Abstract
Background
Folate and cobalamin are essential cofactors for homocysteine (HCY) metabolism. Hyperhomocysteinemia, a multifactorial condition, may reflect B vitamin deficiency and is associated with increased risk of cardiovascular disease, thrombosis, and neurodegenerative and chronic gastrointestinal diseases in humans. Hyperhomocysteinemia has been reported in Greyhounds with suspected chronic enteropathy.
Objectives
To evaluate the frequencies of and the association between hypofolatemia and hyperhomocysteinemia in Greyhounds.
Animals
Data and serum samples from 559 Greyhounds.
Methods
Nested case‐control study. The frequency of hypofolatemia in Greyhounds was determined by a laboratory database search. The relationship between hyperhomocysteinemia (measured by gas chromatography‐mass spectrometry) and hypocobalaminemia and hypofolatemia was evaluated, and its frequency compared between healthy Greyhounds and Greyhounds with thrombosis or chronic diarrhea.
Results
Hypofolatemia was identified in 172 of 423 (41%) Greyhounds and was more common in hypo‐ than in normocobalaminemic dogs (49% vs. 35%; P = .0064). Hyperhomocysteinemia was detected in 53 of 78 (68%) of Greyhounds, being more common in hypo‐ than in normofolatemic dogs (88% vs. 59%; P = .0175). All healthy Greyhounds, 21 of 30 (70%) of dogs with chronic diarrhea and 6 of 8 (75%) of those with thrombosis, were hyperhomocysteinemic. Serum HCY concentrations were inversely correlated with serum folate concentration (ρ = −0.28; P = .0386) and were positively associated with serum albumin concentration (ρ = 0.66; P = .0022).
Conclusions and Clinical Relevance
Hyperhomocysteinemia occurs frequently in the Greyhound population. Its association with hypofolatemia suggests decreased intracellular availability of B vitamins, but the functional implications warrant further investigation. Hyperhomocysteinemia in Greyhounds potentially may serve as a spontaneous canine model to further investigate hyperhomocysteinemia in humans.
Keywords: Dog, Hypocobalaminemia, vitamin B12, vitamin B9
Abbreviations
- CI
confidence interval
- HCY
homocysteine
- IQR
interquartile range
- OR
odds ratio
- OSU
Ohio State University
- RI
reference interval
- SD
standard deviation
- TAMU
Texas A&M University
A recent large database survey indicated that hypocobalaminemia is frequently observed in Greyhounds (40% compared to 21% in dogs of other breeds).1 It is unknown whether intestinal malabsorption of cobalamin or other micronutrients (e.g, folate) as a consequence of chronic gastrointestinal disease may play a causative role for this finding in the breed. Cobalamin (vitamin B12) and folate (vitamin B9) are absorbed in the distal and proximal small intestine, respectively, and decreased concentrations of both B vitamins are usually a reflection of chronic gastrointestinal disease associated with these intestinal segments.2
In humans, hypocobalaminemia and hypofolatemia have been associated with an increase in serum concentrations of homocysteine (HCY), a sulfurated intermediate amino acid that is synthesized from dietary methionine and then either remethylated to methionine or metabolized to cysteine.3, 4, 5 The B vitamins folic acid (vitamin B9), cobalamin (vitamin B12), and pyridoxine (vitamin B6) are essential cofactors for enzymes required for HCY metabolism3, 4, 5, 6 and for redox‐methylation balance (methoxistasis).7 Hyperhomocysteinemia thus reflects a lack of intracellular availability of cobalamin, folic acid, or both for the synthesis of methionine4, 5 and represents a sensitive marker for the detection of B vitamin deficiency5, 6, 8 and perturbation of methoxistasis.7
In humans, hyperhomocysteinemia is an incompletely understood multifactorial condition.3, 4, 5 There is evidence indicating that HCY has direct toxic effects on neurons and endothelial cells5, 8 and that HCY can induce DNA‐strand damage, oxidative stress, and apoptosis.9 In line with these findings, hyperhomocysteinemia has been shown to be associated with cardiovascular disease and with increased risk of venous and arterial thrombosis.4, 5, 6 Neurodegenerative diseases (e.g, cognitive impairment, stroke), chronic gastrointestinal disease, and metabolic and endocrine disorders have also been associated with hyperhomocysteinemia in humans.4, 5, 8, 10, 11
Recently, cardiovascular and thrombotic diseases12, 13, 14, 15, 16, 17, 18, 19 and an increased frequency of hypocobalaminemia1 also have been described in Greyhounds. Furthermore, hyperhomocysteinemia in dogs with suspected chronic gastrointestinal disease is associated with hypocobalaminemia,20 which in turn has been linked to hypoalbuminemia.2, 21 Thus, hypoalbuminemia may affect systemic HCY concentrations, as has been shown previously in people11 and dogs.22 It is unknown whether low concentrations of cobalamin, folate, or both as well as hyperhomocysteinemia, are present in Greyhounds with chronic gastrointestinal disease, Greyhounds with thrombotic disease, or healthy Greyhounds.
The aims of our study were to evaluate (1) the frequency of hypofolatemia in hypocobalaminemic Greyhounds and (2) serum HCY concentrations in hypocobalaminemic and hypofolatemic Greyhounds. As a secondary aim of this study (3), serum HCY, cobalamin, and folate concentrations were evaluated in Greyhounds with chronic diarrhea or thrombotic disease as well as in a group of healthy Greyhounds. We hypothesized that hypofolatemia is frequently observed in Greyhounds and that hypocobalaminemia, hypofolatemia, or both are associated with increased HCY concentrations in this breed. We further hypothesized that, compared to healthy Greyhounds, hyperhomocysteinemia would be more common in those dogs with chronic gastrointestinal disease or thrombotic disease.
Materials and Methods
Ethics Approval
According to the guidelines of the clinical research review committee (2007–2011) and the Institutional Animal Care and Use Committee (2011–2013) at Texas A&M University (TAMU), formal ethical approval of this study and written owner consent were not needed because all dogs included in the study had been sampled during routine diagnostic investigation and no additional samples had been obtained or interventions performed for the purpose of the study.
Study Populations and Sampling
Prevalence of Hypofolatemia
Sample submissions from Greyhounds (n = 423) to the Gastrointestinal Laboratory at TAMU between March 2006 and February 2010 (48 months) for analysis of serum cobalamin and folate concentrations were retrospectively reviewed. Hypocobalaminemic Greyhounds (serum cobalamin concentration <251 ng/L; reference interval [RI], 251–908 ng/L1) were identified and investigated for the proportion of dogs with concurrent hypofolatemia (serum folate concentration <7.7 μg/L; RI, 7.7–24.4 μg/L1). Data extracted from the laboratory database included breed, age, sex, and serum concentrations of cobalamin and folate. Information about the submitting veterinarian and the name of the dog and owner was used to exclude multiple submissions from the same dog.
Frequency of Hyperhomocysteinemia
For the second part of the study, surplus serum samples from 82 Greyhounds, submitted to the Gastrointestinal Laboratory at TAMU between October 2012 and March 2013 (6 months), were used to measure serum HCY concentrations (RI, 5.0–22.1 μmol/L20) in addition to the serum concentrations of cobalamin and folate. Data extracted from the laboratory database included breed, age, sex, and the serum concentrations of cobalamin and folate. Information about the veterinarian and the name of the dog and owner was used to ensure that serial results were removed and only 1 sample included from each dog. Furthermore, all owners or veterinarians of the dogs included in this part of the study were contacted to verify the signalment for each dog.
Serum HCY Concentrations in Greyhounds with Thrombosis or Chronic Diarrhea and from Healthy Greyhounds
For the third part of the study, serum samples from Greyhounds with a history of thrombotic events (n = 8; 6 dogs with thrombotic events involving the central nervous system and 1 dog each with a thrombotic event involving a central blood vessel [aorta] or smaller peripheral vessels [blood supply of a limb]), Greyhounds with chronic diarrhea (n = 30), and healthy control Greyhounds enrolled in a blood donor program (n = 16) were collected at the Ohio State University (OSU; n = 27) and Texas A&M University (TAMU; n = 27) between January 2007 and September 2013 (80 months). Serum samples obtained from Greyhounds at OSU were shipped as 1 batch to TAMU overnight on ice packs, and all serum specimens were stored at −80°C until further analysis of serum HCY, cobalamin, and folate at the TAMU Gastrointestinal Laboratory.
Information about the Greyhounds recruited at OSU (n = 27; all 3 groups of Greyhounds) was extracted from the dogs’ medical records. Retrieval of information about the dogs recruited at TAMU (n = 27; only Greyhounds with chronic diarrhea) was by phone contact with the veterinarian and return of a completed standard study questionnaire (by fax or email) designed to verify the dog's signalment and evaluate its medical history and current health status. Health status in the control group of Greyhounds was determined on the basis of historical information and the results of a complete physical examination and routine blood work (hematology2 and serum biochemistry profile3 ).
Quantification of Serum Cobalamin, Folate, and HCY Concentrations
Serum cobalamin and folate concentrations were measured by means of validated automated solid‐phase chemiluminescence assays.4 , 5 The lower detection limits of the assays are 150 ng/L and 1.0 μg/L, respectively.23
Serum HCY concentrations were measured by use of an established and validated stable isotope dilution gas chromatography‐mass spectrometry (GC/MS) assay as previously described.24 Briefly, standard calibration solutions (200, 100, 50, 25, 12.5, 6.3, and 3.13 μmol/L) were prepared fresh daily with nonisotopic homocystine6 in 1 M HCl, and deuterium‐labeled homocystine isotope7 (100 μmol/L) served as an internal standard. After incubation of serum samples in deuterated homocystine: NaOH (3.4 mM): dithiothreitol (34% [w/v]): ddH2O (26.7: 3.3: 3.4: 66.6) for 1 hour at 37°C, HCY was extracted with a chromatography column8 packed with an ion exchange resin9 and conditioned with methanol/ddH2O. After elution of HCY with 0.4 M acetic acid in methanol, samples were vacuum‐dried in nitrogen at 64°C for 30 minutes. Homocysteine then was derivatized by silylation with N‐methyl‐N‐tert‐butyldimethylsilyl‐trifluoroacetamide: acetonitrile (50 : 50) for 30 minutes at 64°C. Samples were analyzed in a gas chromatograph10 with a mass‐selective detector,11 with samples being injected into a capillary column12 at a temperature of 250°C and helium used as carrier gas. The starting temperature was 140°C and was followed by a linear temperature ramp of 30°C/min to 300°C, then 20°C/min to 325°C, and a hold time of 62 seconds. The mass spectrometer source was operated at 230°C, and HCY and its deuterated isotope were quantified by the ions at m/z 420 and 424, respectively. All samples were extracted, derivatized, and analyzed in batches of 20 samples each. This assay has a lower detection limit of 5.0 μmol/L.
Statistical Analyses
Measurements for continuous variables were first investigated for normality of their distribution by a Shapiro‐Wilk W test. Summary statistics for continuous variables are presented as medians and interquartile ranges (IQR) for nonparametric data and as means ± standard deviations (SD) for parametric data. Categorical variables are presented as proportions or percentages.
A Fisher's exact test, with calculation of the odds ratio (OR) and 95% confidence interval (95% CI), was used to test the possibility of an association between: (1) hypocobalaminemia and concurrent hypofolatemia, (2) hyperhomocysteinemia and either hypocobalaminemia or hypofolatemia alone, and (3) hyperhomocysteinemia and concurrent hypocobalaminemia and hypofolatemia. A Mann‐Whitney U‐test was utilized to compare serum HCY concentrations in hypocobalaminemic and hypofolatemic Greyhounds with those in normocobalaminemic and normofolatemic Greyhounds.
Age and serum HCY, cobalamin, and folate concentrations were compared among the 3 groups of Greyhounds by an anova or a nonparametric Kruskal‐Wallis test, as applicable. Proportions of categorical variables were compared among the different groups of dogs by use of a chi‐square test. Serum HCY concentrations also were assessed with respect to other continuous variables. Correlation analysis was performed to test for any possible correlation between the serum concentration of HCY and the 2 B vitamins (folate and cobalamin) in all Greyhounds and between serum HCY concentrations and 14 clinicopathologic variables (RBC count, hemoglobin concentration, platelet count, WBC count, serum concentrations of BUN, creatinine, phosphorus, total protein, albumin, globulin, and cholesterol, serum Ca×P product, and serum ALT and AST activities) previously shown to have breed‐specific deviations from generic canine reference intervals25 in 19 Greyhounds by calculating a Spearman rank‐sum correlation coefficient ρ for nonparametric data. For all testing, significance was set at a P < .05, and the cutoff for statistical significance was adjusted according to the number of correlations (n = 14) from P < .05 to P < .0035 by a Bonferroni correction for multiple statistical comparisons.13 A commercially available software package14 was used for all statistical analyses.
Results
Prevalence of Hypofolatemia
In the database review, hypofolatemia was identified in 172 of the 423 serum samples (41%) from Greyhounds that were submitted for serum cobalamin and folate analysis over a 48‐month period; hypofolatemia was more frequently observed in hypocobalaminemic Greyhounds (82/168, 49%) than in normocobalaminemic Greyhounds (90/255, 35%; odds ratio [OR] [95% CI]: 1.8 [1.2–2.6]; P = .0064; Fig 1).
Figure 1.
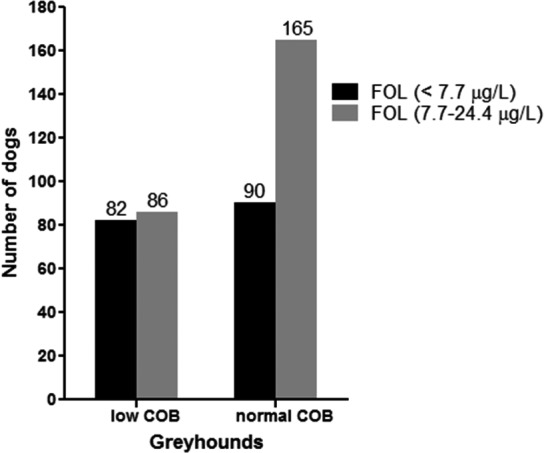
Prevalence of hypofolatemia in Greyhounds (n = 423). Shown are the proportions of hypofolatemic (n = 172, 41%; black bars) or normofolatemic Greyhounds (n = 251, 59%; gray bars) divided by concurrent hypocobalaminemia (low COB) or normocobalaminemia (normal COB).
Frequency of Hyperhomocysteinemia
Four of the 82 dogs considered for inclusion in this part of the study were identified as Italian Greyhounds and thus were excluded from further analyses. Hyperhomocysteinemia was identified in 53 of the 78 serum samples (68%) from Greyhounds that were submitted for cobalamin and folate analysis over a 6‐month period; hyperhomocysteinemia was detected in 11 of 12 (92%) hypocobalaminemic and hypofolatemic Greyhounds and in 28 of 46 (61%) normocobalaminemic and normofolatemic Greyhounds (P = .0806). Although not statistically significant, serum HCY concentrations were numerically higher in hypocobalaminemic and hypofolatemic Greyhounds (n = 12; median, 42.6 μmol/L; interquartile range [IQR], 33.7–55.0 μmol/L) compared to normocobalaminemic and normofolatemic Greyhounds (n = 46; median, 30.8 μmol/L; IQR, 15.0–56.0 μmol/L; P = .1476).
Regardless of the serum folate concentration, hyperhomocysteinemia was identified in 15 of 20 (75%) hypocobalaminemic Greyhounds and in 38 of 58 (64%) normocobalaminemic Greyhounds (P = .5808). If only serum folate concentrations were considered for the classification of dogs (regardless of the serum cobalamin concentration), hyperhomocysteinemia was identified more frequently in hypofolatemic Greyhounds (21/24, 88%) than in Greyhounds with normofolatemia (32/54, 59%; OR [95% CI], 4.8 [1.3–18.1]; P = .0175). Neither age (P = .6105) nor sex (P = .1031) had an effect on serum HCY concentrations, although serum concentrations were numerically higher in males (median, 40.9 μmol/L; IQR, 25.0–60.9 μmol/L) compared to female Greyhounds (median, 30.6 μmol/L; IQR, 13.0–53.3 μmol/L). Serum HCY concentrations were all <9.2 μmol/L (median, 8.0 μmol/L; IQR, 7.1–8.4 μmol/L) in the 4 Italian Greyhounds identified that were excluded from the study.
Comparison of Serum HCY Concentrations between Diseased Groups and Healthy Greyhounds
In the group comparison part of the study, serum HCY concentrations differed among the 3 different groups of Greyhounds (P = .0012) (Table 1), with healthy Greyhounds having significantly higher serum HCY concentrations than Greyhounds with diarrhea (P < .01) or thrombosis (P < .05; Fig 2). All healthy Greyhounds had serum HCY concentrations above the upper limit of the previously reported non‐breed‐specific RI (>22.1 μmol/L), whereas 21 of 30 (70%) of the dogs with chronic diarrhea and 6 of 8 (75%) of those with thrombosis were found to be hyperhomocysteinemic in relation to that RI.
Table 1.
Characteristics of healthy Greyhounds and Greyhounds with chronic diarrhea or thrombosis (n = 54)
| Characteristic | Group | |||
|---|---|---|---|---|
| Healthy Controls | Diarrhea | Thrombosis | P‐valuea | |
| Total number, n | 16 | 30 | 8 | – |
| Age in years, median (IQR)c | 5 (4–7)A | 8 (5–10)A,B | 10 (9–11)B | .0055b |
| Sex, male/female | 8/8 | 19/11 | 4/4 | .6159 |
| Serum HCY concentration (in μmol/L), median (IQR)c | 66.9 (46.3–83.1)A | 36.1 (21.1–54.4)B | 32.3 (26.5–41.6)B | .0012b |
| Serum folate concentration (in μg/L), median (IQR) | 7.1 (6.6–10.0) | 9.2 (5.6–13.2) | 9.7 (6.7–13.1) | .6907 |
| Serum cobalamin concentration (in ng/L), median (IQR) | 362 (331–449) | 316 (246–435) | 339 (280–408) | .2554 |
n, count; IQR, interquartile range; HCY, homocysteine.
Global P‐value.
Significant difference among the 3 groups of Greyhounds.
For each parameter, medians (IQR) not sharing a common superscript are significantly different at P < .05.
Figure 2.
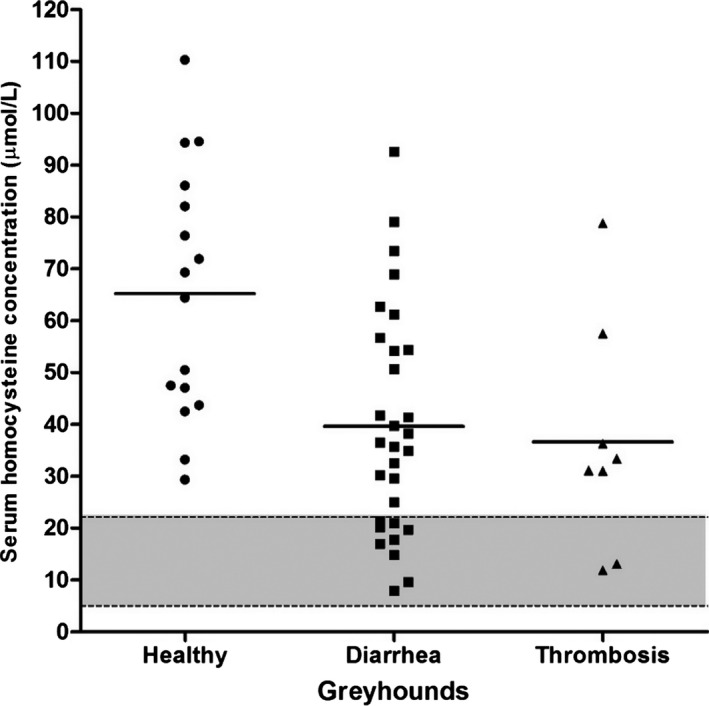
Serum homocysteine (HCY) concentrations in 3 groups of Greyhounds (n = 54). Serum HCY concentrations were significantly higher in healthy Greyhounds (mean ± sd: 65.2 ± 24.2 μmol/L) compared to those in Greyhounds with diarrhea (39.7 ± 21.7 μmol/L; P < .01) or thrombosis (36.7 ± 22.1 μmol/L; P < .05). Solid lines: means; symbols (●, ■, and ▲): serum HCY concentrations in individual dogs; gray‐shaded area between dashed lines: previously reported non‐breed‐specific reference interval (5.0–22.1 μmol/L).
Serum cobalamin and folate concentrations did not differ among the 3 groups of Greyhounds (both: P > .05). Serum cobalamin concentrations were within the RI in all healthy Greyhounds, whereas 10 of 30 (33%) of the Greyhounds with chronic diarrhea and 1 of 8 (13%) of those dogs with thrombosis were hypocobalaminemic. Hypofolatemia was associated with hypocobalaminemia (P = .0459) and was detected in 10 of 16 (63%) healthy controls, 11 of 30 (37%) Greyhounds with chronic diarrhea, and in 3 of 8 (38%) Greyhounds with thrombosis.
No correlation was observed between the serum HCY and cobalamin concentrations (P = .6008), but an inverse correlation was identified between the concentrations of HCY and folate in serum (ρ = −0.28; P = .0386) when samples from dogs of all groups were analyzed together (Fig 3). Serum HCY concentrations also were negatively correlated with age (ρ = −0.42; 95% CI, −0.62 to −0.17; P = .0020), but Greyhounds with thrombosis were significantly older in this study than those dogs in the control group (P < .01). Serum HCY concentrations were numerically higher in male dogs (n = 23; median, 56.7 μmol/L; IQR, 33.6–75.4 μmol/L) than in female dogs (n = 31; median, 35.8 μmol/L; IQR, 29.5–52.5 μmol/L), but the difference did not reach statistical significance (P = .0643).
Figure 3.
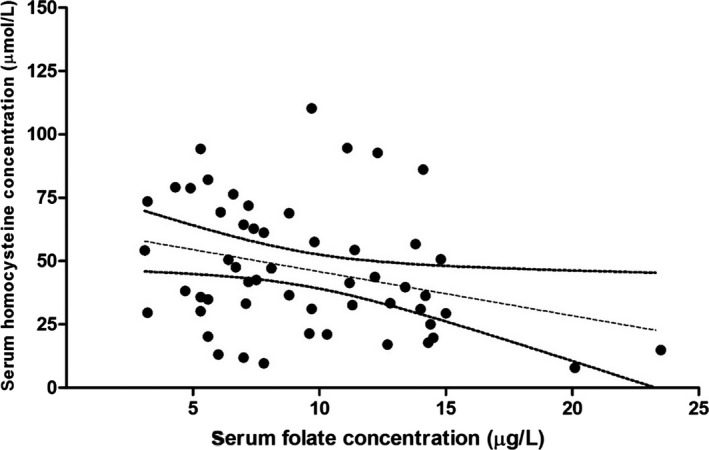
Relationship between the serum concentration of homocysteine (HCY) and folate in Greyhounds with chronic diarrhea or thrombosis (n = 38) and healthy Greyhounds (n = 16). An inverse correlation was detected between the concentration of HCY and folate in serum (Spearman ρ = −0.28; 95%CI: −0.52 to −0.01; P = .0386). Symbols (●): serum HCY and folate concentrations in individual dogs; dotted line: regression line; solid lines: 95% confidence interval of the regression line.
Relevant clinicopathologic variables in 19 of the 54 Greyhounds are summarized in Table 2. Evaluation of the relationship between serum HCY concentrations and selected clinicopathologic variables showed a positive association between the serum concentrations of HCY and albumin (ρ = 0.66; 95% CI, 0.28 to 0.86; P = .0022) but no other significant correlations (for all: P > .0035). Only 1 Greyhound (with thrombosis) was found to be hypoalbuminemic (serum albumin concentration, 2.6 g/dL; RI, 2.9–4.2 g/dL). This dog was panhypoproteinemic (serum globulin concentration, 2.0 g/dL; RI, 2.2–2.9 g/dL) and was 1 of the 2 dogs in this group with a normal serum HCY concentration. Hypoglobulinemia was found in 11 of the 19 Greyhounds (58%) from which a serum biochemistry panel was performed. Serum concentrations of HCY were not associated with serum creatinine concentrations (P = .2212).
Table 2.
Relevant clinicopathologic data in 19 Greyhounds
| Parameter | Median (IQR) | Reference Interval (RI)b | Values Below RI | Values Above RI |
|---|---|---|---|---|
| Hematologya | ||||
| RBC count (×1012/L) | 7.9 (7.3–8.3) | 4.8–8.1 | 0 (0%) | 5 (39%) |
| Hb concentration (g/dL) | 18.3 (17.9–19.5) | 12.1–18.8 | 0 (0%) | 6 (46%) |
| Platelet count (×109/L) | 171 (153–186) | 108–433 | 0 (0%) | 0 (0%) |
| WBC count (×109/L) | 5.6 (4.3–6.4) | 4.1–15.4 | 3 (23%) | 0 (0%) |
| Clinical chemistry | ||||
| BUN (mg/dL) | 21.0 (17.5–22.5) | 5.0–20.0 | 0 (0%) | 10 (53%) |
| Creatinine (mg/dL) | 1.7 (1.5–2.1) | 0.6–1.6 | 0 (0%) | 11 (58%) |
| Phosphorus (mg/dL) | 3.3 (3.1–3.7) | 3.2–8.1 | 9 (47%) | 0 (0%) |
| Ca×P product (mg2/dL2) | 33.2 (30.5–36.6) | ≤60 | – | 1 (5%) |
| Total protein (g/dL) | 5.7 (5.6–6.1) | 5.1–7.1 | 3 (16%) | 0 (0%) |
| Albumin (g/dL) | 3.6 (3.3–3.7) | 2.9–4.2 | 1 (5%) | 0 (0%) |
| Globulin (g/dL) | 2.1 (1.9–2.3) | 2.2–2.9 | 11 (58%) | 0 (0%) |
| Cholesterol (mg/dL) | 174 (144–190) | 80–315 | 0 (0%) | 0 (0%) |
| ALT activity (U/L) | 46 (39–62) | 10–55 | 0 (0%) | 7 (37%) |
| AST activity (U/L) | 42 (32–49) | 12–40 | 0 (0%) | 10 (53%) |
IQR, interquartile range; Hb, hemoglobin, RBC, red blood cell, BUN, blood urea nitrogen, ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Only available for n = 13 dogs.
OSU Clinical pathology RI (not breed‐specific).
Discussion
Our study evaluated serum folate, cobalamin, and HCY concentrations in a large population of Greyhounds. Similar to hypocobalaminemia,1 hypofolatemia also was found to occur frequently in Greyhounds, with combined B hypovitaminoses being more commonly seen than isolated hypocobalaminemia or hypofolatemia. Although this finding may reflect small intestinal malabsorption of both B vitamins as a result of chronic disease,2 increased demand for these vitamins, or the possibility that systemic concentrations of these vitamins may not reflect their intracellular levels, is a possible alternative explanation.
Although hyperhomocysteinemia was observed in hypofolatemic and hypocobalaminemic Greyhounds in our study, it also was present in Greyhounds with normal serum concentrations of both B vitamins. Our study did identify an association between hypofolatemia and hyperhomocysteinemia, whereas hypocobalaminemia and hyperhomocysteinemia were not statistically related. These findings also suggest that hyperhomocysteinemia in Greyhounds occurs as a consequence of the lack of these 2 B vitamins, in particular vitamin B9 (folate), at the intracellular level rather than merely the systemic level.
In keeping with the finding that hyperhomocysteinemia is associated with folate status and oxidative stress,4, 6, 7 the inverse correlation observed in our study between serum concentrations of HCY and folate, but not cobalamin, agrees with findings in people with hypertension,26 cerebral venous thrombosis,27 epilepsy,28 and inflammatory bowel disease.29, 30 However, these findings are in contrast with results in dogs with systemic inflammatory response syndrome or sepsis,23 dogs with chronic enteropathy,31 and in some people with inflammatory bowel disease.11
The relationship with hypofolatemia in our study suggests that hyperhomocysteinemia in Greyhounds could be a cause or consequence of pathologic conditions (e.g, decreased methylation capacity). However, whether the hyperhomocysteinemia is associated with an increased risk of clinically relevant pathology (such as an increased risk of vascular dysfunction,32 thrombosis, or other cardiovascular disease) or represents a peculiarity in the Greyhound breed, similar to other breed‐specific clinicopathological characteristics likely associated with the unique physiology of this breed,25, 33 cannot be evaluated by the results of our study and requires further investigation. Thus, the clinical relevance and potential need for B vitamin supplementation (particularly folic acid) in Greyhounds requires further study because clinical trials of HCY‐lowering treatment in people produced mixed results and did not indicate a clear clinical benefit for all outcomes evaluated.4, 5, 7, 8 Nevertheless, the finding of hyperhomocysteinemia in all healthy Greyhounds in this study suggests that this breed might be explored as a novel spontaneous canine model to further investigate hyperhomocysteinemia in humans. However, further research is needed to characterize this model.
The transsulfuration (vitamin B6‐dependent) pathway presents a way to compensate for decreased remethylation of HCY to methionine. However, vitamin B6 concentrations or the individual methylation potential7 was not determined as part of our investigation. Whether the transsulfuration pathway plays a role in the hyperhomocysteinemia observed in Greyhounds warrants further study. Also, evaluation of potentially synergistic genetic variations (e.g, enzymes involved in the metabolism of HCY: methylenetetrahydofolate reductase, methionine synthase, methionine synthase reductase, or cystathionine β‐synthase and their cofactors) as well as nutritional (e.g, dietary protein content, supplementation) and environmental factors is warranted in Greyhounds with hyperhomocysteinemia.
Despite relative hypocoagulability,25, 34 the activity of plasma antithrombin has been shown to be decreased and von Willebrand factor collagen binding to be increased in some Greyhounds,25 potentially rendering homeostasis a delicate balance between a state of “downregulated” or “upregulated” coagulation in this breed. A procoagulant effect of hyperhomocysteinemia (e.g, induction of tissue factor, activation of factor V, and inhibition of protein C activation and von Willebrand factor secretion) has been identified in people,35, 36, 37, 38 but routine hemostasis or platelet function testing, thromboelastograms, or evaluation of specific coagulation factors or coagulation inhibitors were not included in this study. Thus, the possibility of an association between hyperhomocysteinemia and markers of coagulation, fibrinolysis, and endothelial cell activation and vascular dysfunction in Greyhounds needs to be further investigated. Also, a possible effect of systemic arterial hypertension on serum HCY concentrations in Greyhounds has not been evaluated and warrants further study.
An unexpected finding in our study was a higher frequency of hyperhomocysteinemia in healthy Greyhounds compared to Greyhounds with chronic diarrhea or thrombotic disease. The positive correlation of serum HCY with serum albumin concentration in Greyhounds observed in our study agrees with a previous investigation by this group in dogs with hypocobalaminemia suspected to be associated with chronic enteropathies22 and in people with end‐stage renal disease39 or nephrotic syndrome,40 but it contrasts with findings in people with inflammatory bowel disease.11 A possible explanation for these findings is that the majority (approximately 90%) of systemic total HCY has been shown to be bound to albumin, with the remainder being bound to globulins.41 Thus, protein‐losing diseases (e.g, protein‐losing enteropathy or nephropathy), which were also shown to create a hypercoagulable state,42, 43 may be associated with a lower degree of hyperhomocysteinemia than expected. Nevertheless, the percentage of hyperhomocysteinemic Greyhounds with chronic diarrhea in this study (70%) was similar to that in people in inflammatory bowel disease (50–60%).11, 44
In humans, hyperhomocysteinemia also can be associated with decreased glomerular filtration rate in patients with renal insufficiency.5, 45 Although staging of possible chronic kidney disease was not included in our study, a correlation between serum HCY and serum creatinine concentration was not observed, and healthy Greyhounds previously were reported to have increased glomerular filtration rates.46 Furthermore, our study showed that both age and sex do not appear to affect serum HCY concentrations in Greyhounds, which is dissimilar to studies in people where a predominance of hyperhomocysteinemia has been reported in older people and in males.5, 8 However, the possibility must be considered that the significant age differences seen among the 3 groups of Greyhounds may have had a confounding effect on the relationship between aging and serum HCY concentrations. Also, with the large percentage of neutered Greyhounds included in our study (approximately 90%), an effect of sex hormones on serum HCY concentrations may have been masked. A high frequency of concurrent hypoglobulinemia and normoalbuminemia as seen in our study also is consistent with previous reports in Greyhounds.33
We acknowledge that our study suffered from some limitations. First, the frequency of hypofolatemia, and also hypocobalaminemia, may have been overestimated because of selection bias with the use of data and samples from a laboratory database. The specimens from Greyhounds had been submitted to the laboratory for cobalamin and folate analysis, but the specific indication in individual dogs could not be evaluated. Thus, the proportion of hypofolatemic or hypocobalaminemic dogs or both may not reflect the general Greyhound population. Second, the possibility of B vitamin supplementation in individual dogs cannot be excluded. Third, different underlying conditions leading to thrombosis (e.g, protein‐losing diseases) could present a confounding factor for the group of dogs with thrombotic events. Consequently, the correlation with hyperhomocysteinemia in this study could be coincidental. Fourth, the intra‐ and interindividual biologic variation as well as the minimum critical difference or reference change value for serum HCY concentrations has not been evaluated and is unknown in dogs. Lastly, quantification of serum methylmalonic acid would have been useful to evaluate the cellular availability of cobalamin but could not be performed in this retrospective study because of the poor long‐term stability of methylmalonic acid in serum samples.
In conclusion, hypofolatemia in Greyhounds was associated with hypocobalaminemia, and increased serum HCY concentrations were observed in hypofolatemic and hypocobalaminemic Greyhounds, but also in Greyhounds with normal serum concentrations of both B vitamins, suggesting a lack of these vitamins at the intracellular rather than the serum concentration. The functional implication of these findings in Greyhounds warrants further study. Healthy Greyhounds had higher serum HCY concentrations than Greyhounds with chronic diarrhea or thrombotic disease, and all healthy Greyhounds were hyperhomocysteinemic, suggesting that hyperhomocysteinemia in healthy Greyhounds might represent a novel spontaneous canine model to further investigate hyperhomocysteinemia in humans. Additional studies are warranted to characterize the potential of this model.
Acknowledgments
Serum samples included in the study were collected at the Gastrointestinal Laboratory at Texas A&M University or the College of Veterinary Medicine at the Ohio State University. Data analysis and manuscript writing were carried out at the College of Veterinary Medicine at the University of Leipzig, Texas A&M University, and the Ohio State University. We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was not supported by a grant or otherwise.
Part of the data were presented at the Annual Congress of the 23rd European College of Veterinary Internal Medicine—Companion Animals (ECVIM‐CA), Liverpool, UK (12–14 September, 2013); and the 33rd Annual Meeting of the American College of Veterinary Internal Medicine (ACVIM), Indianapolis, IN, USA (June, 3–6, 2015).
Footnotes
Gastrointestinal Laboratory at Texas A&M University (available at: http://vetmed.tamu.edu/gilab/service/assays/b12folate)
Cell‐Dyn 3500 Automated Hematology Analyzer, Abbott Diagnostics, Lake Forest, IL
Cobas® c501 Clinical Chemistry Analyzer, Roche Diagnostics, Indianapolis, IN
Immulite®2000, Vitamin B12, Siemens Healthcare Diagnostics Inc., Deerfield, IL
Immulite®2000, Folic Acid, Siemens Healthcare Diagnostics Inc., Deerfield, IL
l‐homocystine, Sigma Chemical Co, St. Louis, MO
DL‐Homocystine‐3,3,3,3,4,4,4,4‐d8, C/D/N Isotopes Inc, Pointe‐Claire, QC, Canada
Poly‐Prep® disposable chromatography column, Bio‐Rad, Hercules, CA
AG®1‐MP ion exchange resin, Bio‐Rad, Hercules, CA
Agilent model 7890A gas chromatograph, Agilent Technologies, Santa Clara, CA
Agilent 5975C Series mass‐selective detector, Agilent Technologies, Santa Clara, CA
DB‐1 ms 100% dimethylpolysiloxane column, Agilent Technologies, Santa Clara, CA
Bonferroni correction for multiple statistical comparisons (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm)
GraphPad Prism v6, GraphPad Software, La Jolla, CA
References
- 1. Grützner N, Cranford SM, Norby B, et al. Evaluation of serum cobalamin concentrations in dogs of 164 breeds (2006–2010). J Vet Diagn Invest 2012;24:1105–1114. [DOI] [PubMed] [Google Scholar]
- 2. Berghoff N, Steiner JM. Laboratory tests for the diagnosis and management of chronic canine and feline enteropathies. Vet Clin Small Anim 2011;41:311–328. [DOI] [PubMed] [Google Scholar]
- 3. Duthie SJ, Horgan G, de Roos B, et al. Blood folate status and expression of proteins involved in immune function, inflammation, and coagulation: Biochemical and proteomic changes in the plasma of humans in response to long‐term synthetic folic acid supplementation. J Proteome Res 2010;9:1941–1950. [DOI] [PubMed] [Google Scholar]
- 4. Cullen CE, Carter GT, Weiss MD, et al. Hypohomocysteinemia: A potentially treatable cause of peripheral neuropathology? Phys Med Rehabil Clin N Am 2012;23:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Lippi G, Plebani M. Hyperhomocysteinemia in health and disease: Where we are now, and where do we go from here? Clin Chem Lab Med 2012;50:2075–2080. [DOI] [PubMed] [Google Scholar]
- 6. Stanger O, Herrmann W, Pietrzik K, et al. DACH‐LIGA homocysteine (German, Austrian and Swiss homocysteine society): Consensus paper on the rational clinical use of homocysteine, folic acid and B‐vitamins in cardiovascular and thrombotic diseases: Guidelines and recommendations. Clin Chem Lab Med 2003;41:1392–1403. [DOI] [PubMed] [Google Scholar]
- 7. Joseph J, Loscalzo J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013;5:3235–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Folstein M, Liu T, Peter I, et al. The homocysteine hypothesis of depression. Am J Psychiatry 2007;164:861–867. [DOI] [PubMed] [Google Scholar]
- 9. Tiemeier H, van Tuijl HR, Hofman A, et al. Vitamin B12, folate, and homocysteine in depression: The Rotterdam study. Am J Psychiatry 2002;159:2099–2101. [DOI] [PubMed] [Google Scholar]
- 10. Coll P, Guttormsen AB, Berstad A. Gastrointestinal disease with elevated plasma homocysteine level. Tidsskr Nor Laegeforen 1999;119:3577–3579. [PubMed] [Google Scholar]
- 11. Erzin Y, Uzun H, Celik AF, et al. Hyperhomocysteinemia in inflammatory bowel disease patients without past intestinal resections: Correlations with cobalamin, pyridoxine, folate concentrations, acute phase reactants, disease activity, and prior thromboembolic complications. J Clin Gastroenterol 2008;42:482–486. [DOI] [PubMed] [Google Scholar]
- 12. Baines EA, Watson PJ, Stidworthy MF, Herrtage ME. Gross pulmonary thrombosis in a Greyhound. J Small Anim Pract 2001;42:448–452. [DOI] [PubMed] [Google Scholar]
- 13. Lord LK, Yaissle JE, Marin L, Couto CG. Results of a web‐based healthy survey of retired racing Greyhounds. J Vet Intern Med 2007;21:1243–1250. [DOI] [PubMed] [Google Scholar]
- 14. Fonfara S, de la Heras Alegret L, German AJ, et al. Underlying diseases in dogs referred to a veterinary teaching hospital because of dyspnea: 229 cases (2003–2007). J Am Vet Med Assoc 2011;239:1219–1224. [DOI] [PubMed] [Google Scholar]
- 15. Lowrie M. Vestibular disease: Diseases causing vestibular signs. Compend Contin Educ Vet 2012;34:E2. [PubMed] [Google Scholar]
- 16. Major AC, Caine A, Rodriguez SB, Cherubini GB. Imaging diagnosis—magnetic resonance imaging findings in a dog with sequential brain infarction. Vet Radiol Ultrasound 2012;53:576–580. [DOI] [PubMed] [Google Scholar]
- 17. Surman S, Couto CG, DiBartola SP, Chew DJ. Arterial blood pressure, proteinuria, and renal histopathology in clinically healthy retired racing greyhounds. J Vet Intern Med 2012;26:1320–1329. [DOI] [PubMed] [Google Scholar]
- 18. Innerå M. Cutaneous vasculitis in small animals. Vet Clin North Am Small Anim Pract 2013;43:113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kent M, Glass EN, Haley AC, et al. Ischemic stroke in Greyhounds: 21 cases (2007–2013). J Am Vet Med Assoc 2014;245:113–117. [DOI] [PubMed] [Google Scholar]
- 20. Grützner N, Heilmann RM, Stupka KC, et al. Serum homocysteine and methylmalonic acid concentrations in Chinese Shar‐Pei dogs with cobalamin deficiency. Vet J 2013;197:420–426. [DOI] [PubMed] [Google Scholar]
- 21. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 22. Grützner N, Suchodolski JS, Steiner JM. Relationship between cobalamin‐dependent metabolites and both serum albumin and alpha1‐proteinase inhibitor concentrations in hypocobalaminemic dogs of 7 different breeds. Vet Clin Pathol 2014;43:561–566. [DOI] [PubMed] [Google Scholar]
- 23. Patterson BE, Barr JW, Fosgate GT, et al. Homocysteine in dogs with systemic inflammatory response syndrome. J Small Anim Pract 2013;54:620–624. [DOI] [PubMed] [Google Scholar]
- 24. Stabler SP, Marcell PD, Podell ER, Allen RH. Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography‐mass spectrometry. Anal Biochem 1987;162:185–196. [DOI] [PubMed] [Google Scholar]
- 25. Zaldívar‐López S, Marín LM, Iazbik MC, et al. Clinical pathology of Greyhounds and other sighthounds. Vet Clin Pathol 2011;40:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scazzone C, Bono A, Tornese F, et al. Correlation between low folate levels and hyperhomocysteinemia, but not with vitamin B12 in hypertensive patients. Ann Clin Lab Sci 2014;44:286–290. [PubMed] [Google Scholar]
- 27. Nagaraja D, Noone ML, Bharatkumar VP, Christopher R. Homocysteine, folate and vitamin B12 in puerperal cerebral venous thrombosis. J Neurol Sci 2008;272:43–47. [DOI] [PubMed] [Google Scholar]
- 28. Eldeen ON, Eldayem SMA, Shatla RH, et al. Homocysteine, folic acid and vitamin B12 levels in serum of epileptic children. Egypt J Med Hum Genet 2012;13:275–280. [Google Scholar]
- 29. Papa A, De Stefano V, Danese S, et al. Hyperhomocysteinemia and prevalence of polymorphisms of homocysteine metabolism‐related enzymes in patients with inflammatory bowel disease. Am J Gastroenterol 2001;96:2677–2682. [DOI] [PubMed] [Google Scholar]
- 30. Akbulut S, Altiparmak E, Topal F, et al. Increased levels of homocysteine in patients with ulcerative colitis. World J Gastroenterol 2010;16:2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossi G, Breda S, Giordano A, et al. Association between hypocobalaminaemia and hyperhomocysteinaemia in dogs. Vet Rec 2013;172:365. [DOI] [PubMed] [Google Scholar]
- 32. Martinez JT, Rogers LK, Kellogg C, et al. Plasma vasoprotective eicosanoid concentrations in healthy Greyhounds and non‐Greyhound dogs. J Vet Intern Med 2016;30:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fayos M, Couto G, Iazbik MC, Wellman ML. Serum protein electrophoresis in retired racing Greyhounds. Vet Clin Pathol 2005;34:397–400. [DOI] [PubMed] [Google Scholar]
- 34. Vilar P, Couto CG, Westendorf N, et al. Thromboelastographic tracings in retired racing Greyhounds and Non‐Greyhound dogs. J Vet Intern Med 2008;22:374–379. [DOI] [PubMed] [Google Scholar]
- 35. Rodgers GM, Kane WH. Activation of endogenous factor V by a homocysteine‐induced vascular endothelial cell activator. J Clin Invest 1986;77:1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lentz SR, Sadler JE. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J Clin Invest 1991;88:1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lentz SR, Sadler JE. Homocysteine inhibits von Willebrand factor processing and secretion by preventing transport from the endoplasmic reticulum. Blood 1993;81:683–689. [PubMed] [Google Scholar]
- 38. Fryer RH, Wilson BD, Gubler DB, et al. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler Thromb 1993;13:1327–1333. [DOI] [PubMed] [Google Scholar]
- 39. Suliman ME, Stenvinkel P, Bárány P, et al. Hyperhomocysteinemia and its relationship to cardiovascular disease in ESRD: Influence of hypoalbuminemia, malnutrition, inflammation and diabetes mellitus. Am J Kidney Dis 2003;41(3Suppl1):S89–S95. [DOI] [PubMed] [Google Scholar]
- 40. Aminzadeh MA, Gollapudi P, Vaziri ND. Effect of nephrotic syndrome on homocysteine metabolism. Nephrol Dial Transplant 2011;26:1244–1247. [DOI] [PubMed] [Google Scholar]
- 41. Hortin GL, Seam N, Hoehn GT. Bound homocysteine, cysteine, and cysteinylglycine distribution between albumin and globulins. Clin Chem 2006;52:2258–2264. [DOI] [PubMed] [Google Scholar]
- 42. Goodwin LV, Goggs R, Chan DL, Allenspach K. Hypercoagulability in dogs with protein‐losing enteropathy. J Vet Intern Med 2011;25:273–277. [DOI] [PubMed] [Google Scholar]
- 43. Lennon EM, Hanel RM, Walker JM, Vaden SL. Hypercoagulability in dogs with protein‐losing nephropathy as assessed by thromboelastography. J Vet Intern Med 2013;27:462–468. [DOI] [PubMed] [Google Scholar]
- 44. Casella G, Antonelli E, Di Bella C, et al. Hyperhomocysteinemia in patients with Crohn's disease. Tech Coloproctol 2013;17:497–500. [DOI] [PubMed] [Google Scholar]
- 45. Wu CC, Zheng CM, Lin YF, et al. Role of homocysteine in end‐stage renal disease. Clin Biochem 2012;45:1286–1294. [DOI] [PubMed] [Google Scholar]
- 46. Drost WT, Couto CG, Fischetti AJ, et al. Comparison of glomerular filtration rate between Greyhounds and non‐Greyhound dogs. J Vet Intern Med 2006;20:544–546. [DOI] [PubMed] [Google Scholar]


