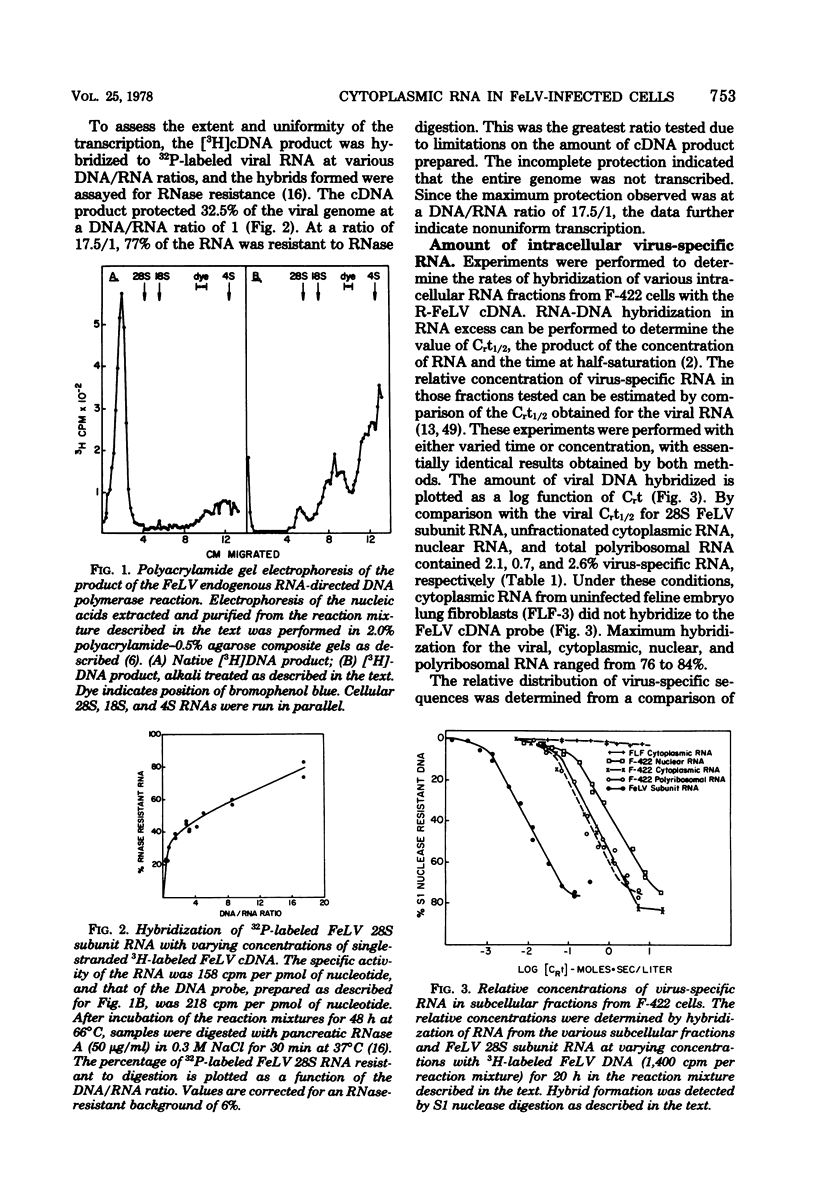
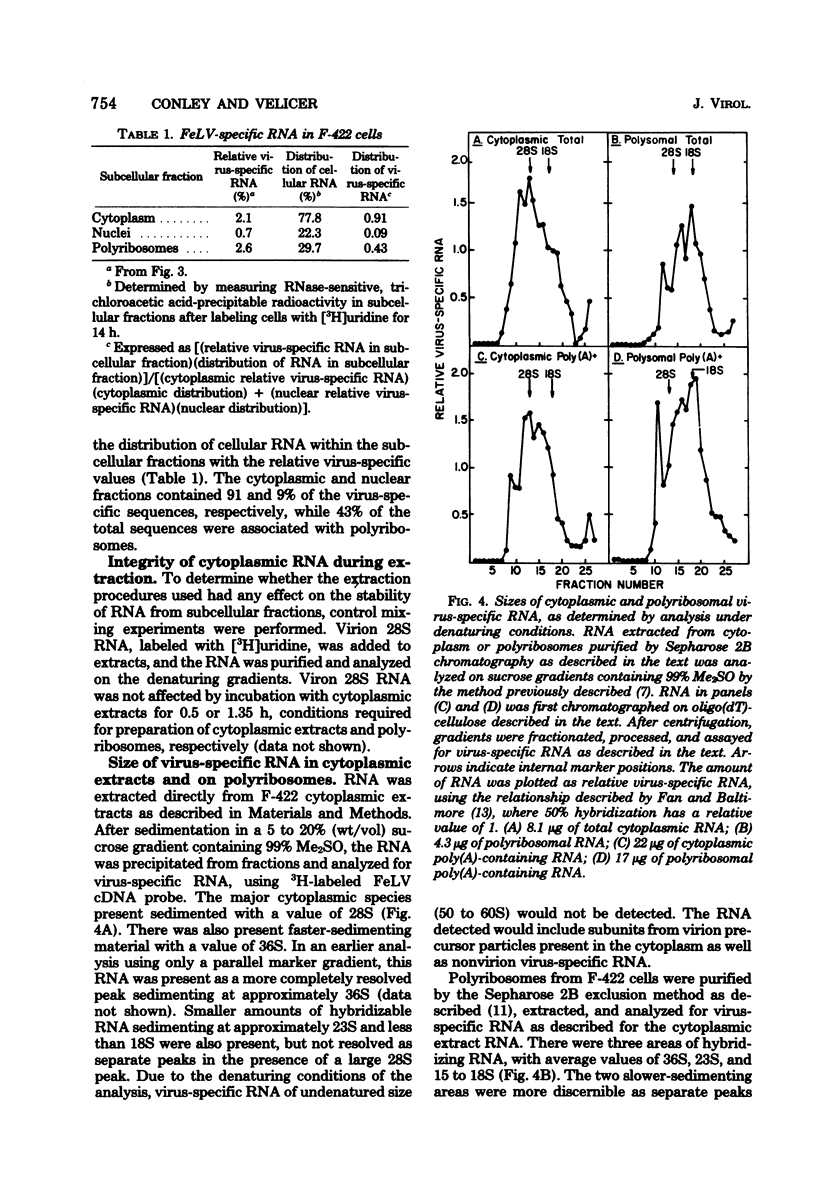
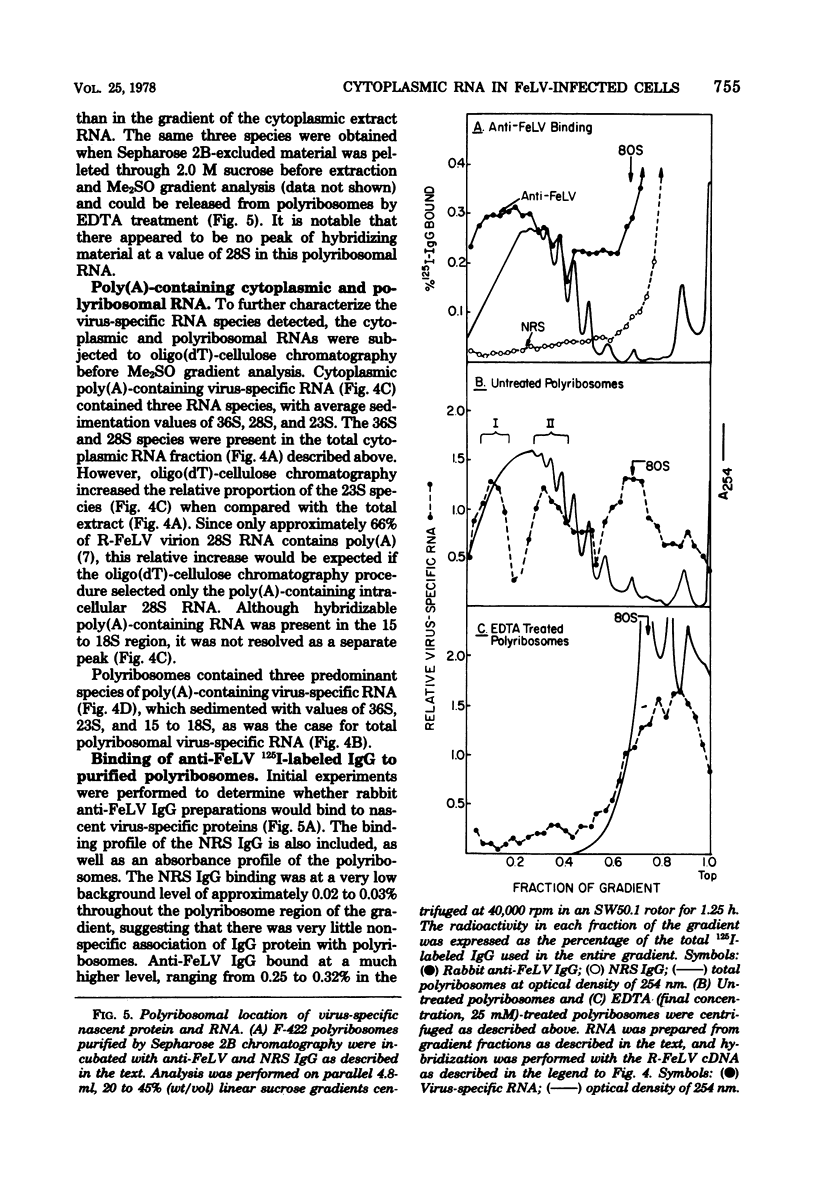
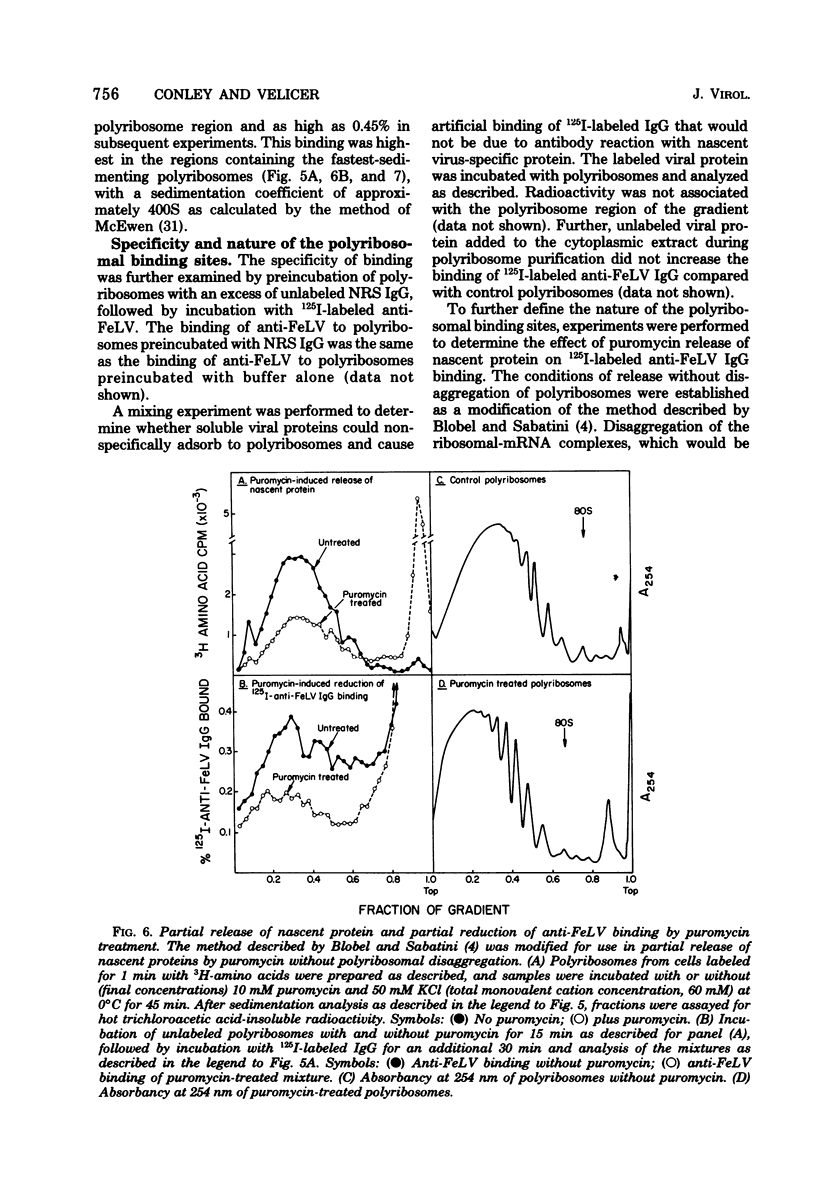
Abstract
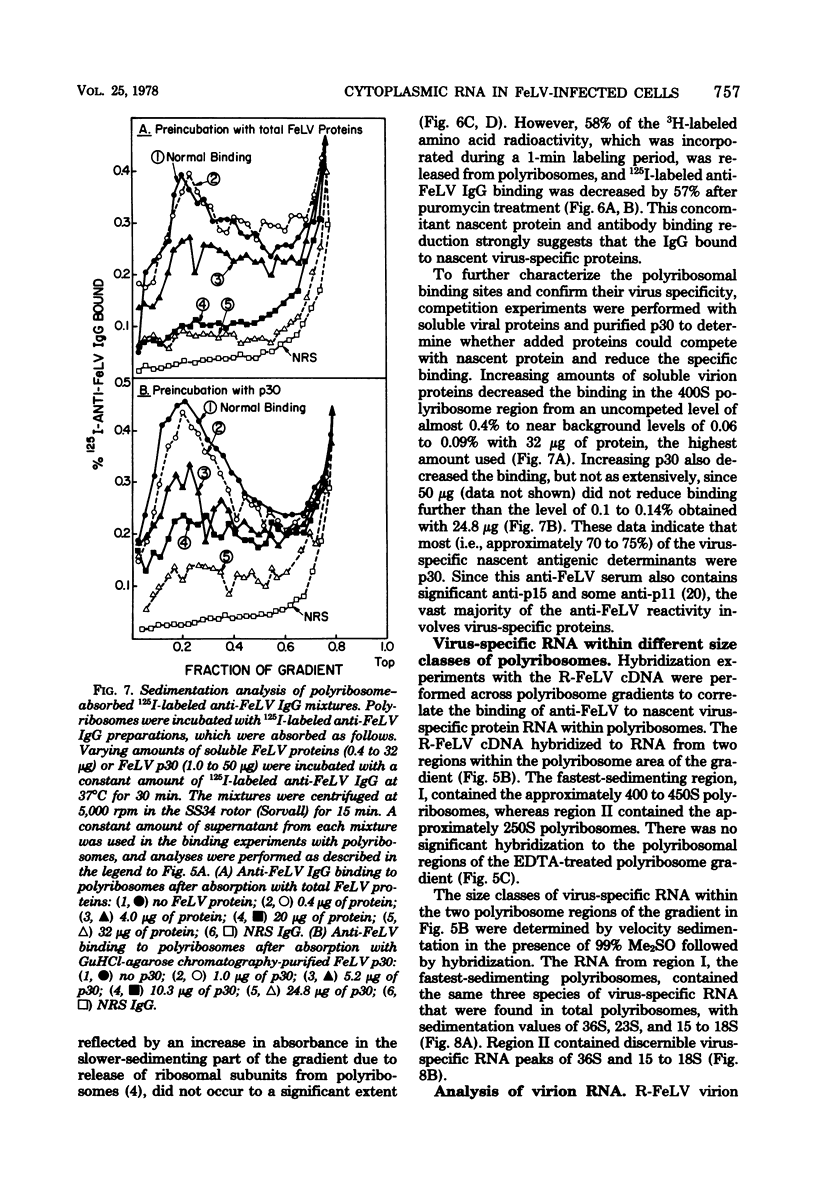
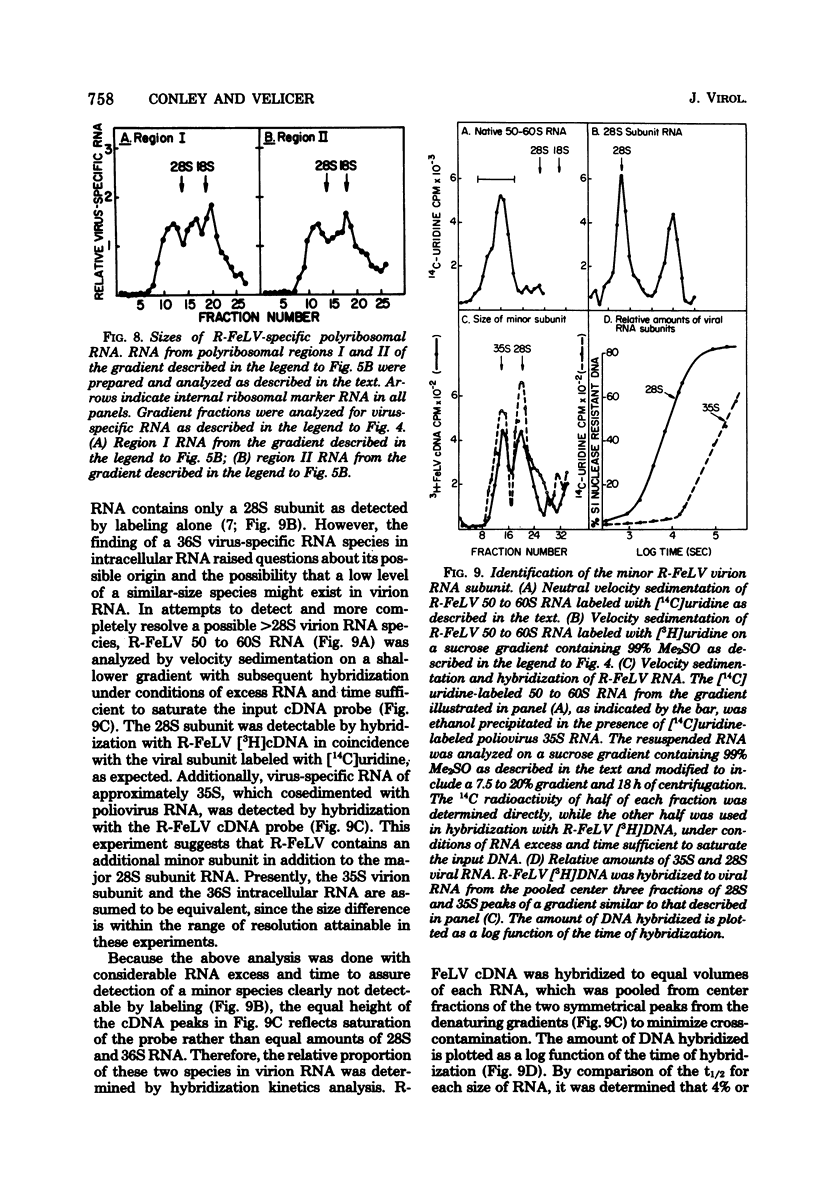
Cytoplasmic virus-specific RNA and polyribosomes from a chronically infected feline thymus tumor cell line, F-422, were analyzed by using in vitro-synthesized feline leukemia virus (Rickard strain) (R-FeLV) complementary DNA (cDNA) probe. By hybridization kinetics analysis, cytoplasmic, polyribosomat, and nuclear RNAs were found to be 2.1, 2.6, and 0.7% virus specific, respectively. Size classes within subcellular fractions were determined by sucrose gradient centrifugation in the presence of dimethyl sulfoxide followed by hybridization. The cytoplasmic fraction contained a 28S size class, which corresponds to the size of virion subunit RNA, and 36S, 23S, and 15 to 18S RNA species. The virus-specific 36S, 23S, and 15 to 18S species but not the 28S RNA were present in both the total and polyadenylic acid-containing polyribosomal RNA. Anti-FeLV gamma globulin bound to rapidly sedimenting polyribosomes, with the peak binding at 400S. The specificity of the binding for nascent virus-specific protein was determined in control experiments that involved mixing polyribosomes with soluble virion proteins, absorption of specific gamma globulin with soluble virion proteins, and puromycin-induced nascent protein release. The R-FeLV cDNA probe hybridized to RNA in two polyribosomal regions (approximately 400 to 450S and 250S) within the polyribosomal gradients before but not after EDTA treatment. The 400 to 450S polyribosomes contained three major peaks of virus-specific RNA at 36S, 23S, and 15 to 18S, whereas the 250S polyribosomes contained predominantly 36S and 15 to 18S RNA. Further experiments suggest that an approximately 36S minor subunit is present in virion RNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant M., Hashimoto S., Green M. Methylation pattern of genomic RNA from Moloney murine leukemia virus. J Virol. 1976 Sep;19(3):998–1005. doi: 10.1128/jvi.19.3.998-1005.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H., Smith K. D., Noyes A. N., Mullen M. A. Immunological characterization of rabbit hemoglobin alpha and beta chain-synthesizing polysomes. J Biol Chem. 1974 Nov 25;249(22):7210–7219. [PubMed] [Google Scholar]
- Brian D. A., Thomason A. R., Rottman F. M., Velicer L. F. Properties of feline leukemia virus. III. Analysis of the RNA. J Virol. 1975 Sep;16(3):535–545. doi: 10.1128/jvi.16.3.535-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of DNA from the 70S RNA of Rous sarcoma virus: identification and characterization of various size classes of DNA transcripts. J Virol. 1975 Nov;16(5):1220–1228. doi: 10.1128/jvi.16.5.1220-1228.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- East J. L., Knesek J. E., Allen P. T., Dmochowski L. Structural characteristics and nucleotide sequence analysis of genomic RNA from RD-114 virus and feline RNA tumor viruses. J Virol. 1973 Nov;12(5):1085–1091. doi: 10.1128/jvi.12.5.1085-1091.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Patterson R. J. Polysome isolation of sepharose column chromatography. Prep Biochem. 1975;5(3):247–255. doi: 10.1080/00327487508061575. [DOI] [PubMed] [Google Scholar]
- Fan H., Mueller-Lantzsch N. RNA metabolism of murine leukemia virus. III. Identification and quantitation of endogenous virus-specific mRNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1976 May;18(2):401–410. doi: 10.1128/jvi.18.2.401-410.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. RNA metabolism of murine leukemia virus: size analysis of nuclear pulse-labeled virus-specific RNA. Cell. 1977 Jun;11(2):297–305. doi: 10.1016/0092-8674(77)90046-0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Van Zaane D., Bloemers H. P., Bloemendal H. Synthesis of Rauscher murine leukemia virus-specific polypeptides in vitro. Proc Natl Acad Sci U S A. 1976 Feb;73(2):356–360. doi: 10.1073/pnas.73.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C., Velicer L. F. Properties of feline leukemia virus. I. Chromatographic separation and analysis of the polypeptides. J Virol. 1974 Aug;14(2):349–365. doi: 10.1128/jvi.14.2.349-365.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- Harewood K. R., Chang P., Higdon C., Larson D. The endogenous reverse transcriptase activity of Gibbon ape lymphoma virus: characterization of the DNA product. Biochim Biophys Acta. 1975 Sep 12;407(1):14–23. doi: 10.1016/0005-2787(75)90020-9. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Baltimore D. Size of murine RNA tumor virus-specific nuclear RNA molecules. J Virol. 1976 Aug;19(2):331–337. doi: 10.1128/jvi.19.2.331-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G., Karshin W. L., Naso R. B., Arcement L. J., Arlinghaus R. B. Proteins of Rauscher murine leukemia virus: resolution of a 70,000-dalton, Nonglycosylated polypeptide containing p30 peptide sequences. Virology. 1975 Nov;68(1):135–145. doi: 10.1016/0042-6822(75)90155-5. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J., Fraenkel-Conrat H. Identification of the 5' end of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3347–3350. doi: 10.1073/pnas.72.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Wood G., Saunders T. E., Arlinghaus R. B. The cell-free translation of Rauscher leukemia virus RNA into high molecular weight polypeptides. Biochim Biophys Acta. 1975 Mar 10;383(2):195–206. doi: 10.1016/0005-2787(75)90261-0. [DOI] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M. RD 114 virus-specific sequences in feline cellular RNA: detection and characterization. J Virol. 1973 Nov;12(5):984–994. doi: 10.1128/jvi.12.5.984-994.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasinki G. F., Velicer L. F. Analysis of intracellular feline leukemia virus proteins. I. Identification of a 60,000-dalton precursor of feline leukemia virus p30. J Virol. 1976 Oct;20(1):96–106. doi: 10.1128/jvi.20.1.96-106.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasinski G. F., Velicer L. F. Analysis of intracellular feline leukemia virus proteins II. Generation of feline leukemia virus structural proteins from precursor polypeptides. J Virol. 1977 Apr;22(1):74–85. doi: 10.1128/jvi.22.1.74-85.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Pawlowski P. J., Gillette M. T., Martinell J., Lukens L. N. Identification and purification of collagen-synthesizing polysomes with anti-collagen antibodies. J Biol Chem. 1975 Mar 25;250(6):2135–2142. [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton R. E., Liberti P., Baglioni C. Isolation of messenger RNA from polysomes by chromatography on oligo(dT)-cellulose. Anal Biochem. 1975 May 26;66(1):18–28. doi: 10.1016/0003-2697(75)90720-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Haseltine W. A., Baltimore D. 5'-terminus of Moloney murine leukemia virus 35s RNA is m7G5' ppp5' GmpCp. J Virol. 1976 Oct;20(1):324–329. doi: 10.1128/jvi.20.1.324-329.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salden M. H., Gielkens A. L., Bloemendal H. Translation of Rauscher leukemia virus RNA in heterologous cell-free systems. Biochim Biophys Acta. 1976 Mar 4;425(2):208–219. doi: 10.1016/0005-2787(76)90027-7. [DOI] [PubMed] [Google Scholar]
- Schechter I. Use of antibodies for the isolation of biologically pure messenger ribonucleic acid from fully functional eukaryotic cells. Biochemistry. 1974 Apr 23;13(9):1875–1885. doi: 10.1021/bi00706a016. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Green M. The virus-specific RNA species in free and membrane-bound polyribosomes of transformed cells replicating murine sarcoma-leukemia viruses. Biochem Biophys Res Commun. 1974 Feb 4;56(3):697–702. doi: 10.1016/0006-291x(74)90661-5. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dimock K. Evidence of methylation of B77 avian sarcoma virus genome RNA subunits. J Virol. 1976 May;18(2):586–595. doi: 10.1128/jvi.18.2.586-595.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Specific binding of albumin antibody to rat liver polysomes. J Biol Chem. 1974 Jun 10;249(11):3597–3601. [PubMed] [Google Scholar]
- Thomason A. R., Brian D. A., Velicer L. F., Rottman F. M. Methylation of high-molecular-weight subunit RNA of feline leukemia virus. J Virol. 1976 Oct;20(1):123–132. doi: 10.1128/jvi.20.1.123-132.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Green M. Intracellular and virion 35 S RNA species of murine sarcoma and leukemia viruses. Virology. 1974 May;59(1):258–265. doi: 10.1016/0042-6822(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Vecchio G., Tsuchida N., Shanmugam G., Green M. Virus-specific messenger RNA and nascent polypeptides in polyribosomes of cells replicating murine sarcoma-leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2064–2068. doi: 10.1073/pnas.70.7.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaane D. V., Gielkens A. L., Hesselink W. G., Bloemers H. P. Identification of Rauscher murine leukemia virus-specific mRNAs for the synthesis of gag- and env-gene products. Proc Natl Acad Sci U S A. 1977 May;74(5):1855–1859. doi: 10.1073/pnas.74.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaanie D., Gielkens A. L., Dekker-michielsen M. J., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus. Virology. 1975 Oct;67(2):544–552. doi: 10.1016/0042-6822(75)90454-7. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]



