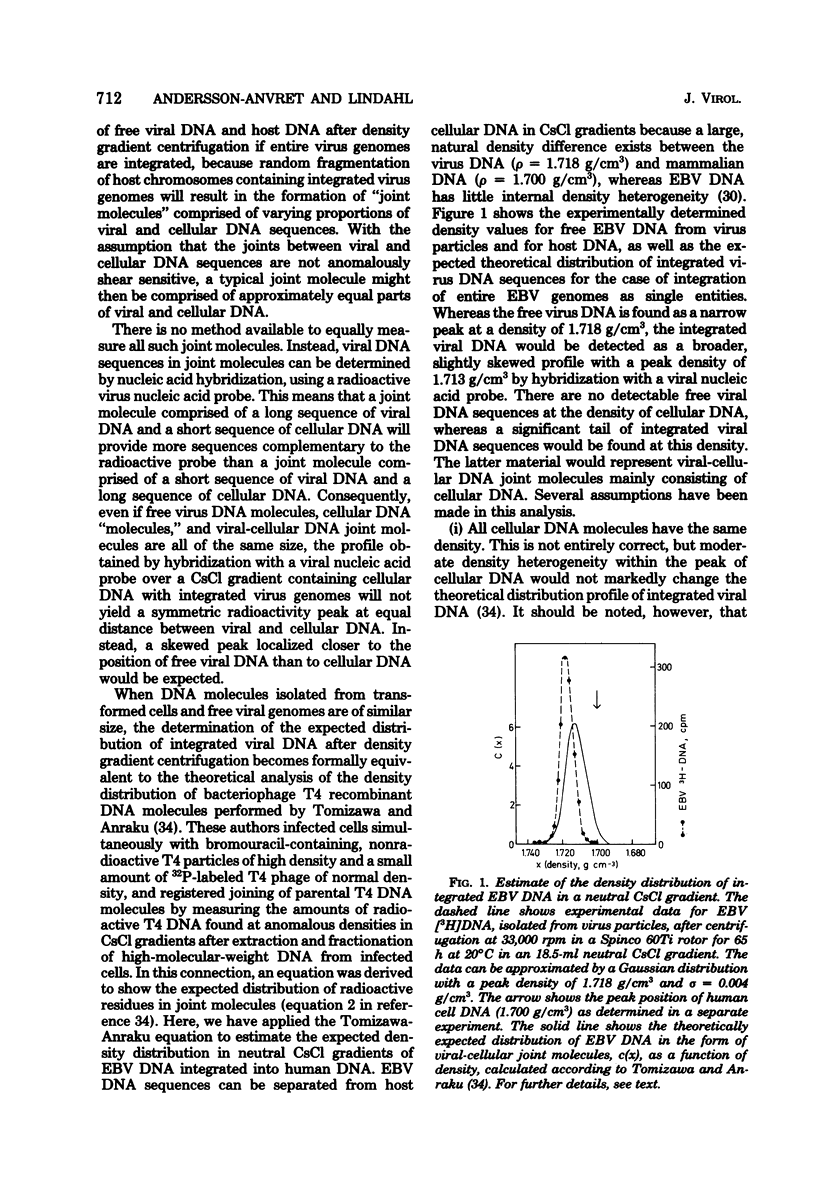
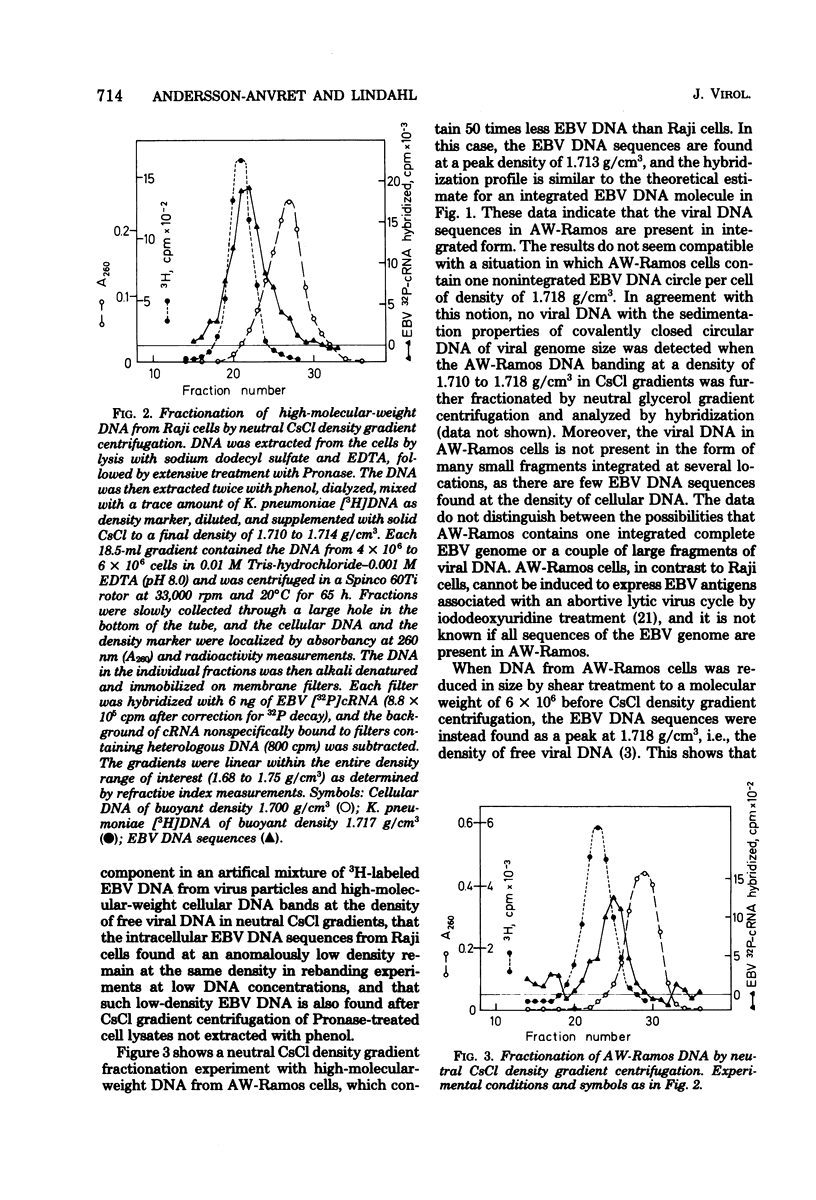
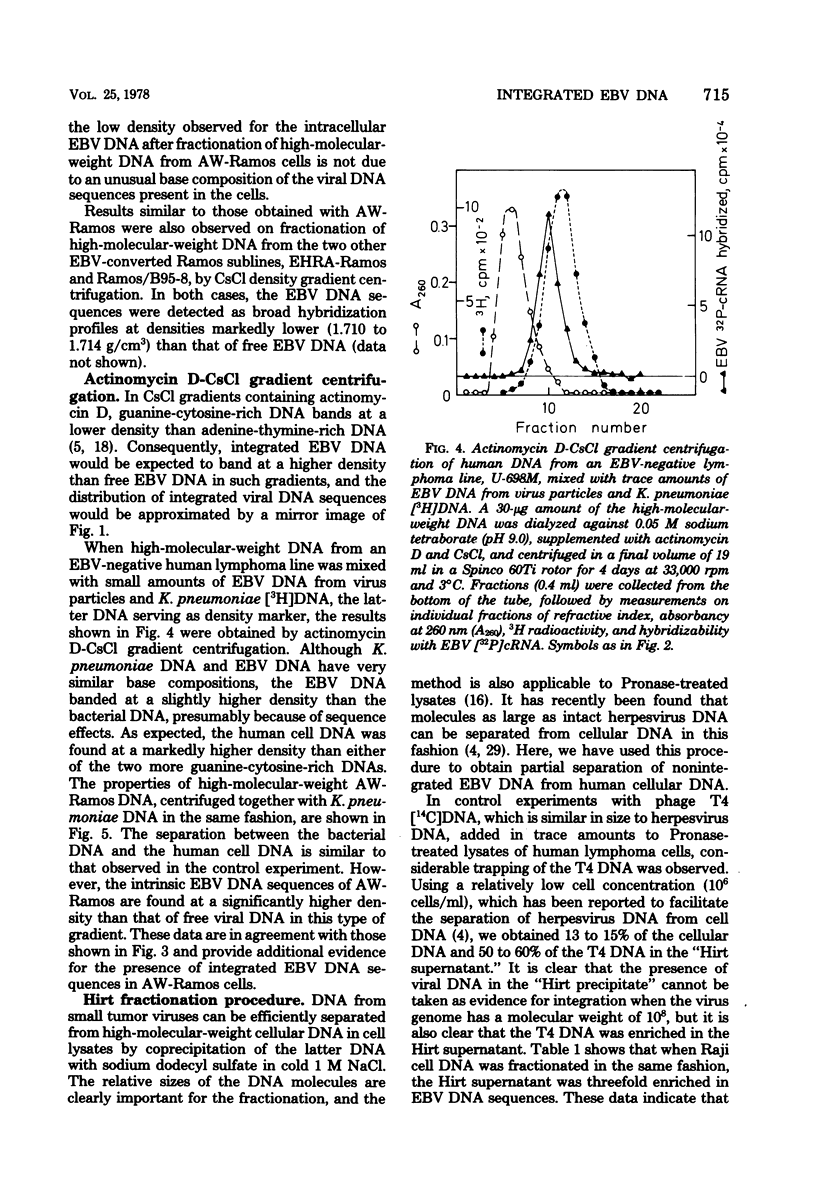
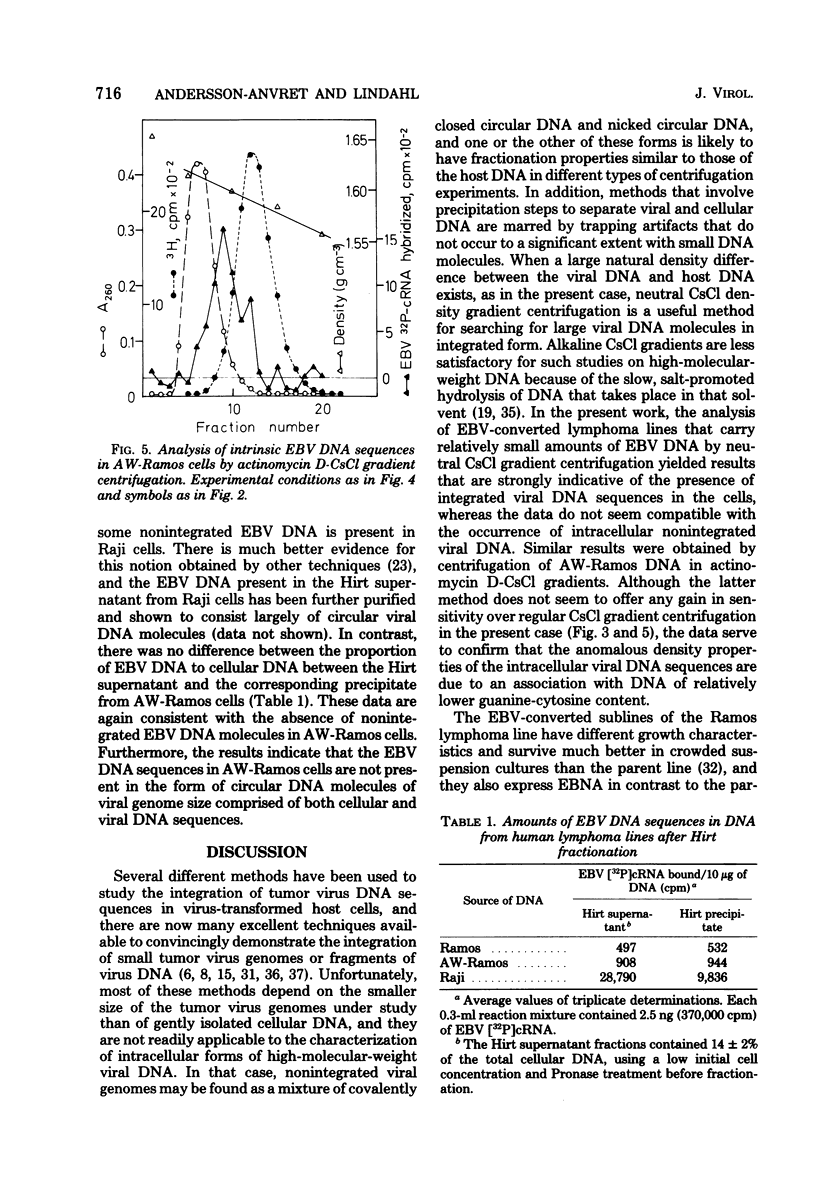
Abstract
Most human lymphoid cell lines contain multiple copies of circular, nonintegrated Epstein-Barr virus (EBV) DNA molecules as well as viral DNA sequences with properties of integrated DNA. The physical state of the EBV DNA in a human lymphoma line that only contains one virus genome equivalent per cell has now been studied by three different methods, neutral CsCl density gradient centrifugation, actinomycin D-CsCl gradient centrifugation, and Hirt fractionation. This cell line, AW-Ramos, has been obtained by EBV infection in vitro of the apparently EBV-negative Ramos lymphoma line. The results indicate that the EBV DNA in AW-Ramos is present exclusively in a linearly integrated form. Similar data were obtained with two other EBV-converted sublines of Ramos cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Intracellular forms of Epstein-Barr virus DNA in Raji cells. IARC Sci Publ. 1975;(11 Pt 1):125–132. [PubMed] [Google Scholar]
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M., Lindahl T. Epstein-Barr virus DNA in human lymphoid cell lines: in vitro conversion. Virology. 1976 Aug;73(1):96–105. doi: 10.1016/0042-6822(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S., Stehn B., Rubenstein A. S. Concatemeric forms of intracellular herpesvirus DNA. Virology. 1976 Feb;69(2):547–560. doi: 10.1016/0042-6822(76)90484-0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M., Telford J., Weinberg E., Stafford D. Isolation and some properties of the genes coding for histone proteins. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2900–2904. doi: 10.1073/pnas.71.7.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Klein G., Povey S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int J Cancer. 1975 Jul 15;16(1):125–133. doi: 10.1002/ijc.2910160114. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Integration of viral DNA into the host genome. Curr Top Microbiol Immunol. 1975;71:1–78. doi: 10.1007/978-3-642-66193-8_1. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Merkt B., Bornkamm G. W., Hausen H. Heterogeneity of Epstein-Barr virus originating from P3HR-1 cells. I. Studies on EBNA induction. Int J Cancer. 1977 Mar 15;19(3):317–323. doi: 10.1002/ijc.2910190306. [DOI] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg J., Chardonnet Y., Doerfler W. Integrated viral sequences in adenovirus type 12-transformed hamster cells. Cell. 1977 Jan;10(1):101–111. doi: 10.1016/0092-8674(77)90144-1. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Kersten W., Kersten H., Szybalski W. Physicochemical properties of complexes between deoxyribonucleic acid and antibiotics which affect ribonucleic acid synthesis (actinomycin, daunomycin, cinerubin, nogalamycin, chormomycin, mithramycin, and olivomycin). Biochemistry. 1966 Jan;5(1):236–244. doi: 10.1021/bi00865a031. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Young E. T., 2nd, Sinsheimer R. L. Purification and properties of intracellular lamba DNA rings. J Mol Biol. 1968 Apr 28;33(2):395–413. doi: 10.1016/0022-2836(68)90197-6. [DOI] [PubMed] [Google Scholar]
- Kirk J. T. Effect of methylation of cytosine residues on the buoyant density of DNA in caesium chloride solution. J Mol Biol. 1967 Aug 28;28(1):171–172. doi: 10.1016/s0022-2836(67)80087-1. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Khoury G. Integration of DNA tumor virus genomes. Curr Top Microbiol Immunol. 1976;73:35–65. doi: 10.1007/978-3-642-66306-2_2. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Replication and molecular recombination of T-phage. Annu Rev Microbiol. 1975;29:355–376. doi: 10.1146/annurev.mi.29.100175.002035. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Giovanella B. C., Stehlin J. S., Klein G. Tumorigenicity of human hematopoietic cell lines in athymic nude mice. Int J Cancer. 1977 Mar 15;19(3):337–344. doi: 10.1002/ijc.2910190309. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Pontén J. Classification and biological nature of established human hematopoietic cell lines. Int J Cancer. 1975 Feb 15;15(2):321–341. doi: 10.1002/ijc.2910150217. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Hyman R. W., Rapp F. Isolation of herpes simplex virus DNA from the "hirt supernatant". Virology. 1976 Dec;75(2):481–483. doi: 10.1016/0042-6822(76)90046-5. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Comparison between growth characteristics of an Epstein--Barr virus (EBV)-genome-negative lymphoma line and its EBV-converted subline in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3518–3520. doi: 10.1073/pnas.72.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMIZAWA J. I., ANRAKU N. MOLECULAR MECHANISMS OF GENETIC RECOMBINATION IN BACTERIOPHAGE. II. JOINING OF PARENTAL DNA MOLECULES OF PHAGE T4. J Mol Biol. 1964 Apr;8:516–540. doi: 10.1016/s0022-2836(64)80009-7. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA J., ANRAKU N. MOLECULAR MECHANISMS OF GENETIC RECOMBINATION IN BACTERIOPHAGE. IV. ABSENCE OF POLYNUCLEOTIDE INTERRUPTION IN DNA OF T4 AND LAMBDA PHAGE PARTICLES, WITH SPECIAL REFERENCE TO HETEROZYGOSIS. J Mol Biol. 1965 Mar;11:509–527. doi: 10.1016/s0022-2836(65)80007-9. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Oncogenic Herpes viruses. Biochim Biophys Acta. 1975 Mar 20;417(1):25–53. doi: 10.1016/0304-419x(75)90007-4. [DOI] [PubMed] [Google Scholar]



