Abstract
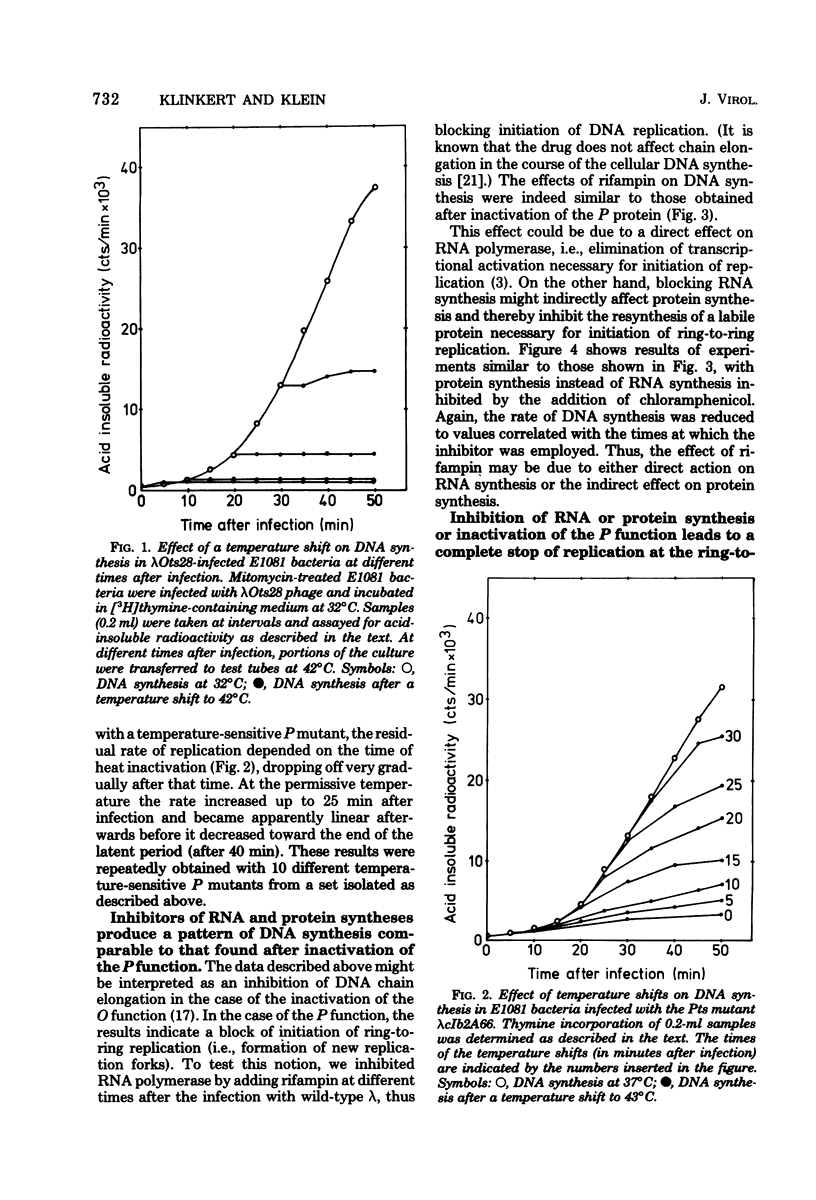
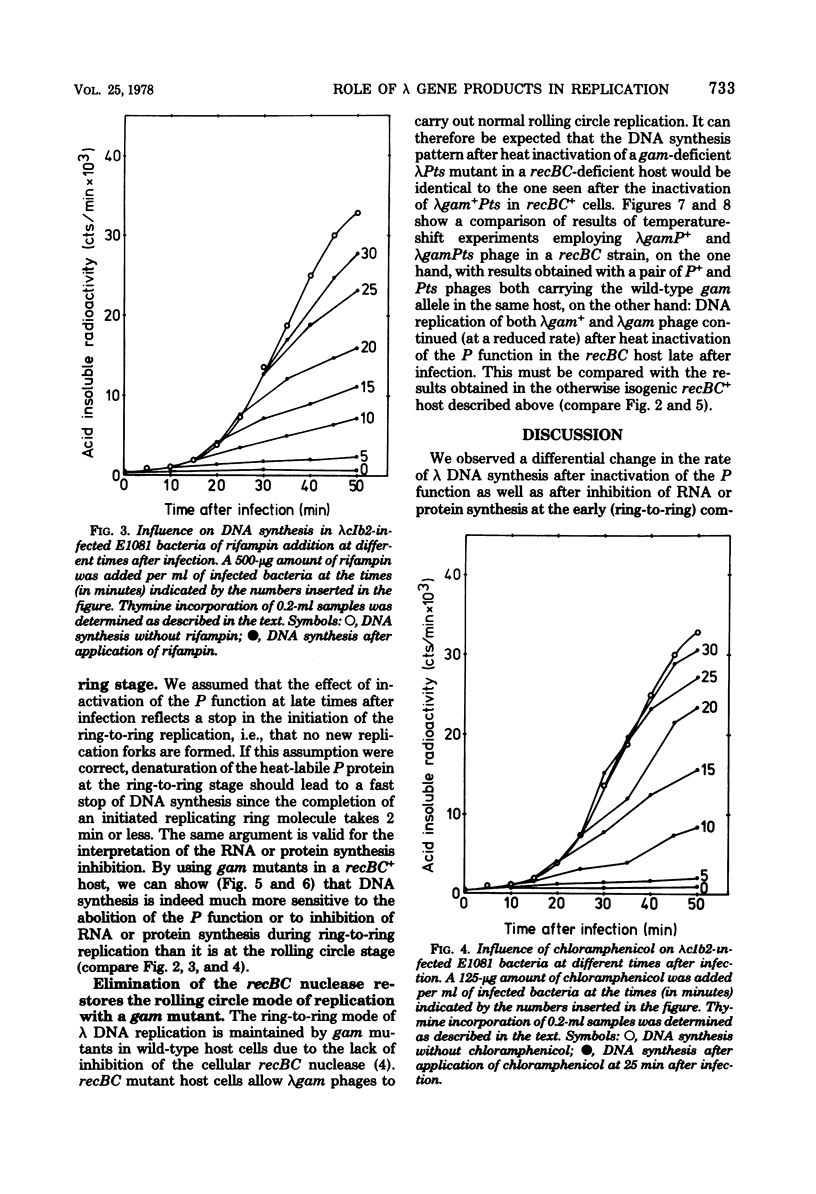
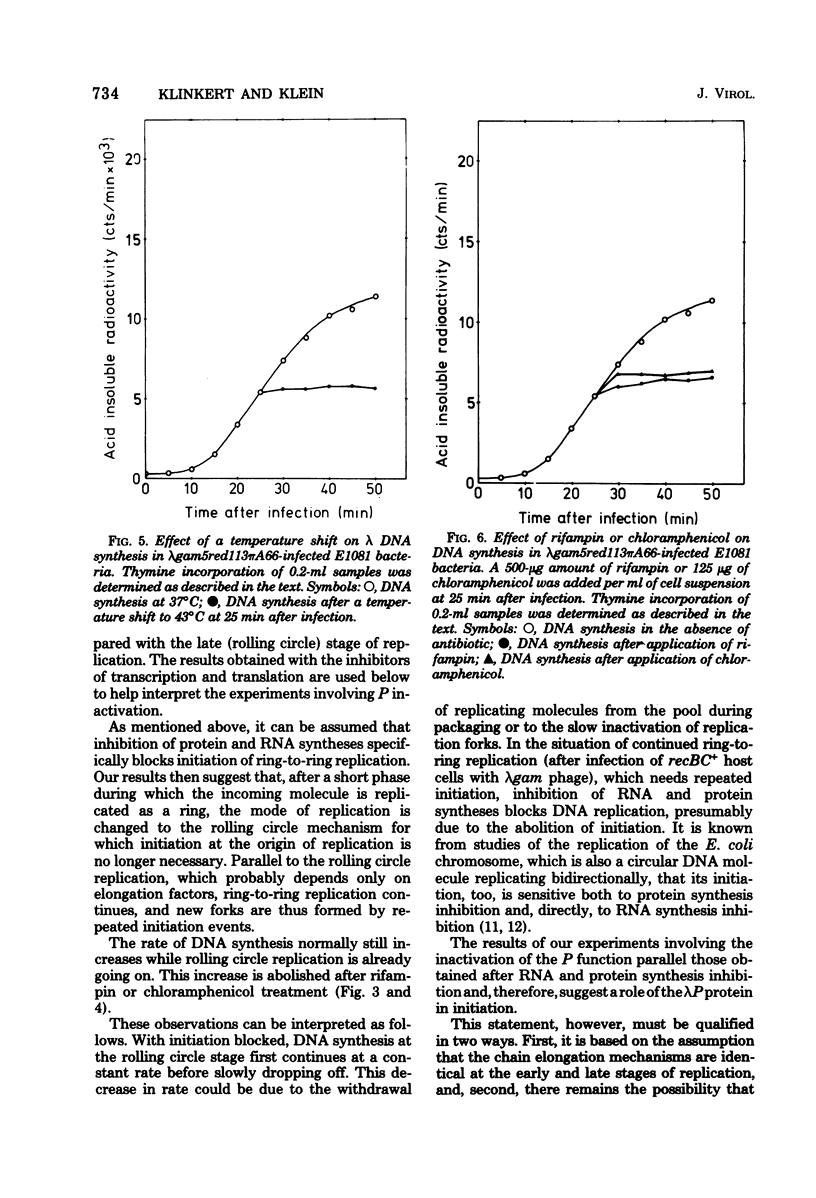
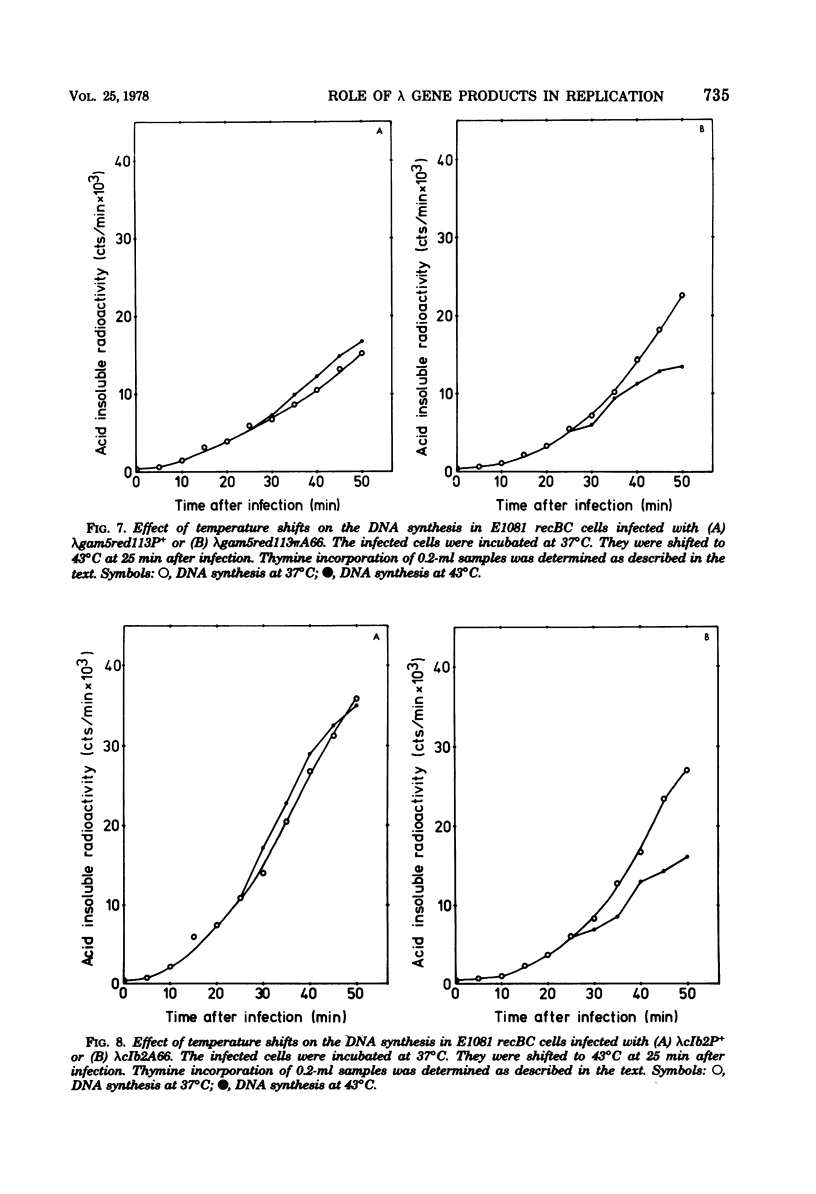
Ring-to-ring (early) replication of bacteriophage lambda DNA was blocked after heat inactivation of the P protein. Rolling circle (late) replication continued for several rounds at the rate reached when the temperature shift was carried out. The same differential effect was observed after inhibition of RNA or protein synthesis during the two different phases of replication. In contrast, inactivation of the O protein resulted in a fast stop of lambda DNA synthesis at early and late times after infection. The results were consistent with the following interpretations. (i) The lambda P gene product plays a role in the initiation of the ring-to-ring replication. (ii) Ring-to-ring replication continues parallel to rolling circle replication, possibly diminishing with time after infection. (iii) The O function is stable in and necessary for the structural integrity of an elongation complex. It is unstable in free form and probably released from such a replication complex after each round of replication at the ring-to-ring stage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D., Sueoka N. Studies on the late replication of phage lambda: rolling-circle replication of the wild type and a partially suppressed strain, Oam29 Pam80. J Mol Biol. 1975 Oct 25;98(2):305–320. doi: 10.1016/s0022-2836(75)80120-3. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Shaw B. D., Smith M. G. Two stages in the replication of bacteriophage lambda DNA. Biochim Biophys Acta. 1969 Dec 16;195(2):494–505. doi: 10.1016/0005-2787(69)90656-x. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Klein A., Powling A. Initiation of lambda DNA replication in vitro. Nat New Biol. 1972 Sep 20;239(90):71–73. doi: 10.1038/newbio239071a0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Greenstein M., Skalka A. The circle mode of replication of bacteriophage lambda: the role of covalently closed templates and the formation of mixed catenated dimers. J Mol Biol. 1976 May 25;103(3):537–562. doi: 10.1016/0022-2836(76)90216-3. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Role of genes O and P in the replication of bacteriophage lambda DNA. J Mol Biol. 1975 May 25;94(3):385–396. doi: 10.1016/0022-2836(75)90209-0. [DOI] [PubMed] [Google Scholar]
- Takahaski S. Physiological transition of a coliphage lambda DNA replication. Biochim Biophys Acta. 1975 Jul 7;395(3):306–313. [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt W. M., Inokuchi H. Stability of lambda O and P replication functions. Virology. 1974 Mar;58(1):313–315. doi: 10.1016/0042-6822(74)90168-8. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. NOVEL Escherichia coli dnaB mutant: direct involvement of the dnaB252 gene product in the synthesis of an origin-ribonucleic acid species during initiaion of a round of deoxyribonucleic acid replication. J Bacteriol. 1977 Mar;129(3):1476–1486. doi: 10.1128/jb.129.3.1476-1486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



