Abstract
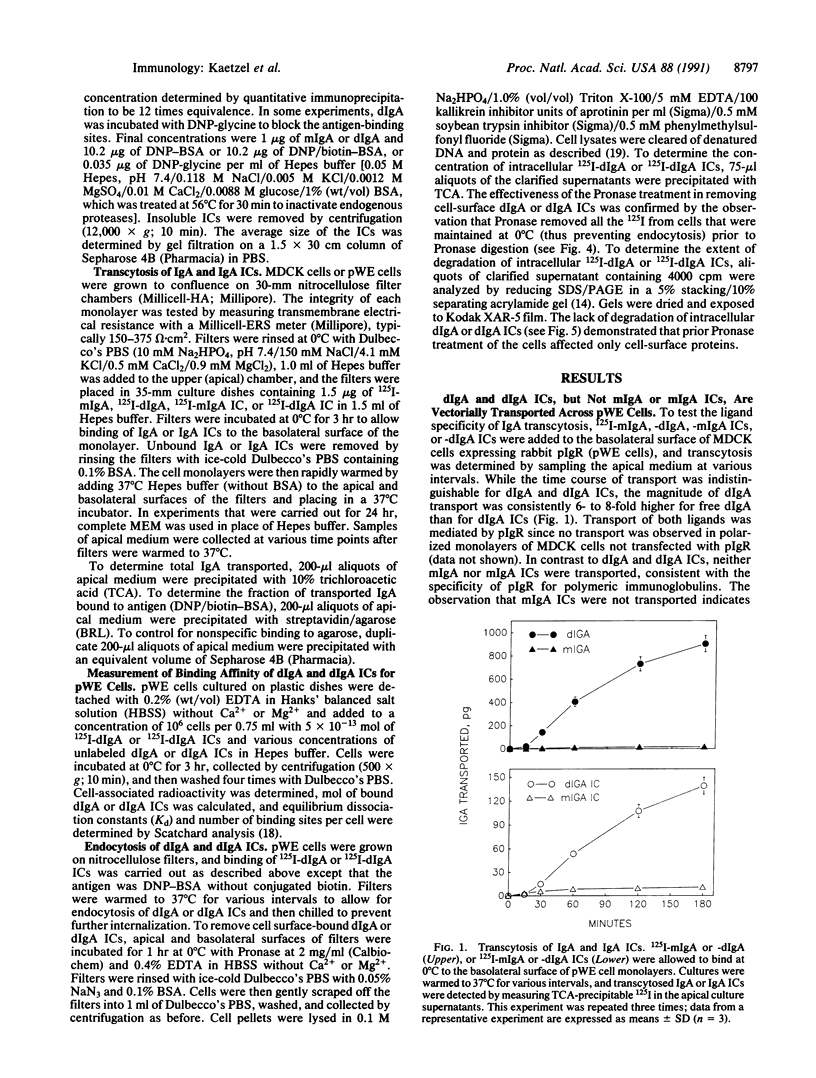
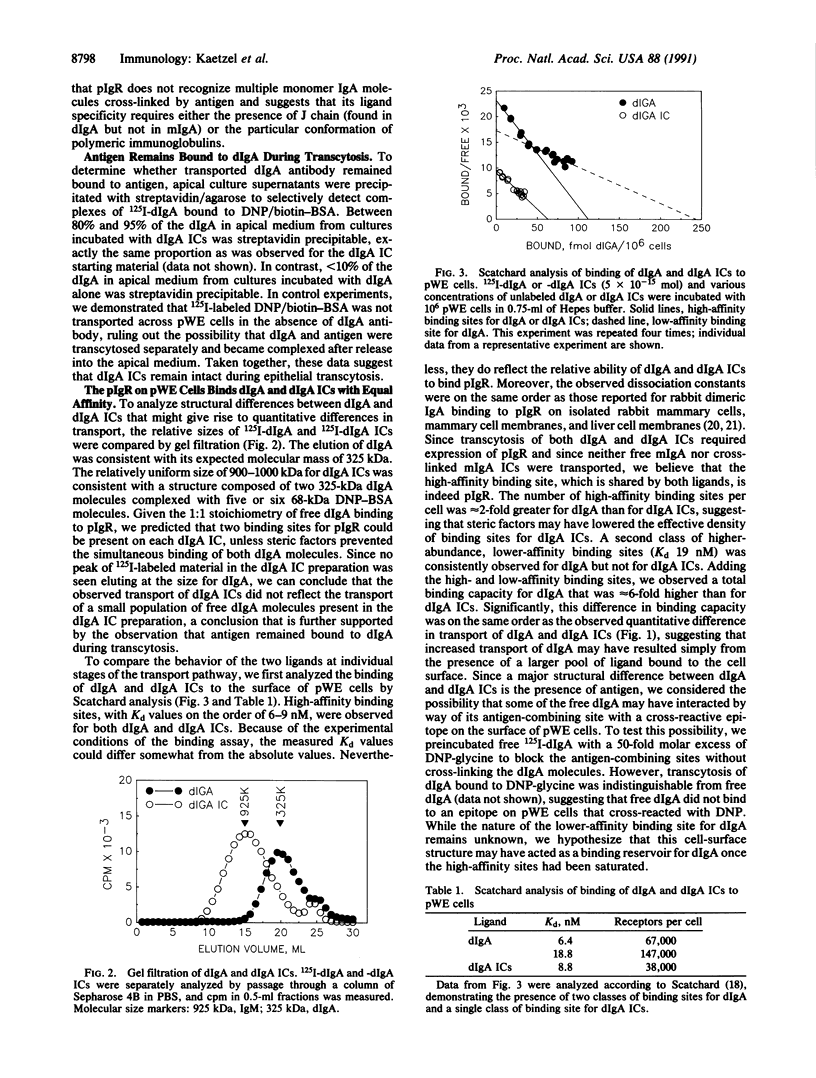
The polymeric immunoglobulin receptor (pIgR) on mucosal epithelial cells binds dimeric IgA (dIgA) on the basolateral surface and mediates transport of dIgA to the apical surface. Using Madin-Darby canine kidney epithelial cells stably transfected with pIgR cDNA, we found that soluble immune complexes (ICs) of 125I-labeled rat monoclonal antidinitrophenyl (DNP) dIgA (125I-dIgA) and DNP/biotin-bovine serum albumin were transported from the basolateral to the apical surface and then released. Monomeric IgA ICs were not transported, consistent with the specificity of pIgR for polymeric immunoglobulins. Essentially all the 125I-dIgA in apical culture supernatants was streptavidin precipitable, indicating that dIgA remained bound to antigen during transcytosis. While both dIgA and dIgA ICs bound pIgR with equal affinity (Kd approximately 8 nM), the number of high-affinity binding sites per cell was 2- to 3-fold greater for dIgA than for dIgA ICs. The extent of endocytosis of dIgA and dIgA ICs was correlated with the number of high-affinity binding sites. SDS/PAGE analysis of intracellular dIgA and dIgA ICs demonstrated that in both cases IgA remained undegraded during transport. The results suggest that the pathways of epithelial transcytosis of free dIgA and dIgA ICs are the same. Given the high population density of mucosal IgA plasma cells and the enormous surface area of pIgR-expressing mucosal epithelium, it is likely that significant local transcytosis of IgA ICs occurs in vivo. Such a process would allow direct elimination of IgA ICs at the mucosal sites where they are likely to form, thus providing an important defense function for IgA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitfeld P. P., Casanova J. E., Harris J. M., Simister N. E., Mostov K. E. Expression and analysis of the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells using retroviral vectors. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Harris J. M., Mostov K. E. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J Cell Biol. 1989 Aug;109(2):475–486. doi: 10.1083/jcb.109.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers N. K., Bruce M. G., McGhee J. R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Hodgson H. J., McPherson A., Dive C., Vaerman J. P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J Clin Invest. 1982 Aug;70(2):230–241. doi: 10.1172/JCI110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N. PREPARATION OF PURIFIED ANTI-2,4-DINITROPHENYL ANTIBODIES. Methods Med Res. 1964;10:94–102. [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. IgA nephropathy: overproduction or decreased clearance of immune complexes? Lab Invest. 1989 Oct;61(4):365–367. [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989 Feb;60(2):168–183. [PubMed] [Google Scholar]
- Emancipator S. N., Lamm M. E. Pathways of tissue injury initiated by humoral immune mechanisms. Lab Invest. 1986 May;54(5):475–478. [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Hoppe C. A., Connolly T. P., Hubbard A. L. Transcellular transport of polymeric IgA in the rat hepatocyte: biochemical and morphological characterization of the transport pathway. J Cell Biol. 1985 Dec;101(6):2113–2123. doi: 10.1083/jcb.101.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvale D., Løvhaug D., Sollid L. M., Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988 May 1;140(9):3086–3089. [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. Role of secretory component, a secreted glycoprotein, in the specific uptake of IgA dimer by epithelial cells. J Biol Chem. 1979 Nov 10;254(21):11072–11081. [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. The membrane receptor for polymeric immunoglobulin is structurally related to secretory component. Isolation and characterization of membrane secretory component from rabbit liver and mammary gland. J Biol Chem. 1981 Dec 10;256(23):12490–12495. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Intracellular transport and processing of secretory component in cultured rat hepatocytes. Gastroenterology. 1987 Dec;93(6):1194–1204. doi: 10.1016/0016-5085(87)90244-7. [DOI] [PubMed] [Google Scholar]
- Nagura H., Smith P. D., Nakane P. K., Brown W. R. IGA in human bile and liver. J Immunol. 1981 Feb;126(2):587–595. [PubMed] [Google Scholar]
- Peppard J., Orlans E., Payne A. W., Andrew E. The elimination of circulating complexes containing polymeric IgA by excretion in the bile. Immunology. 1981 Jan;42(1):83–89. [PMC free article] [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Rifai A., Mannik M. Clearance of circulating IgA immune complexes is mediated by a specific receptor on Kupffer cells in mice. J Exp Med. 1984 Jul 1;160(1):125–137. doi: 10.1084/jem.160.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai A., Schena F. P., Montinaro V., Mele M., D'Addabbo A., Nitti L., Pezzullo J. C. Clearance kinetics and fate of macromolecular IgA in patients with IgA nephropathy. Lab Invest. 1989 Oct;61(4):381–388. [PubMed] [Google Scholar]
- Rifai A., Schena F. P., Montinaro V., Mele M., D'Addabbo A., Nitti L., Pezzullo J. C. Clearance kinetics and fate of macromolecular IgA in patients with IgA nephropathy. Lab Invest. 1989 Oct;61(4):381–388. [PubMed] [Google Scholar]
- Rits M., Cormont F., Bazin H., Meykens R., Vaerman J. P. Rat monoclonal antibodies. VI. Production of IgA secreting hybridomas with specificity for the 2,4-dinitrophenyl (DNP) hapten. J Immunol Methods. 1986 May 1;89(1):81–87. doi: 10.1016/0022-1759(86)90034-7. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Mestecky J. Role of serum IgA. Hepatobiliary transport of circulating antigen. J Exp Med. 1981 Apr 1;153(4):968–976. doi: 10.1084/jem.153.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socken D. J., Simms E. S., Nagy B. R., Fisher M. M., Underdown B. J. Secretory component-dependent hepatic transport of IgA antibody-antigen complexes. J Immunol. 1981 Jul;127(1):316–319. [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. II. Localization of its intracellular forms by cell fractionation studies. J Cell Biol. 1985 Apr;100(4):1255–1261. doi: 10.1083/jcb.100.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Intracellular and transcellular transport of secretory component and albumin in rat hepatocytes. J Cell Biol. 1983 Nov;97(5 Pt 1):1582–1591. doi: 10.1083/jcb.97.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]