Abstract
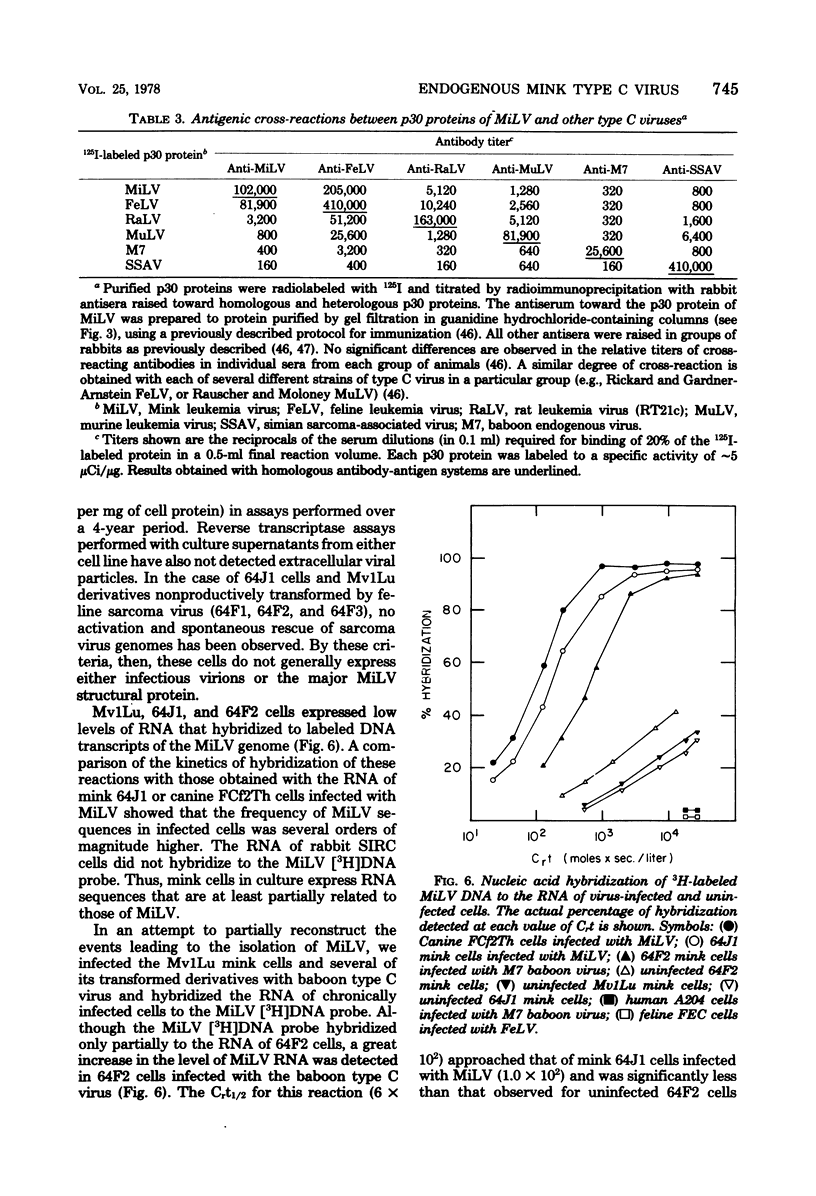
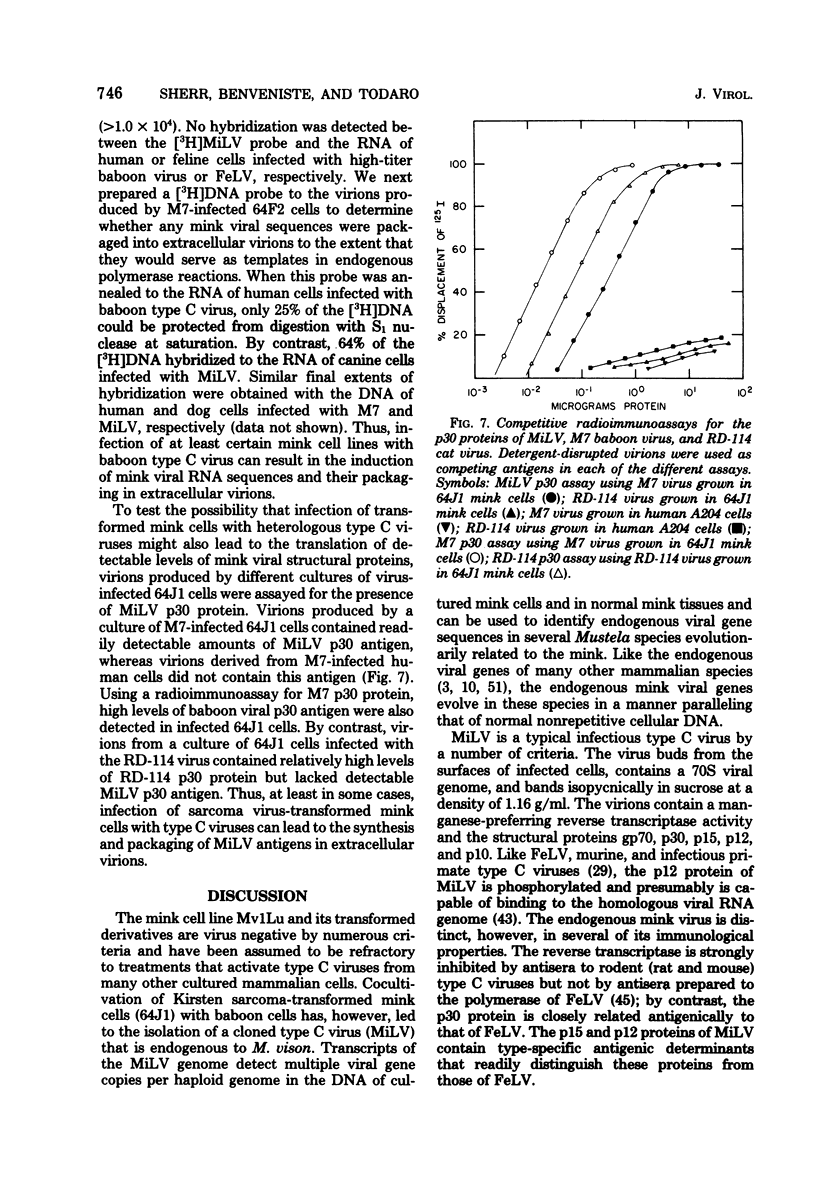
A previously described type virus stock (designated PP-1R), isolated by cocultivating baboon cells with mink cells transformed by Kirsten sarcoma virus (64J1), has been further cloned and characterized. End point-diluted stocks of PP-1R have been obtained that are free of focus-forming activity and lack both Kirsten sarcoma and primate type C viral sequences. Nucleic acid hybridization experiments show that the cloned virus (MiLV) is an endogenous, genetically transmitted virus of the mink (Mustela vison). MiLV replicates in canine, feline, and 64J1 mink cells but not in an untransformed mink cell line. Multiple viral gene copies can be detected in the DNA of normal mink cells in culture and in normal mink tissues; related endogenous viral genes are also detected in several related Mustela species. The virus codes for a p30 protein very closely related antigenically to that of feline leukemia virus but contains p15 and p12 proteins that are antigenically distinct. The mink cell line, Mv1Lu, and its Kirsten sarcoma-transformed derivatives, 64J1, express relatively low levels of type C viral RNA related to MiLV and normally do not produce detectable levels of MiLV p30 protein or complete, infectious viral particles. Infection of sarcoma virus-transformed mink cells with baboon type C virus, however, can augment the level of expression of endogenous mink viral RNA and can result in the synthesis and packaging of mink viral RNA and p30 antigen in extracellular virions. Since the Mv1Lu cell line and its tranformed derivatives have become widely used in studies of retroviruses, the possibility of activating endogenous mink viral genes should be considered by investigators working with these cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Sherr C. J., Todaro G. J. Isolation of a type C virus (FS-1) from the European Wildcat (Felis sylvestris). Virology. 1975 Jul;66(1):117–127. doi: 10.1016/0042-6822(75)90183-x. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E. Induction of GIX antigen and gross cell surface antigen after infection by ecotropic and xenotropic murine leukemia viruses in vitro. J Virol. 1976 Dec;20(3):545–554. doi: 10.1128/jvi.20.3.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal B. K., McAllister R. M., Gardner M. B., Roy-Burman P. Comparative studies on the structural phosphoproteins of mammalian type C viruses. J Virol. 1975 Jul;16(1):123–131. doi: 10.1128/jvi.16.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Peeples P. T., Gerwin B. I., Papageorge A. G., Smith S. G. Murine sarcoma virus defectiveness. Viral polymerase expression murine and nonmurine host cells transformed by S+L-type murine sarcoma virus. Virology. 1975 Oct;67(2):344–355. doi: 10.1016/0042-6822(75)90436-5. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C. Endogenous ecotropic mouse type C viruses deficient in replication and production of XC plaques. J Virol. 1976 May;18(2):411–417. doi: 10.1128/jvi.18.2.411-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnic E. M., Goldberg R. J., Parks W. P. A biochemical and genetic analysis of mammalian RNA-containing sarcoma viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):885–895. doi: 10.1101/sqb.1974.039.01.103. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Goldberg R. J., Williams D. Characterizatiion of rat genetic sequences of Kirsten sarcoma virus: distinct class of endogenous rat type C viral sequences. J Virol. 1976 May;18(2):559–566. doi: 10.1128/jvi.18.2.559-566.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Maryak J., Vass W., Goldberg R. J., Parks W. P. Type C particle-positive and type C particle-negative rat cell lines: characterization of the coding capacity of endogenous sarcoma virus-specific RNA. J Virol. 1976 Dec;20(3):570–582. doi: 10.1128/jvi.20.3.570-582.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Benveniste R. E., Lieber M. M., Todaro G. J. Type C viruses from Kirsten sarcoma-transformed mink cells co-cultivated with primate cells and expressing p30 antigens related to feline leukemia virus. J Virol. 1976 Aug;19(2):346–358. doi: 10.1128/jvi.19.2.346-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Benveniste R. E., Todaro G. J. Interspecies antigenic determinants of the reverse transcriptases and p30 proteins of mammalian type C viruses. J Virol. 1975 Jun;15(6):1440–1448. doi: 10.1128/jvi.15.6.1440-1448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Todaro G. J. Radioimmunoassay of the major group specific protein of endogenous baboon type C viruses: relation to the RD-114-CCC group and detection of antigen in normal baboon tissues. Virology. 1974 Sep;61(1):168–181. doi: 10.1016/0042-6822(74)90252-9. [DOI] [PubMed] [Google Scholar]
- Sliski A. H., Essex M., Meyer C., Todaro G. Feline oncornavirus-associated cell membrane antigen: expression in transformed nonproducer mink cells. Science. 1977 Jun 17;196(4296):1336–1339. doi: 10.1126/science.194310. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Golub M. M., Stephenson J. R., Aaronson S. A. Distribution and expression in mammals of genes ralated to an endogenous type C RNA virus of Odocoileus hemionus. J Virol. 1977 Jul;23(1):1–9. doi: 10.1128/jvi.23.1.1-9.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. E., Vasington P. J., Petricciani J. C., Hopps H. E., Lorenz D. E., Kadanka Z. Development and characterization of cell lines from subhuman primates. In Vitro. 1973 Mar-Apr;8(5):333–341. doi: 10.1007/BF02619057. [DOI] [PubMed] [Google Scholar]



