Abstract
Background
Duodenitis‐proximal jejunitis (DPJ) is an acute sporadic gastrointestinal disorder of horses of unknown cause.
Hypothesis/Objectives
We hypothesize that Clostridium difficile toxins are involved in the pathogenesis of DPJ in horses. The objective of this study was to determine whether experimentally delivered C. difficile toxins cause clinical signs and histologic lesions similar to those of naturally occurring DPJ.
Animals
Six healthy mature mixed breed horses.
Methods
Experimental study: animal model of animal disease. Fasted horses were administered crude C. difficile toxins via gastroscopy and monitored for up to 48 hour. Blood was collected for complete blood cell count, biochemistry profile, and plasma fibrinogen assay, and abdominal fluid was collected for cytologic analysis and total solids before and after toxin administration. Physical examination and abdominal ultrasonography were performed throughout the study period. Tissues were collected from the gastrointestinal tract and processed for routine histologic analysis, and lesions were scored.
Results
Clinical signs were observed in 2 of 6 horses that are typical although not specific for horses with naturally occurring DPJ. Histopathologic lesions were observed in 6 of 6 horses and were similar to those reported in horses with naturally occurring DPJ. Two horses were severely affected.
Conclusions and Clinical Importance
Duodenitis‐proximal jejunitis is likely a syndrome with multiple causes that result in the same clinical and pathologic findings, and our data suggest that the toxins of C. difficile represent one cause of this syndrome. Toxin dose and variation in individual animal susceptibility might affect the clinical signs and lesions after administration of C. difficile toxins.
Keywords: Enteritis, Exotoxins, Gastrointestinal
Abbreviations
- DPJ
duodenitis‐proximal jejunitis
- H&E
hematoxylin and eosin stain
- SAA
serum amyloid A
- WBC
white blood cell
- WNL
within normal limits
Duodenitis‐proximal jejunitis (DPJ) is an acute sporadic gastrointestinal disease of horses, clinically characterized by depression, colic, ileus, endotoxemia, and nasogastric reflux because of fluid accumulation in the stomach and proximal small intestine.1, 2 Complications including cardiac arrhythmias, increased serum activity of liver‐derived enzymes, and laminitis occur.3, 4, 5 The cause of this condition remains unknown. Clostridia, Salmonella spp., mycotoxins, and ischemia associated with use of nonsteroidal anti‐inflammatory drugs have been suggested as possible etiologies,6, 7 although evidence supporting Salmonella spp. or mycotoxins as the cause of DPJ is scarce.7, 8 Typical lesions described in DPJ cases including ulceration of the proximal small intestine and often the pars esophagea of the stomach are consistent with ischemia‐induced injury although this has not been definitively demonstrated.9
A clostridial cause has long been suspected but specific bacterial species have not been consistently and repeatedly isolated from affected horses.7, 10 Subsequent study suggested that Clostridium difficile, a known enteropathogen of various species, might be involved, because toxigenic C. difficile were isolated from the gastric contents of 10 of 10 horses with DPJ but only 1 of 16 clinically normal horses.11 Clostridium difficile is an anaerobic spore‐forming bacterium that is mainly associated with colitis in horses,12, 13 but severe small‐intestinal lesions have been reported in naturally and experimentally infected foals.14 This organism has also been isolated from the small intestine of horses and ponies with various small‐intestinal disorders.15 Clostridium difficile‐associated enteritis also occur in humans, and there have been increasing numbers of C. difficile‐associated ileitis reports in recent years.16, 17
Clostridium difficile can produce various toxins, including toxin A (TcdA), toxin B (TcdB), and binary toxin (CDT). In vitro C. difficile toxins A and B can profoundly affect the motility of the small intestine. Purified toxins of C. difficile cause significant muscle excitation‐contraction coupling alterations in intestinal smooth muscle.18 This transitory increase in intestinal motility is followed by an absence of intestinal motility by 18 hours after toxin A exposure.19 This suggests that C. difficile toxins alter small‐intestinal motility in horses leading to ileus and potentially contribute to subsequent development of clinical signs of DPJ.
The objective of this study was to determine whether horses inoculated with C. difficile toxins develop clinical signs and histopathologic lesions similar to those of naturally occurring DPJ.
Materials and Methods
Toxin Inoculum Preparation
A toxigenic C. difficile strain ribotype 027 (A+B+CDT+) isolated from the gastric reflux of a horse clinically diagnosed with DPJ was grown anaerobically at 37°C for 5–7 days in dialysis tubing suspended in 4‐L flasks as described previously.20 Briefly, an overnight culture (1 mL) in brain‐heart infusion broth of the toxigenic C. difficile strain was used to inoculate a sterilized dialysis bag (molecular weight cutoff 10,000 daltons) containing approximately 500 mL PBS. The dialysis bag was then suspended in 3–3.5 L of brain‐heart infusion broth in a stoppered and vented 4‐L conical flask. After incubation for 5–7 days, the contents of the dialysis bag were harvested and centrifuged (10,000 × g × 15 minute). The supernatant was then filtered with a PTHK membrane (100,000 nominal molecular weight limit1 ), and sterile filtrates were stored at −80°C. An aliquot was cultured on blood agar plates aerobically and anaerobically for at least 72 hour to ensure that the crude toxin supernatant was free of C. difficile vegetative cells, spores, and other bacterial contaminants. The presence of toxins A and B was confirmed by a commercial immunoassay (C. DIFF QUIK CHEK COMPLETE® 2), and the presence of toxin B was confirmed through a cell cytotoxicity assay.21
The toxin supernatant was divided into ½‐L aliquots into 1‐L vials, snap‐frozen in liquid nitrogen, and then concentrated to half of its original volume by freeze‐drying at −50°C and <100 mbar overnight. The final toxin preparations were transferred to 1‐L plastic bottles and stored at −80°C until use. The concentrations of the toxins in the inoculum were not determined. The unconcentrated toxins are referred to as 1×, whereas toxins that were concentrated from 6 L to 3 L by lyophilization are referred as 2×.
Animals and Toxin Administration
Six healthy mature mixed breed horses (5 females, 1 castrated male) with a mean age of 8.75 years (range 4–17) and average body weight of 499 kg (range 460–560 kg) were included in this study. Horses were clinically normal with no history of gastrointestinal disease or antimicrobial exposure in the preceding 8 weeks. Housing and all experimental procedures for this study were approved by the Animal Care Committee of the University of Guelph and conformed to the standards of the Canadian Council on Animal Care.
Blood samples were collected for complete blood cell count, serum biochemistry profile, and plasma fibrinogen assay 1 day before toxin administration and before euthanasia, which was performed either as a result of development of disease or at the conclusion of the study period (24 or 48 hours after inoculation). Abdominocentesis was performed at the midline of the cranioventral abdomen before both toxin administration and euthanasia, and samples were submitted for cytologic analysis and total protein concentration.
All horses were fasted for 14–18 hour before toxin administration. For toxin administration, horses were sedated with 0.3 mg/kg of xylazine, and a 300‐cm video endoscope (SIF 1403 ) was advanced through the nasal cavity into the stomach. Fifteen minutes before toxin infusion, 500 mL of 1 M sodium bicarbonate solution was infused into the stomach to neutralize gastric pH and minimize toxin degradation. The endoscope was then advanced through the gastric pylorus into the proximal duodenum, where the C. difficile toxin concentrate was infused through the endoscope's biopsy channel with a pressure bag. Horses 1 and 2 received 500 mL and 1 L of nonconcentrated (1×) toxins, respectively. Horses 3–6 received 3 L of concentrated toxin (2×) preparation (see Table S1 for specific volumes and location of dosing). Horses were fed 2 hours after toxin administration and had access to free choice hay and water throughout the study period.
Full physical examination, including assessment of changes in demeanor, heart and respiratory rate, rectal temperature, hydration status, and gastrointestinal sounds, was performed at −24, −1, 2, 4, 8, 12 hours after toxin administration, then every 6 hours. All horses were monitored continuously for signs of colic including pawing, flank watching, kicking at the abdomen, getting up and down, and rolling during the first 8 hours. Horses that showed clinical signs of colic or discomfort were immediately euthanized (horses 3 and 5). Horses that did not show clinical signs of colic or discomfort were euthanized at 24 (horses 1 and 2) or 48 hours (horses 4 and 6) after toxin administration.
Fecal samples were collected per rectum the day before toxin administration, and both small‐ and large‐intestinal content samples were collected during postmortem examination for C. difficile culture by selective enrichment method.22
Assessment of Small‐Intestinal Motility
Small‐intestinal motility was monitored by transabdominal ultrasonographic scanning with an ultrasound machine equipped with a 2‐ to 5‐MHz dynamic range convex probe (GE Logiq 5 Expert 4) performed in each horse before toxin administration, then at 1, 3, 6, 12, 18 and 24 hours after toxin administration. Ultrasonographic visualization of the small intestine was attempted at 3 sites during each time point, and the number of small‐intestinal muscular contractions was recorded at each site for a period of 2 minutes. Scanning was performed on the right side of the abdomen to assess motility of the duodenum, and on the ventral abdomen to assess motility of the jejunum/ileum in least 2 different locations. An approximately 20 × 20 cm scanning window was made by clipping the 3 sites to consistently scan the same locations. Site 1: the duodenum was visualized at the level of the 17th thoracic vertebrae, next to the right kidney; site 2: immediately cranioventral to the right stifle; and site 3: immediately caudal to the xiphoid process of the sternum in the mid‐ventral abdomen.
Postmortem Examination and Tissue Collection
Immediately after euthanasia with an overdose of pentobarbital, a complete postmortem examination was performed and tissue samples were collected from glandular and squamous portion of the stomach, gastric pylorus, proximal duodenum, jejunum, ileum, cecum, right ventral colon, and pelvic flexure. All tissues were fixed in 10% neutral buffered formalin before routine embedding in paraffin. Four‐micrometre‐thick sections were cut and stained with hematoxylin and eosin (H&E) for histologic scoring. All sections were independently and blindly (to the relative amount of toxins administered to the horses) reviewed and scored for lesions by a board‐certified veterinary anatomic pathologist (BLP).
Histologic Scoring
All gastrointestinal tissue sections were scored based on a modified system previously used to evaluate Clostridium perfringens enterotoxin‐induced lesions in the colon of rabbits.23 Briefly, each H&E‐stained section was scored on a scale from 0 (no lesions present) to 5 (severe lesions present) for each of the following histologic features: epithelial desquamation/necrosis, lamina proprial necrosis, villous blunting (small intestine only), inflammation, hemorrhage, and submucosal edema. Where multiple sections of the same tissue were present for evaluation, the most severe score was recorded. Scores were then added for each tissue type (small intestine, large intestine, stomach) and reported as a tissue lesion score (maximum score of 30 for small intestine and 25 for large intestine and stomach). Tissue lesion scores for each tissue type were then added and reported as an overall lesion score for each animal (maximum score 190).
Results
Throughout the trial period, all vital variables remained within normal limits (WNL) for horses 1, 2, 4, and 6. Horse 3 experienced a gradual increase in rectal temperature from 37.4°C to 39.3°C (reference range: 37–38°C), increased heart rate from 44 to 100 bpm (reference range: 24–40 bpm), increased respiratory rate of 36–40 breaths per minute (reference range: 8–16 breaths per minute), and marked depression between 6 and 10 hours after toxin administration. Horse 5 also experienced gradual increase in rectal temperature from 37.9 to 39.4°C, increased heart rate from 44 to 100 bpm, and increased respiratory rate of 12–40 breaths per minute between 8 and 12 hours after toxin administration. Horses 3 and 5 both became clinically dehydrated (estimated at 8–10%) and displayed intermittent mild colic signs of abdominal discomfort including agitation, pawing, walking in circles in the stall, and chewing movements. Horse 3 and 5 horses were euthanized at 10 and 12 hours after toxin administration, respectively.
Abdominal ultrasonographic examination was performed at the 3 described locations at each time point in all horse, but the small intestine was not consistently visualized. Overall, the small intestine was observed in 66% (81 of 123) of the 3 scanned sites in all horses, in 30 (73%), 27 (66%), and 24 (59%) (of 41 total scan times) in sites 1, 2, and 3, respectively, for all horses. In horses 3 and 5, amotile and distended loops of small intestine (up to 5 cm in diameter) were observed before euthanasia. No abnormalities were detected in the other 4 horses.
The complete blood count, serum biochemistry profile, plasma fibrinogen, and peritoneal fluid analysis were WNL for all horses before administration of the toxin preparation, and for horses 1, 2, 4, and 6 throughout the trial. Serum amyloid A (SAA) ranged from 0 to 1.2 mg/L (reference range: 0–20 mg/L) in all 6 horses before toxin administration. Serum amyloid A increased in horse 5 (109.8 mg/L) at 24 hours after toxin administration, and in horse 6 (363 and 955 mg/L) at 24 and 48 hours after toxin administration, respectively. No increases in SAA were noted for the other 4 horses. Plasma fibrinogen ranged from 1.5 to 2.3 g/L (reference range: 1.29–2.59 g/L) before challenge in all 6 horses in this study, and no significant changes were noted after toxin administration for any horse during this study (1.0–2.47 g/L).
The hematocrit was WNL for all horses before toxin administration (reference range: 0.28–.44 L/L). Hematocrit was increased in horse 3 (0.60 L/L) and in horse 5 (0.68 L/L) at 10 hours after toxin administration but remained WNL for all other horses during the duration of the study (range 0.41–0.46 L/L). The white blood cell (WBC) count was within the reference range (5.1–11.0 × 109/L) in all 6 horses before toxin administration, but decreased to 4.5 × 109/L in horse 3, and to 3.6 × 109/L in horse 5 by 10 hours after toxin administration, but remained within the reference range in horses 1, 2, 4, and 6. The total solids and total nucleated cell count in the abdominal fluid of all 6 horses were within the reference range (<25 g/L and <5.0 × 109/L, respectively) before and after toxin administration.
At postmortem examination of horses 3 and 5, no gastric or small‐intestinal distention/fluid accumulation was noted and the only gross abnormality was dark red discoloration of the serosal surface and hemorrhage of the mucosal surface of the proximal duodenum (Fig 1). In all other horses, no gross abnormalities or lesions were observed. Microscopic lesions were observed in all horses, and the most severe overall lesion scores were observed in horses 3 and 5 (Table S2). Lesions noted in tissues of affected horses included lamina proprial vascular congestion, hemorrhage, edema, and neutrophil infiltration (Fig 2). Higher tissue lesion scores occurred in the duodenum (Table S3) and jejunum (Table S4) where surface epithelial flattening, dysplasia, or both with superficial lamina proprial neutrophil infiltration, fibrin or both were the most common lesions observed. In horses 1, 2, 4, and 6 the lesions were mild with submucosal edema, inflammation, and hemorrhage observed in various sections of the gastrointestinal tract (for other tissues, see Tables S5–S9).
Figure 1.
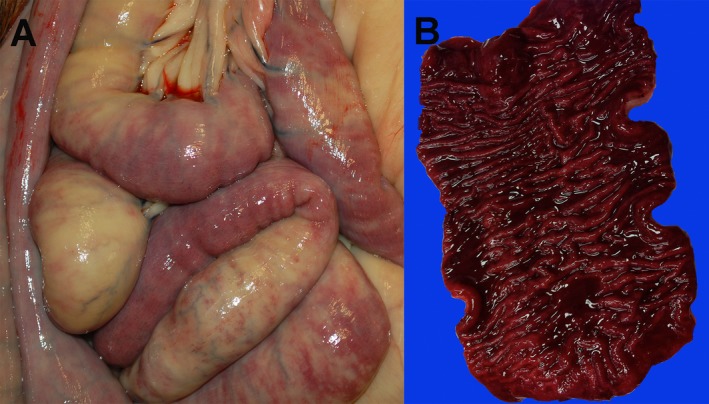
Gross lesions in a horse inoculated with Clostridium difficile toxins. Small‐intestinal loops have multiple areas of hyperemia and congestion visible on the serosal surface (A). In an opened section of duodenum, there is diffuse hemorrhage as well as thickening and corrugation of the mucosal surface (B). Lesions presented are from horse 5.
Figure 2.
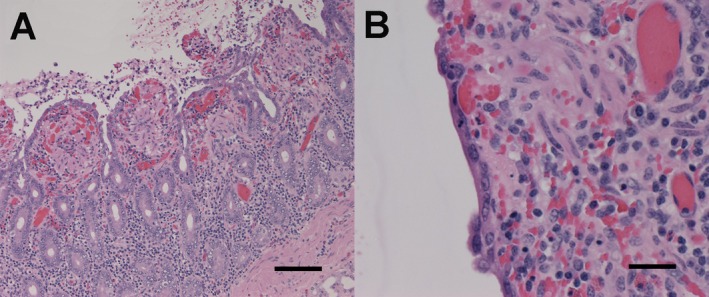
Microscopic lesions in the duodenum of a horse inoculated with Clostridium difficile toxins. In (A), there is blunting of villi with loss of epithelium lining villous tips. The lamina propria is expanded by congestion and hemorrhage. The lumen contains inflammatory cells intermixed with sloughed epithelial cells, erythrocytes, and fibrin. Magnification 10×, hematoxylin and eosin (H&E) stain. In (B), the intact epithelial cells are flattened and stretched across the surface of an affected villus. Magnification 40×, H&E stain. Lesions presented are from horse 5. Figure A length of the scale bar = 100 μm. Figure B length of the scale bar = 25 μm.
Clostridium difficile was not isolated from any fecal sample collected before toxin administration or from intestinal contents collected during postmortem examination.
Discussion
Direct administration of a concentrated crude mixture of C. difficile toxins to healthy horses elicited clinical signs consistent with DPJ in 2 of 6 horses, and histologic lesions in all 6 horses, with the most severe histologic lesions observed in the 2 horses displaying clinical signs of DPJ. Importantly, the duodenum and jejunum were most severely affected, similar to lesions in horses with naturally occurring DPJ.8, 10 The clinical signs observed in horses administered toxins in this study are typical although not specific for those described in horses with naturally occurring disease. Nonspecific signs of depression, tachycardia, and fever are among the most consistent clinical findings in horses with DPJ.10 Mild colic signs and decreased gastrointestinal motility were documented in 2 of 6 horses in this study; however, these are considered to be inconsistent clinical signs in horses with DPJ. No signs of colic were noted during hospital admission in 12 of 20 DPJ cases, and 8 of 12 of those horses only exhibited signs of depression.10 Nasogastric reflux is widely considered one of the hallmark clinical findings of DPJ although reflux might not be observed until at least 24–72 hours after the onset of ileus and abdominal pain.10 Therefore, whether the horses administered toxins in this study would have developed ileus and gastric reflux if the disease was allowed to progress for a longer period of time is not known. Medical treatment/management, including nasogastric intubation, was not attempted in this study in the clinically affected horses because they exhibited rapid clinical deterioration and were required to be euthanized when they reached the predetermined clinical score set to minimize suffering.
Sedation and pretreatment with sodium bicarbonate are possible factors that could have altered gastrointestinal motility in these horses. Light sedation was used in this study for all horses to facilitate the endoscopic procedure; however, the effects of such sedatives are mild and short‐lived (<30 min), and therefore, we believe unlikely to have negatively impacted gastrointestinal motility or other variables monitored during this experiment.24 Treatment with 1 M sodium bicarbonate neutralizes gastric pH and prevents C. difficile toxin degradation in rats, mice, and hamsters.25 The amount and concentration of sodium bicarbonate used in this study was an extrapolation from that study and was estimated to achieve neutralization without negatively affecting gastrointestinal motility. Horses received approximately 41 g of sodium bicarbonate in total, and in other studies, horses received over 10 times this amount without experiencing adverse effects including altered gastrointestinal motility.26
An estimate of the amount of toxins required to cause clinical signs of DPJ in horses is unknown and could not be accurately extrapolated from toxin administration studies in other species. Therefore, the first 2 horses in this study were given a lower concentration of toxins. Because both of the initial horses in this study remained clinically healthy during the first 24 hours after toxin administration except for brief mild colic signs in horse 2, the toxins were concentrated (2×) before administration into the remaining 4 horses. The biological activity of the toxins was confirmed before and after the lyophilization process and after toxin administration through the cell cytotoxicity assay. The 4 horses that received concentrated toxin preparations (3 L) developed the most severe histologic lesions and clinical signs. The distribution and severity of lesions among horses were not uniform in this study. This could reflect differences in toxin transit along the intestine, the precise amount of toxins within each preparation, and variation in individual susceptibility among horses.
Toxins A and B are the most studied C. difficile toxins, and the C. difficile strain used in this study produced both. However, this strain also possessed genes encoding binary toxin (CDT) production, and the role of this toxin in disease is still unclear. In humans, CDT is more frequently observed in C. difficile strains associated with increased severity of infection,27 but whether this is caused by the effects of CDT or the coincidental presence of CDT genes in strains that are more virulent for other reasons remains unknown.
Microscopically, the anatomic sites and severity of the lesions in horses of this study resembled those described for horses with naturally occurring DPJ.10 A potential dose‐dependent effect of toxins was noted, similar to the dose‐dependent effects caused by C. perfringens enterotoxin in rabbits, associated with the development of histologic lesions in the small and large intestine.23 In our study, although the most severe lesions occurred in the duodenum and jejunum of the inoculated horses, other tissues including the stomach (pylorus) proximally and the cecum and large colon (pelvic flexure) distally were also mildly affected. The lesions observed in the distal part of the GI tract could have been caused by biologically active toxins that reached these segments. This suggestion remains speculative, however, particularly because a control group was not included in the study.
The scoring system used to characterize the histologic lesions in the small intestine and colon was adapted from a study of the effects of C. perfringens enterotoxin in the rabbit colon.23 This scoring system has not been used in horses or to assess C. difficile toxin‐induced lesions; however, it includes the most relevant variables and is thus likely suitable to achieve subjective but repeatable quantification of histologic lesions in horses.
Clinical and experimental evidence shows that C. difficile affects the small intestine in humans and several animal species, affecting intestinal physiologic function and causing structural damage. Clostridium difficile was previously suggested as a potential causal agent for DPJ because the toxins alter intestinal motility, and toxigenic strains of C. difficile have been recovered in gastric reflux of horses with naturally occurring DPJ (Arroyo et al.11). This earlier work motivated the current study, to specifically investigate the effects of experimental C. difficile toxin administration in healthy horses. This study demonstrates that direct administration of a crude C. difficile toxin preparation causes clinical signs (2/6) and histologic lesions (6/6) that are consistent with, but not specific for, features reported in horses with naturally occurring DPJ.
Although the clinical and histologic changes observed were attributed to the toxins that were administered, the possibility that those changes could have occurred because of another reason should be considered. An important limitation to this study is the lack of toxin quantification in the inocula administered to the horses in our study. The concentration of C. difficile toxins A and B, and their synergism in vivo, has been studied in laboratory animals25 but not in horses; this will be important to understand in future work.
The potential effect of variables such as sedation and bicarbonate administration could have been better accounted for if control animals would have been included in the study. There is, however, no evidence that bicarbonate or sedatives, for example, are associated with the clinical and pathologic findings observed in this study and they are unlikely to have had an impact.
These data are evidence for a role of C. difficile toxins in the pathogenesis of DPJ in horses, although toxin dose dependency and individual animal susceptibility could also be important factors in the host response to toxins. The interplay of these factors remains speculative because of the limited number of animals in this study. It is plausible that C. difficile is one of multiple potential etiologies that can result in the clinical and pathologic signs known collectively as the syndrome DPJ.
Supporting information
Table S1. Horse information, dose levels and clinical findings.
Table S2. Cumulative histologic lesion scores for the different gastrointestinal tract segments of 6 horses inoculated with Clostridium difficile toxins.
Table S3. Histologic scores of duodenal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S4. Histologic scores of jejunal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S5. Histologic scores of stomach lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S6. Histologic scores of pylorus lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S7. Histologic scores of ileal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S8. Histologic scores of cecal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S9. Histologic scores of large colon lesions of 6 horses inoculated with Clostridium difficile toxins.
Acknowledgments
The authors thank Michelle Beaudoin‐Kimble, Nicole Kudo, and Amanda Hathaway for their various contributions to this research project. Financial support for the project was obtained from Equine Guelph for the research costs and from the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the Ontario Veterinary College for graduate student stipend support.
Grant support: This study was supported by funding from Equine Guelph (project # 14‐2011).
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was performed at the Comparative Clinical Research Facility, Department of Clinical Studies, Ontario Veterinary College, Guelph, ON, Canada.
This research was presented in part at the Eleventh Equine Colic Research Symposium, Trinity College Dublin, Dublin, Ireland, on July 7–9, 2014, and a research report abstract was presented at the 2016 ACVIM Forum in Denver, CO, USA, on June 8–11, 2016.
Footnotes
Millipore Corp., Etobicoke, ON, Canada
TechLab, Blacksburg, VA
Olympus Canada Inc, Richmond Hill, ON, Canada
GE Medical Systems, Mississauga, ON, Canada
References
- 1. Blackwell RB, White NA. Duodenitis/proximal jejunitis in the horse Proceedings of the Equine Colic Research Symposium, Vol. 1. Athens, GA: College of Veterinary Medicine, University of Georgia; 1982. [Google Scholar]
- 2. Freeman DE. Duodenitis‐proximal jejunitis. Equine Vet Educ 2000;12:322–332. [Google Scholar]
- 3. Cohen ND, Parson EM, Seahorn TL, Carter GK. Prevalence and factors associated with development of laminitis in horses with duodenitis/proximal jejunitis: 33 cases (1985–1991). J Am Vet Med Assoc 1994;204:250–254. [PubMed] [Google Scholar]
- 4. Cornick JL, Seahorn TL. Cardiac arrhythmias identified in horses with duodenitis/proximal jejunitis: Six cases (1985–1988). J Am Vet Med Assoc 1990;197:1054–1059. [PubMed] [Google Scholar]
- 5. Davis JL, Blikslager AT, Catto K, Jones SL. A retrospective analysis of hepatic injury in horses with proximal enteritis (1984–2002). J Vet Intern Med 2003;17:896–901. [DOI] [PubMed] [Google Scholar]
- 6. Schumacher J, Mullen J, Shelby R, et al. An investigation of the role of Fusarium moniliforme in duodenitis/proximal jejunitis of horses. Vet Hum Toxicol 1995;37:39–45. [PubMed] [Google Scholar]
- 7. Griffiths NJ, Walton JR, Edwards GB. An investigation of the toxigenic types of Clostridium perfringens in horses with anterior enteritis: Preliminary results. Anaerobe 1997;3:121–125. [DOI] [PubMed] [Google Scholar]
- 8. Tyler DE, White NA, Blackwell RB, Allen D. Pathology of equine duodenitis‐proximal jejunitis (anterior enteritis) Proceedings of the Equine Colic Research Symposium, Vol. 2. Lawrenceville, NJ: Veterinary Learning Systems; 1985. [Google Scholar]
- 9. Uzal F, Plattner BL, Hostetter JM. Alimentary system and peritoneum In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals, 6th ed St. Louis, MO: Elsevier; 2016:1–257. [Google Scholar]
- 10. White NA 2nd, Tyler DE, Blackwell RB, Allen D. Hemorrhagic fibrinonecrotic duodenitis‐proximal jejunitis in horses: 20 cases (1977–1984). J Am Vet Med Assoc 1987;190:311–315. [PubMed] [Google Scholar]
- 11. Arroyo LG, Stämpfli HR, Weese JS. Potential role of Clostridium difficile as a cause of duodenitis‐proximal jejunitis in horses. J Med Microbiol 2006;55:605–608. [DOI] [PubMed] [Google Scholar]
- 12. Jones RL. Clostridial enterocolitis. Vet Clin North Am Equine Pract 2000;16:471–485. [DOI] [PubMed] [Google Scholar]
- 13. Baverud V. Clostridium difficile infections in animals with special reference to the horse. A review. Vet Q 2002;24:203–219. [DOI] [PubMed] [Google Scholar]
- 14. Jones RL, Shideler RK, Cockerell GL. Association of Clostridium difficile with foal's diarrhea In: Caroll D, ed. Equine Infectious Disease V. Lexington, KY: University Press of Kentucky; 1988:236–240. [Google Scholar]
- 15. Braun M, Herholz C, Straub R, et al. Detection of the ADP‐ribosyltransferase toxin gene (cdtA) and its activity in Clostridium difficile isolates from Equidae. FEMS Microbiol Lett 2000;184:29–33. [DOI] [PubMed] [Google Scholar]
- 16. Freiler JF, Durning JS, Ender PT. Clostridium difficile small bowel enteritis occurring after total colectomy. Clin Infect Dis 2001;33:1429–1431. [DOI] [PubMed] [Google Scholar]
- 17. Elinav E, Planer D, Gatt ME. Prolonged ileus as a sole manifestation of pseudomembranous enterocolitis. Int J Colorectal Dis 2004;19:273–276. [DOI] [PubMed] [Google Scholar]
- 18. Gilbert RJ, Pothoulakis C, LaMont JT. Effect of purified Clostridium difficile toxins on intestinal smooth muscle. II. Toxin B. Am J Physiol 1989;256:767–772. [DOI] [PubMed] [Google Scholar]
- 19. Lima CC, Carvalho‐de‐Souza JL, Lima AA, Leal‐Cardoso JH. Ileal smooth muscle motility depression on rabbit induced by toxin A from Clostridium difficile . Dig Dis Sci 2008;53:1636–1643. [DOI] [PubMed] [Google Scholar]
- 20. Sullivan NM, Pellett S, Wilkins TD. Purification and characterization of toxins A and B of Clostridium difficile . Infect Immun 1982;35:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyerly DM, Neville LM, Evans DT, et al. Multicenter evaluation of the Clostridium difficile TOX A/B TEST. J Clin Microbiol 1998;36:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arroyo LG, Rousseau J, Willey BM, et al. Use of a selective enrichment broth to recover Clostridium difficile from stool swabs stored under different conditions. J Clin Microbiol 2005;43:5341–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia JP, Li J, Shrestha A, et al. Clostridium perfringens type A enterotoxin damages the rabbit colon. Infect Immun 2014;82:2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merritt AM, Burrow JA, Hartless CS. Effect of xylazine, detomidine, and a combination of xylazine and butorphanol on equine duodenal motility. Am J Vet Res 1998;59:619–623. [PubMed] [Google Scholar]
- 25. Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun 1985;47:349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor EA, Beard WL, Douthit T, Pohlman L. Effect of orally administered sodium bicarbonate on caecal pH. Equine Vet J 2014;46:223–226. [DOI] [PubMed] [Google Scholar]
- 27. Gerding DN, Johnson S, Rupnik M, Aktories K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014;5:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Horse information, dose levels and clinical findings.
Table S2. Cumulative histologic lesion scores for the different gastrointestinal tract segments of 6 horses inoculated with Clostridium difficile toxins.
Table S3. Histologic scores of duodenal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S4. Histologic scores of jejunal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S5. Histologic scores of stomach lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S6. Histologic scores of pylorus lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S7. Histologic scores of ileal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S8. Histologic scores of cecal lesions of 6 horses inoculated with Clostridium difficile toxins.
Table S9. Histologic scores of large colon lesions of 6 horses inoculated with Clostridium difficile toxins.


