperadrenocorticism, hypothyroidism, and diabetes mellitus may predispose dogs to GBM formation.2, 3, 4
Leptin, which is one of the most important hormones derived from mature adipose tissue, plays a role in energy metabolism at a central level.5 The past 20 years of research on leptin have identified a network that links leptin with metabolism, nutrition, and various diseases. Leptin works primarily as a fasting hormone through regulation of food intake and energy consumption and is thus associated with obesity,6 and it also serves as a physiological modulator in association with several diseases such as pancreatitis,7, 8 endocrinopathy,9, 10, 11 and tumors.12, 13 Indeed, several studies comparing the expression of leptin between patient and control groups have suggested a relationship of these diseases with dysregulation of leptin.
The expression of leptin or leptin receptors has been reported in various organs such as the stomach,14 pancreas,15 and small intestine,16 with leptin emerging as a key factor responsible for various physiological functions in each organ. Numerous studies have described the relationship between leptin and the gallbladder. Leptin regulates, either directly or indirectly, physiological mechanisms in the gallbladder, including modulation of gallbladder motility through effects on neurotransmitters,17 as well as secretion and reabsorption of water and electrolytes by upregulation or downregulation of gallbladder genes related to water, chloride, sodium, and bicarbonate transport.18 In addition, earlier studies proposed that leptin dysregulation results in a change in bile components within the gallbladder and causes several diseases of the gallbladder such as cholelithiasis.19, 20 Furthermore, hypokinetic and altered gallbladder contents are known to be correlated with the pathogenesis of GBM.
Although many different physiological and pathological roles of leptin have been reported in various peripheral tissues of animals and humans, to the best of our knowledge, there is little information about the link between leptin and GBM in dogs. On the basis of the above‐mentioned studies, we evaluated the possibility of a link between the pathology of GBM and leptin. We compared serum and tissue leptin and leptin receptor concentrations in dogs with and without GBM by ELISA and real‐time polymerase chain reaction (PCR), respectively.
Materials and Methods
Sample Preparation
Blood samples were collected for measurement of leptin concentration, and gallbladder samples were obtained for mRNA quantification of leptin and leptin receptor. Informed consent was obtained from the dogs' owners and all of the procedures were approved by the Seoul National University (SNU) Institutional Animal Care and Use Committees (SNU‐160120‐2).
Twenty‐two dogs with GBM and 25 healthy dogs were enrolled in this study. The 22 client‐owned dogs with newly diagnosed GBM and 16 healthy dogs were recruited from Veterinary Medicine Teaching Hospital at SNU. An additional 9 healthy laboratory beagle dogs housed at the Department of Veterinary Surgery, College of Veterinary Medicine at SNU, were included for analysis. All dogs underwent physical examination, CBC, serum biochemistry, and abdominal ultrasonography. A body condition score (BCS) for all dogs was recorded by a single investigator using a 1–9 scale system.21 All dogs enrolled were selected based on a normal BCS (BCS of 5 or 6 of 9). We confirmed that all of the gallbladders of the control group were normal based on blood analysis and ultrasonography. The GBM dogs were diagnosed using abdominal ultrasonography according to criteria previously described at the Department of Veterinary Radiology, College of Veterinary Medicine, SNU.1 Diagnosis of hyperadrenocorticism was made according to clinical signs and hormone tests, with either a low‐dose dexamethasone suppression test or adrenocorticotropic hormone stimulation test.22 A dog was diagnosed with hypothyroidism if the thyroid panel (total T4, free T4, thyroid‐stimulating hormone) indicated decreased thyroid gland activity with clinical signs.10 Persistent fasting hyperglycemia and glycosuria concurrent with clinical signs were the diagnostic criteria for diabetes mellitus.23 Patients included in the endocrinopathy group were diagnosed with individual endocrinopathies and managed accordingly.
Blood samples were taken from the jugular vein of 22 dogs that presented with GBM and 25 healthy dogs, including 9 healthy laboratory beagle dogs as controls. The dogs were fasted for 12 h before blood collection. Serum was separated immediately after collection in a serum separation tube. One portion of the sample was used for serum biochemistry analysis, including total cholesterol and triglycerides, and the remainder was frozen at −80°C until further analysis.
Gallbladder samples were collected from 9 dogs with GBM that underwent surgery for therapeutic purposes and 9 healthy laboratory beagle dogs as the normal control group that were euthanized for another approved experiment (SNU‐150624‐7) unrelated to our study. In the GBM patients, cholecystectomy was performed when medical management failed, clinical signs associated with biliary obstruction were present, or gallbladder rupture was suspected. Gallbladder samples were promptly frozen in liquid nitrogen and then stored at −80°C until used for real‐time PCR.
Sandwich ELISA
Serum leptin concentration was measured in duplicate by means of a commercial canine leptin sandwich ELISA (canine leptin ELISA) kit1 according to the manufacturer's instructions. The intra‐ and interassay coefficients of variation were 6% and 9%, respectively. The absorbance at 450 nm of each well was measured with a microplate reader2.
Real‐Time PCR
Total RNA was extracted from the gallbladder samples with a Hybrid‐R RNA extraction kit3, and the RNA concentration was quantified with Implen NanoPhotometer4. Total RNA (1000 g) was reverse‐transcribed with a PrimeScript 1st strand cDNA synthesis kit5, and the synthesized cDNA was amplified in triplicate by real‐time PCR with a SYBR Premix Ex Taq II6 and StepOnePlus Real‐Time PCR System7 according to a slight modification of the manufacturer's protocol. The mRNA levels for leptin and leptin receptor were normalized to that of the housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase. The primer sequences for each gene used in real‐time PCR are shown in Table 1. These primers of leptin24 and the leptin receptor25 were reported previously. The identity of amplified PCR products was confirmed by DNA cloning and sequencing with Sanger's method.
Table 1.
Specific primer sequences for real‐time polymerase chain reaction analysis with product sizes and optimal annealing temperatures
| Target Gene | Accession Number | Direction | Sequence (5′ to 3′) | Product Size (Base Pair) | Annealing Temp. (°C) |
|---|---|---|---|---|---|
| Ob | NM_001003070.1 | Forward | ACCGTATGGGTGTCCTTTATCCT | 63 | 58 |
| Reverse | AGAGTGGCTCTGTGGTGTGAGA | ||||
| Ob‐R | NM_001024634 | Forward | CTTTTGCCTGCTGGAATCTC | 143 | 58 |
| Reverse | TTGCTCCAAAAGCAACAGTG | ||||
| GAPDH | NM_001003142.2 | Forward | CATTGCCCTCAATGACCACT | 105 | 58 |
| Reverse | TCCTTGGAGGCCATGTGGAC |
Ob, leptin; Ob‐R, leptin receptor; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase.
Statistical Analysis
Statistical analyses were performed by SPSS, version 23.08. Normality tests (Shapiro–Wilks) were conducted to determine whether the data were normally distributed. The Mann–Whitney U‐test was used to analyze the differences in serum leptin concentration, age, body weight, and BCS between the healthy and GBM groups. The Kruskal–Wallis test was used to compare serum leptin concentration among 3 groups (healthy, GBM without endocrinopathy, and GBM with endocrinopathy; healthy, nonoperated GBM, and operated GBM). When a significant difference was found, the Mann–Whitney U‐test was used for follow‐up pairwise comparisons with a Bonferroni–Holm multiple comparison adjustment. The data are presented as the median and range or interquartile range. The relative mRNA expression levels of leptin and leptin receptor in gallbladder samples, triglyceride concentrations, and total cholesterol concentrations were compared between the healthy and GBM groups by the independent t‐test, and the data are presented as the mean values of each group ± standard deviation. Associations between serum leptin concentrations and age, breed, sex, body weight, BCS, triglycerides, and cholesterol were evaluated with the Spearman rank correlation test. A P‐value < .05 was considered to represent statistical significance.
Results
Cases
Details of the healthy dogs and the 22 dogs with GBM included for blood analysis are summarized in Table 2. The median body weight of the dogs with GBM was 5.97 kg, which was greater than that of the healthy dogs but without statistical significance (P = .58). The median BCS was 5 of 9 in the GBM group, which was similar to that of the healthy dogs (P = .67).
Table 2.
Comparison of age, sex, breed, BW, BCS, endocrinopathy, and biochemical characteristics between healthy dogs (controls) and dogs with GBM from which blood samples were taken
| Healthy Dogs | Dogs with GBM | |
|---|---|---|
| Age (years) | 8.00 (3.50) | 13.00 (4.25)*** |
| Sex (n) |
Female (2) Spayed females (10) Males (5) Castrated males (8) |
Spayed females (8) Male (1) Castrated males (13) |
| Breed (n) |
Beagles (9) Yorkshire Terriers (4) Maltese (4) Mixed breeds (2) Bichon Frises (1) Chiguagua (1) Spitz (1) Shetland sheepdog (1) Poodle (1) Shih Tzu (1) |
Malteses (6) Cocker Spaniels (4) Schnauzers (4) Poodles (3) Shih Tzus (3) Yorkshire Terrier (1) Pug (1) |
| BW (kg) | 5.30 (6.60) | 5.97 (4.32)*** |
| BCS (/9) | 5 (5–6) | 5 (5–6)*** |
| Endocrinopathy (n) | ― |
Hyperadrenocorticism (6) Hypothyroidism (1) Diabetes mellitus (2) |
| Total cholesterol (mg/dL) | 164.36 ± 49.54 | 322.5 ± 197.62** |
| Triglycerides (mg/dL) | 74.24 ± 31.68 | 197.62 ± 166.98** |
BW, body weight; BCS, body condition score; n, number of patients; GBM, gallbladder mucocele.
Continuous variables are presented as mean and SD, except for age and BW (which are presented as the median and interquartile range). BCS, which is a discontinuous variable, is presented as the median and range.
**P < .01, ***P > .05 compared with controls.
Normal gallbladder tissue samples were obtained from 9 healthy beagle dogs and 9 patients that underwent surgery for GBM. Detailed information of these dogs is summarized in Table 3. The median body weight of the healthy group was 10 kg, which was significantly higher than that of the GBM group (P = .046). The median BCS of the healthy group was 5 of 9, which was not different from that of the GBM group (P = .53).
Table 3.
Comparison of age, sex, breed, BW, BCS, endocrinopathy, and biochemical characteristics between healthy dogs (controls) and dogs with GBM from which gallbladder samples were taken
| Healthy Dogs | Dogs with GBM | |
|---|---|---|
| Age (years) | 7.00 (1.50) | 8.00 (4.00)*** |
| Sex |
Female (1) Spayed females (4) Males (4) |
Spayed females (2) Castrated males (7) |
| Breed (n) | Beagles (9) |
Cocker Spaniels (3) Maltese (3) Poodles (2) Schnauzer (1) |
| BW (kg) | 10.01 (1.05) | 6.80 (5.99)*** |
| BCS (/9) | 5 (5–6) | 5 (5–6)*** |
| Endocrinopathy (n) | ― |
Hyperadrenocorticism (3) Diabetes mellitus (1) |
| Total cholesterol (mg/dL) | 145.33 ± 41.95 | 390.89 ± 240.05* |
| Triglycerides (mg/dL) | 64.44 ± 29.53 | 215.11 ± 86.39** |
BW, body weight; BCS, body condition score; n, number of patients; GBM, gallbladder mucocele.
Continuous variables are presented as mean and SD, except for age and BW (which are presented as the median and interquartile range). BCS, which is a discontinuous variable, is presented as the median and range.
*P < .05, **P < .01, ***P > .05 compared with controls.
Serum Concentrations of Leptin in Healthy Dogs and Dogs With GBM
The serum concentration of leptin was significantly higher in dogs with GBM compared to that in healthy dogs (P < .001; Fig 1). To determine the association between the severity of GBM and the serum leptin concentration, GBM patients were further categorized into operated and nonoperated groups, and the serum leptin concentration was significantly higher in the former group (P = .001; Fig 1). The concurrence of an endocrinopathy with GBM had no significant influence on serum leptin concentration (Fig 3). No correlation was detected between serum leptin concentration and age (P = .356), breed (P = .271), sex (P = .369), body weight (P = .626), or BCS (P = .390) for the control dogs or in patients with GBM that underwent surgery (P = .32, .26, .7, .08, and .86, respectively).
Figure 1.
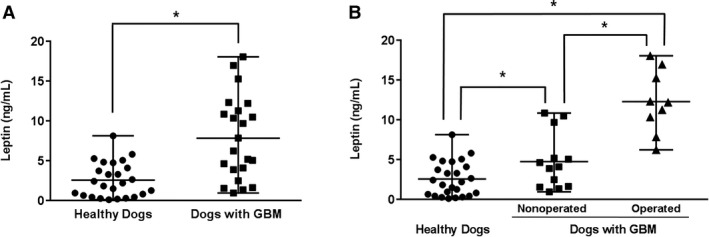
Comparison of the circulating leptin concentration (A) between healthy dogs (n = 25) and dogs with gallbladder mucocele (GBM, n = 22) and (B) between nonoperated (n = 13) and operated (n = 9) patients among the dogs with GBM. The horizontal bars indicate the medians and ranges. *P < .05 between 2 groups.
Figure 3.
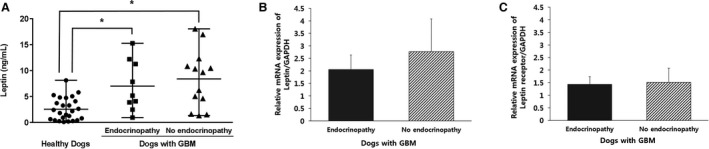
Comparison of (A) the circulating leptin concentration among healthy dogs (n = 25) and dogs with gallbladder mucocele (GBM, n = 22) classified according to the presence of endocrinopathy. The relative mRNA expression levels of (B) leptin and (C) leptin receptor in dogs with GBM (n = 22) depending on the presence of endocrinopathy. The horizontal bars of (A) indicate the medians and ranges. In (B) and (C), the columns represent the mean value of each parameter, and the vertical error bars indicate the standard deviation. *P < .05 between groups.
Relative mRNA Expression Levels of Leptin and Leptin Receptor in Healthy Dogs and Dogs With GBM
The mRNA expression of leptin (P = .003) and leptin receptor (P = .032) was significantly downregulated in healthy dogs compared with that of dogs with GBM (Fig 2). No significant difference was identified in the mRNA expression levels between the GBM groups with or without endocrinopathy (Fig 3).
Figure 2.
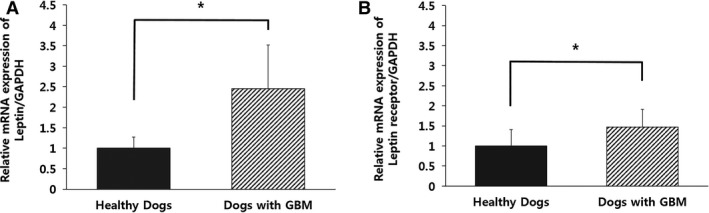
Comparison of the relative mRNA expression levels of (A) leptin and (B) leptin receptor in healthy dogs (n = 9) and dogs with gallbladder mucocele (GBM, n = 9). The columns represent the mean value of each parameter, and the vertical error bars indicate the standard deviation. *P < .05 between groups.
Discussion
The results of our study showed that body weight, BCS, age, breed, and sex are not significantly associated with serum leptin concentrations either in healthy dogs or in dogs with GBM. A previous study showed that circulating plasma concentrations of leptin were significantly higher in dogs with higher BCS but there were no significant relationships between plasma leptin concentrations and breed, age, or sex.26 These findings suggested that plasma leptin concentrations may play a role in the parameters of adiposity regardless of breed, age, and sex.26 To rule out the influence of body fat mass on leptin concentration in dogs,26, 27 all dogs enrolled in the present study were selected based on a normal BCS (BCS of 5 or 6 of 9).
The diagnosis of GBM relies on a combination of imaging studies, laboratory tests, and clinical signs such as anorexia, vomiting, and lethargy. One of the most useful diagnostic tools currently available is abdominal ultrasonography. Ultrasonographically, the GBM usually contains echogenic material with finely striated or stellated bile patterns and differs from biliary sludge by non‐gravity‐dependent bile movement.1, 28 Moreover, ultrasonographic changes in bile patterns according to the progression of GBM have been described, in which immobile echogenic bile gradually changes to a stellate or finely striated pattern.1 However, in some cases, even if these changes are observed on ultrasound examination, they are not always accompanied by changes in clinical signs and laboratory findings. Therefore, sole dependence on diagnostic imaging tests to determine a patient's prognosis has limitations. Our study showed that serum leptin concentrations were significantly upregulated in dogs with GBM compared to healthy dogs. To further determine the association between the severity of GBM and the circulating leptin concentration, the GBM group was divided into operated and nonoperated groups, and significantly higher serum leptin concentrations were observed in the former. This result further supported that serum leptin concentration might be a useful variable for determining the severity of GBM in dogs. Considering that both gallstones and GBM are caused by similar environmental factors within the gallbladder, such as changes in gallbladder motility and bile components, our results are strengthened by previous research showing that patients with gallstones had higher serum leptin concentrations than controls.2, 20, 29 We also found increases in the mRNA expression levels of leptin and leptin receptor in the gallbladder tissues of dogs with GBM compared to controls.
Although the relationship between serum leptin concentration and the severity of GBM has not been evaluated and the potential mechanism remains unknown, previous studies on leptin provide some clues. Leptin was shown to modulate cholecystokinin (CCK) signaling and had cooperative interactions with CCK in vagal afferent neurons (VAN).30, 31, 32 Leptin may potentiate the inhibitory action of CCK on VAN.33 Cholecystokinin acts by binding to CCK receptors distributed in the gallbladder smooth muscle and sphincter of Oddi.34, 35 Previous studies have reported that leptin modulated the contractions and emptying of the gallbladder36 and simultaneously relaxed the sphincter of Oddi.35 These findings suggest that overexpression of leptin and leptin receptors in GBM patients may reflect a compensatory reaction to resolve biliary dyskinesia caused by decreased motility of the gallbladder and sphincter of Oddi. Another study showed that leptin decreased gallbladder volume, bile sodium concentration, and pH by regulating gallbladder genes related to absorption and secretion.18 Specifically, leptin upregulated the expression of aquaporin 1 downregulated aquaporin 4 and influenced sodium channel‐α and sodium–hydrogen exchangers.18 These findings further support our hypothesis that overexpression of leptin and leptin receptors in GBM patients might reflect a compensatory response to maintain homeostasis within the gallbladder in pathological situations. However, there currently is no clear evidence to confirm this hypothesis, and thus, further studies are needed to gain an accurate understanding of the relationship between leptin and GBM.
Previous studies have suggested that leptin has a synergistic interaction with endocrinopathies such as hyperadrenocorticism, hypothyroidism, and diabetes mellitus, which were associated with increased leptin concentrations.9, 10, 11 In addition, a case–control study showed that these endocrinopathies were associated with GBM; in particular, the presence of hyperadrenocorticism increased the morbidity of GBM 29‐fold.4 Therefore, it was hypothesized that leptin concentrations would be even higher in patients with both GBM and an endocrinopathy compared to those with only 1 disease but this was not the case in the our study. The association between leptin and endocrinopathies as well the association between leptin and GBM has been identified in several previous studies.4, 9, 10, 11 Accordingly, we expected to observe a higher serum leptin concentration in GBM patients with concurrent endocrinopathy than in cases of either disease alone but this synergistic effect of GBM and endocrinopathies on leptin concentration could not be clearly identified in our study. Therefore, further studies are needed to better understand the exact mechanism that underlies this interaction between GBM and endocrinopathies.
Hyperlipidemia is defined as an increase in the blood concentration of total cholesterol, triglycerides, or both.37 In the present study, the serum concentrations of total cholesterol and triglycerides were upregulated in GBM patients compared with controls, suggesting that hypercholesterolemia and hypertriglyceridemia are associated with GBM in dogs. This finding is consistent with a previous study,38 in which the authors suggested that hypertriglyceridemia decreased gallbladder motility, and the excessive secretion of cholesterol in the bile with subsequent oversaturation led to the formation of GBM. Another study showed that leptin promoted biliary cholesterol elimination by decreasing bile salt pool size and decreasing the activity of 3‐hydroxy‐3‐methylglutaryl‐CoA reductase in the liver.19 Combining the results of previous research with our results, it appears that the increase in leptin concentrations in GBM patients may occur as a negative feedback mechanism to prevent formation of a mucocele induced by increased cholesterol concentrations.
The association between serum triglyceride or cholesterol and leptin concentrations is controversial. Several previous studies have concluded that the relationship between serum leptin and lipid (cholesterol and triglycerides) concentrations was not statistically significant when adjusted for variations in age and body mass index.39, 40 By contrast, in other studies, leptin was reported to be positively correlated with cholesterol in healthy dogs41 and with triglycerides.42 In addition, leptin was found to play a role in the decrease in very‐low‐density lipoprotein triglyceride after weight loss.43 In our study, triglyceride and cholesterol concentrations were relatively high in the GBM group, and the serum leptin concentration of the GBM group also was higher than that of the control group. In practice, most GBM patients have concurrent hyperlipidemia, and this association among hyperlipidemia, leptin, and GBM also seemed to be present in our study. No direct association was observed, however, within the GBM group, according to the correlation analysis between cholesterol and leptin (P = .069) or between triglycerides and leptin (P = .454).
There are several limitations to our study. One limitation is the relatively small sample size of 22 GBM dogs and 25 normal control dogs. Obtaining a larger sample size would not only help to obtain more reliable results, but also would allow for a multiperspective analysis. In particular, because the focus of our study was on the relationship between GBM and leptin, we did not seek to increase the endocrinopathy‐only group sample size in advance, and the total sample size was too small to allow for subgrouping according to each type of endocrinopathy. Therefore, subgrouping of the endocrinopathy group into diabetes mellitus, hyperadrenocorticism, and hypothyroidism was necessary to evaluate the effect of leptin concentration for each endocrinopathy. In addition, the presence of an endocrinopathy in the non‐GBM group could not be analyzed. Therefore, to best determine the interaction between GBM and endocrinopathy and its effects on leptin concentrations, a larger sample size would be needed. This would permit effective comparisons of leptin concentrations in the GBM‐only group, GBM with concurrent endocrinopathy group, endocrinopathy‐only group, and control group. Moreover, as with most retrospective studies, an accurate grouping and history control could be limited by failure to document a complete diagnostic and medication history in the medical record. Previous studies suggested that treatment could attenuate alterations in leptin in dogs with hypothyroidism44 or hyperadrenocorticism.9 Because the dogs with endocrinopathies already were being treated at the time of sample collection, both the potential misclassification of dogs and treatment of the dogs with endocrinopathies may have influenced the difference in leptin concentration between dogs with and without endocrinopathies. In the process of controlling other factors that could affect leptin concentration, we restricted patients for our study to those with a BCS of 5–6 of 9. However, the fact that the observed association was detected in dogs with normal body condition does not necessarily imply the presence of the same association in underweight or obese dogs. Another limitation is that the quantitative evaluation of the relative expression levels of leptin and leptin receptor was only conducted at the mRNA level by real‐time PCR and not at the protein level.
In conclusion, our results indicated that serum leptin concentrations are associated with GBM in dogs and that dysregulation of leptin might have an influence on the pathogenesis of GBM. Furthermore, serum leptin concentration might be a useful variable for determining the severity of GBM in dogs. The increased expression of leptin and leptin receptors in GBM patients suggests that the dysregulation of leptin, which plays a role in regulating physiological processes in the gallbladder, could be a causative factor in GBM. The results of our study may provide the foundation needed to build knowledge on the relationship between leptin and the pathophysiology of gallbladder diseases in the future. However, further studies are needed to elucidate the specific roles of leptin in the development of GBM.
Acknowledgments
Grant support: This study was partially supported by the Research Institute for Veterinary Science, Seoul National University.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Canine Leptin ELISA, Millipore, Billerica, MA
Model 680 Microplate Reader, Bio‐Rad, Hercules, CA
GeneAll Biotechnology, Seoul, Korea
Implen GmbH, Munich, Germany
Takara Bio, Tokyo, Japan
Takara Bio Inc, Otsu, Japan
Applied Biosystems, Foster City, CA
SPSS Inc., Chicago, IL
References
- 1. Besso J, Wrigley R, Gliatto J, et al. Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Vet Radiol Ultrasound 2000;41:261–271. [DOI] [PubMed] [Google Scholar]
- 2. Tsukagoshi T, Ohno K, Tsukamoto A, et al. Decreased gallbladder emptying in dogs with biliary sludge or gallbladder mucocele. Vet Radiol Ultrasound 2012;53:84–91. [DOI] [PubMed] [Google Scholar]
- 3. Jubb I, Kennedy KVF, Palmer N. Pathology of Domestic Animals. 4th ed San Diego, CA: American Press; 1993. [Google Scholar]
- 4. Mesich ML, Mayhew PD, Paek M, et al. Gall bladder mucoceles and their association with endocrinopathies in dogs: A retrospective case–control study. J Small Anim Pract 2009;50:630–635. [DOI] [PubMed] [Google Scholar]
- 5. Maffei M, Fei H, Lee G‐H, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA 1995;92:6957–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–770. [DOI] [PubMed] [Google Scholar]
- 7. Konturek P, Jaworek J, Maniatoglou A, et al. Leptin modulates the inflammatory response in acute pancreatitis. Digestion 2002;65:149–160. [DOI] [PubMed] [Google Scholar]
- 8. Paek J, Kang JH, Kim HS, et al. Serum adipokine concentrations in dogs with acute pancreatitis. J Vet Intern Med 2014;28:1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho KD, Paek J, Kang JH, et al. Serum adipokine concentrations in dogs with naturally occurring pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2014;28:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazaki‐Tovi M, Feuermann Y, Segev G, et al. Increased serum leptin and insulin concentrations in canine hypothyroidism. Vet J 2010;183:109–114. [DOI] [PubMed] [Google Scholar]
- 11. Kim AY, Kim H‐S, Kang J‐H, et al. Serum adipokine concentrations in dogs with diabetes mellitus: A pilot study. J Vet Sci 2015;16:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C‐reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res 2013;6:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Artac M, Altundag K. Leptin and breast cancer: An overview. Med Oncol 2012;29:1510–1514. [DOI] [PubMed] [Google Scholar]
- 14. Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature 1998;394:790–793. [DOI] [PubMed] [Google Scholar]
- 15. Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic β‐cells. Biochem Biophys Res Commun 1996;224:522–527. [DOI] [PubMed] [Google Scholar]
- 16. Lostao MP, Urdaneta E, Martínez‐Ansó E, et al. Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett 1998;423:302–306. [DOI] [PubMed] [Google Scholar]
- 17. Goldblatt MI, Swartz‐Basile DA, Svatek CL, et al. Decreased gallbladder response in leptin‐deficient obese mice. J Gastrointest Surg 2002;6:438–444. [DOI] [PubMed] [Google Scholar]
- 18. Swartz‐Basile DA, Lu D, Basile DP, et al. Leptin regulates gallbladder genes related to absorption and secretion. Am J Physiol Gastrointest Liver Physiol 2007;293:G84–G90. [DOI] [PubMed] [Google Scholar]
- 19. Hyogo H, Roy S, Paigen B, et al. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem 2002;277:34117–34124. [DOI] [PubMed] [Google Scholar]
- 20. Sarac S, Atamer A, Atamer Y, et al. Leptin levels and lipoprotein profiles in patients with cholelithiasis. J Int Med Res 2015;43:385–392. [DOI] [PubMed] [Google Scholar]
- 21. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract 1997;22:10–15. [Google Scholar]
- 22. Behrend E, Kooistra H, Nelson R, et al. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J Vet Intern Med 2013;27:1292–1304. [DOI] [PubMed] [Google Scholar]
- 23. Rucinsky R, Cook A, Haley S, et al. AAHA diabetes management guidelines for dogs and cats. J Am Anim Hosp Assoc 2010;46:215–224. [DOI] [PubMed] [Google Scholar]
- 24. Fonfara S, Hetzel U, Tew S, et al. Leptin expression in dogs with cardiac disease and congestive heart failure. J Vet Intern Med 2011;25:1017–1024. [DOI] [PubMed] [Google Scholar]
- 25. Mercati F, Maranesi M, Dall'Aglio C, et al. Leptin receptor is expressed by epidermis and skin appendages in dog. Acta Histochem 2014;116:1270–1275. [DOI] [PubMed] [Google Scholar]
- 26. Ishioka K, Hosoya K, Kitagawa H, et al. Plasma leptin concentration in dogs: Effects of body condition score, age, gender and breeds. Res Vet Sci 2007;82:11–15. [DOI] [PubMed] [Google Scholar]
- 27. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: A review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol 2009;38:136–156. [DOI] [PubMed] [Google Scholar]
- 28. Fossum TW. Small animal surgery. 4th ed St. Louis (MO): Elsevier Health Sciences; 2013. [Google Scholar]
- 29. Wang S, Yeh Y, Yu M, et al. Hyperleptinaemia and hypoadiponectinaemia are associated with gallstone disease. Eur J Clin Invest 2006;36:176–180. [DOI] [PubMed] [Google Scholar]
- 30. Barrachina MD, Martínez V, Wang L, et al. Synergistic interaction between leptin and cholecystokinin to reduce short‐term food intake in lean mice. Proc Natl Acad Sci USA 1997;94:10455–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Lartigue G, Lur G, Dimaline R, et al. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 2010;151:3589–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 2006;89:477–485. [DOI] [PubMed] [Google Scholar]
- 33. De Lartigue G, De La Serre CB, Espero E, et al. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 2012;7:e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steigerwalt RW, Goldfine I, Williams JA. Characterization of cholecystokinin receptors on bovine gallbladder membranes. Am J Physiol 1984;247:G709–G714. [DOI] [PubMed] [Google Scholar]
- 35. Krishnamurthy S, Krushnamurthy GT. Biliary dyskinesia: Role of the sphincter of Oddi, gallbladder and cholecystokinin. J Nucl Med 1997;38:1824. [PubMed] [Google Scholar]
- 36. Shiratori K, Watanabe S, Chey W, et al. Endogenous cholecystokinin drives gallbladder emptying in dogs. Am J Physiol 1986;251:G553–G558. [DOI] [PubMed] [Google Scholar]
- 37. Nelson RW, Couto CG. Small Animal Internal Medicine. 5th ed Louis (Mo): Elsevier Health Sciences; 2014. [Google Scholar]
- 38. Kutsunai M, Kanemoto H, Fukushima K, et al. The association between gall bladder mucoceles and hyperlipidaemia in dogs: A retrospective case control study. Vet J 2014;199:76–79. [DOI] [PubMed] [Google Scholar]
- 39. Haluzik M, Fiedler J, Nedvidkova J, et al. Serum leptin concentrations in patients with combined hyperlipidemia: Relationship to serum lipids and lipoproteins. Physiol Res 1998;48:363–368. [PubMed] [Google Scholar]
- 40. Haluzík M, Fiedler J, Nedvídková J, et al. Serum leptin levels in patients with hyperlipidemias. Nutrition 2000;16:429–433. [DOI] [PubMed] [Google Scholar]
- 41. Park HJ, Lee SE, Oh JH, et al. Leptin, adiponectin and serotonin levels in lean and obese dogs. BMC Vet Res 2014;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzaga NC, Medeiros CC, de Carvalho DF, et al. Leptin and cardiometabolic risk factors in obese children and adolescents. J Paediatr Child Health 2014;50:707–712. [DOI] [PubMed] [Google Scholar]
- 43. Magkos F, Fabbrini E, McCrea J, et al. Decrease in hepatic very‐low‐density lipoprotein‐triglyceride secretion after weight loss is inversely associated with changes in circulating leptin. Diabetes Obes Metab 2010;12:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tvarijonaviciute A, Jaillardon L, Ceron JJ, et al. Effects of thyroxin therapy on different analytes related to obesity and inflammation in dogs with hypothyroidism. Vet J 2013;196:71–75. [DOI] [PubMed] [Google Scholar]


