Abstract
Lipoarabinomannan (LAM) and arabinogalactan (AG) are the two major mycobacterial cell wall (lipo)polysaccharides, which contain a structurally similar arabinan domain that is highly branched and assembled in a stepwise fashion by variety of arabinofuranosyltransferases (ArafT). In addition to playing an essential role in mycobacterial physiology, LAM and its biochemical precursor lipomannan possess potent immunomodulatory activities that affect the host immune response. In the search of additional mycobacterial ArafTs that participate in the synthesis of the arabinan segment of LAM, we disrupted aftB (MSMEG_6400) in Mycobacterium smegmatis. The deletion of chromosomal aftB locus could only be achieved in the presence of a rescue plasmid carrying a functional copy of aftB, strongly suggesting that it is essential for the viability of M. smegmatis. Isolation and detailed structural characterization of a LAM molecule derived from the conditional mutant deficient in AftB revealed the absence of terminal β(1 → 2)-linked arabinofuranosyl residues. Furthermore, we demonstrated that truncated LAM displays proinflammatory activity, which is due to its ability to activate Toll-like receptor 2. All together, our results indicate that AftB is an essential mycobacterial ArafT that plays a role in the synthesis of the arabinan domain of LAM.
Despite the existence of treatments for tuberculosis (TB), TB continues to represent a major healthcare challenge, accounting for nearly 9 million new infections and over 1 million deaths each year.1 Indeed, this global health threat is escalating, given the variable efficacy of the vaccine strain BCG, the increased susceptibility of HIV-infected individuals to TB, and the increased prevalence of multi-drug-resistant (MDR) and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis, the causative agent of the disease.1 As current treatments lose their efficacy, it is vital that we address the increasing health burden of TB by replenishing the drug pipeline with new drugs, drug targets, and improved treatment regimes.
The cell envelope of M. tuberculosis is unique in that it contains a thick carbohydrate and lipid rich layer that accounts for its inherent resistance to numerous drugs and contributes to its persistence and virulence. This is due to the key cell envelope structures, notably the mycolyl-arabinogalactan-peptidoglycan complex (mAGP), lipomannan (LM), and lipoarabinomannan (LAM). The latter glycolipids, in addition to their structural roles, exhibit potent immunomodulatory activities and thus are probably important modulators of the host–pathogen interactions during the course of infection.
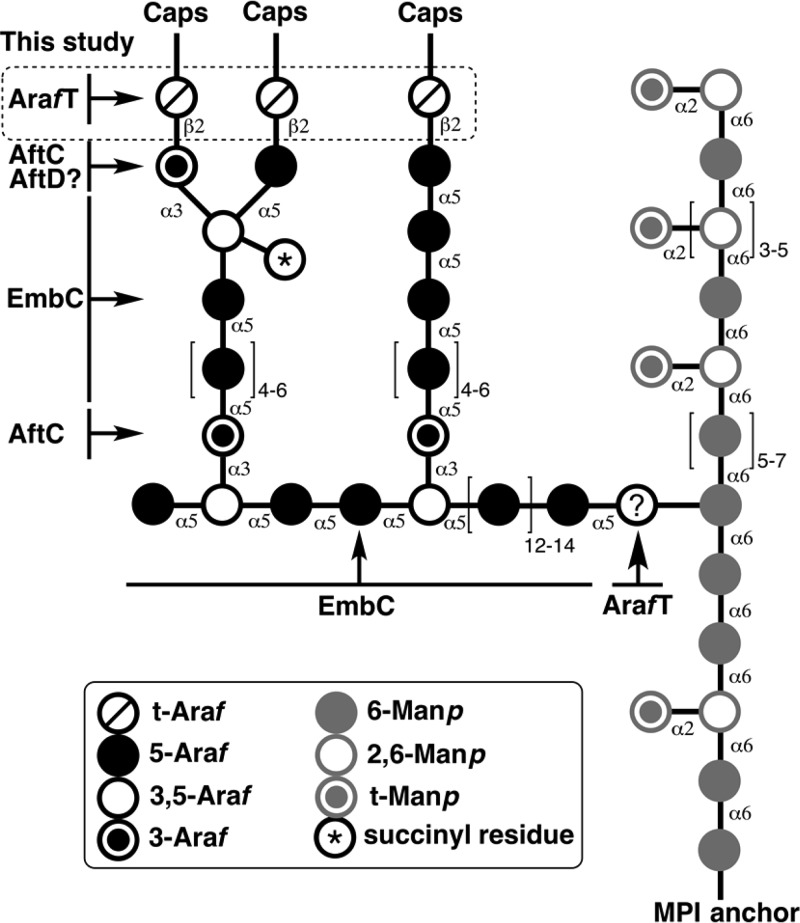
In mycobacteria, LM is composed of approximately 20–25 α(1 → 6) α-d-mannopyranosyl (Manp) residues connected in a linear fashion and are anchored to the inner and outer membrane via their phosphatidyl-myo-inositol (PI) unit.2,3 This mannose core is further decorated with 7–10 singular α(1 → 2) Manp residues. The mature LM then serves as a precursor to the formation of LAM where it is further glycosylated by large arabinan domain consisting of approximately 55–70 Araf residues in a linear α(1 → 5)-d-Araf fashion with 3,5-α-d-Araf branches (Figure 1).4 Recent studies suggest that this single arabinan chain is attached to the mannan core at the O-6 position of the mannosyl residue and not O-2 as previously reported.5,6 Although, the arabinan structure of LAM is more variable than that of AG, two types of highly branched and conserved motifs remain. These are the tetra-arabinoside (β-d-Araf(1 → 2)-α-d-Araf(1 → 5)-α-d-Araf(1 → 5)-α-d-Araf) and hexa-arabinoside ([β-d-Araf(1 → 2)-α-d-Araf]2-3,5-α-d-Araf(1 → 5)-α-d-Araf) motifs, both of which end with a characteristic disaccharide unit (Araf-β(1 → 2)-Araf-α(1 →.).4,5 The termini of the arabinan domain are completed with the capping motifs that are species dependent. The fast growers, such as M. smegmatis, are capped by phosphoinositide units (PILAM). The slow growers including M. tuberculosis are capped with 2–3 mannose residues (Man-LAM), and some species, such as Mycobacterium chelonae have uncapped LAM (AraLAM).5,7,8 In addition, C. glutamicum, a closely related organism to M. tuberculosis and a desired model organism to study mycobacterial AG and LM assembly, produces LAM that lacks a highly branched arabinan domain and contains single Araf residues attached to the mannan core by AftE.9 As a result, although the structure of LAM has been fairly well described, understanding physiological functions and biological activities of lipoglycans prove to be challenging.
Figure 1.
Structural representation of the wild type lipoarabinomannan and the unknown arabinofuranosyltransferase investigated in this study.
Targeting the enzymes responsible for the synthesis and assembly of the arabinan domains of AG and LAM continue to present opportunities for new chemotherapeutics. Indeed, the front-line anti-TB drug ethambutol targets the assembly of these vital cell wall structures by inhibiting the embCAB operon.10 Individual genetic disruption of embA, embB, and embC in M. smegmatis revealed that both EmbA and EmB are α(1 → 5) ArafT that play a key role in the synthesis of the arabinan domain of AG, whereas EmbC is required exclusively for the elongation of the arabinan domain of LAM.10,11 Recently identified α(1 → 6) ArafT, capable of utilizing synthetic (1 → 6)-Manp disaccharide and trisaccaride acceptors in vitro, was suggested to prime the mannan core. However, further analysis is required to identify the enzyme responsible.6 Deletion studies in M. smegmatis identified ArafT with dual function −AftC– that is responsible for the α-1,3-branching of the linear arabinose polymers of AG and LAM.12,13In vitro assays using neoglycolipid acceptors and cell-free extracts from M. smegmatis demonstrated that AftD was able to add α(1 → 3) Araf residues to the linear α-1,5-linked acceptor, resulting in branching of the linear arabinan. It was concluded that AftD participates in the arabinan synthesis of both AG and LAM.14 Finally, the branched arabinose motif of LAM is further modified to either a tetra-arabinoside or a hexa-arabinoside motif by an unknown β(1 → 2) ArafT.
As several ArafT’s have a dual role and are involved in the arabinan synthesis of both AG and LAM polysaccharides, we investigated the potential role of AftB in LAM biosynthesis. Our previous studies in Corynebacterium glutamicum identified AftB as a nonessential ArafT responsible for the terminal β(1 → 2) linkages at the nonreducing end of the arabinan domain of AG.15 However, the disruption and essentiality of aftB in Mycobacterium species or its essential role in LAM biosynthesis has not been reported. Herein, we demonstrate the essentiality of aftB in M. smegmatis using the conditional expression-specialized transduction essentiality test (CESTET) and analyzed the structure of LAM from the conditional M. smegmatisΔaftB::pMV306-aftB mutant. We have shown, for the first time, that AftB has a dual function and is responsible for β(1 → 2)-linked Araf incorporation into LAM. Moreover, we have analyzed the immunological properties of a LAM molecule isolated from the aftB conditional mutant in order to shed the light on the structure–function relationship between mycobacterial lipoglycans and host pattern recognition receptors (PRRs), notably Toll-like receptor 2 (TLR2).
Results and Discussion
Construction and Growth of the M. smegmatis aftB Conditional Mutant
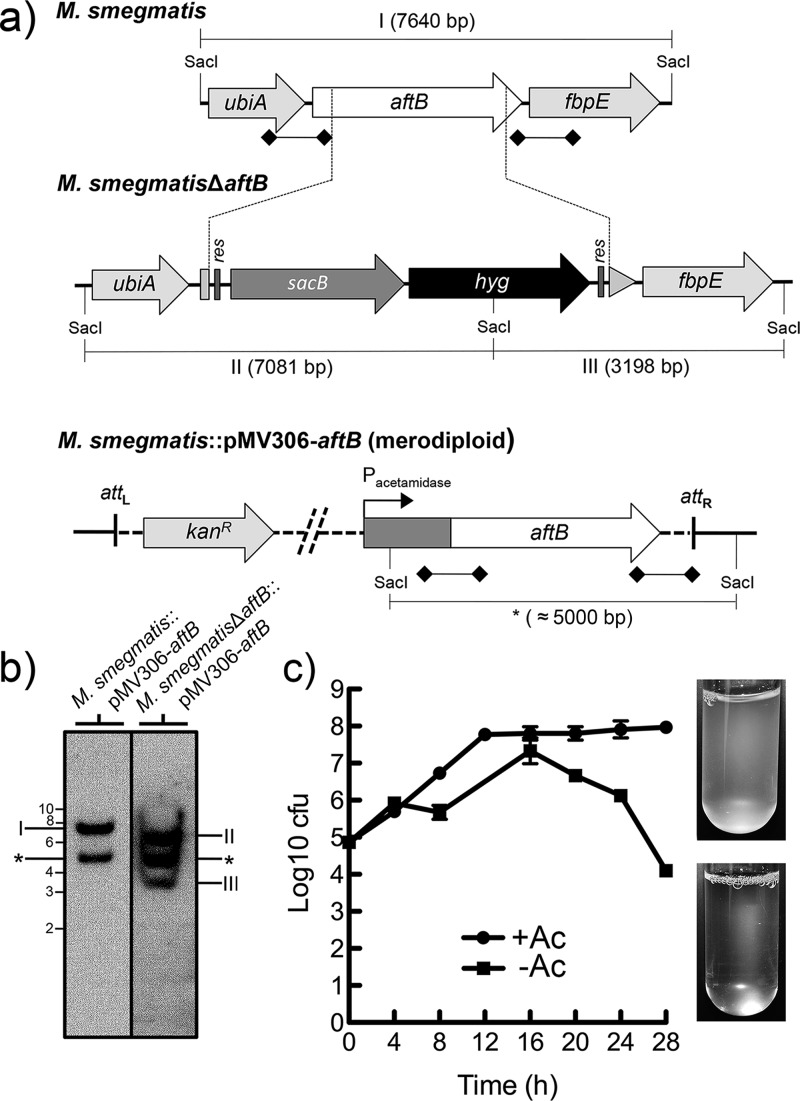
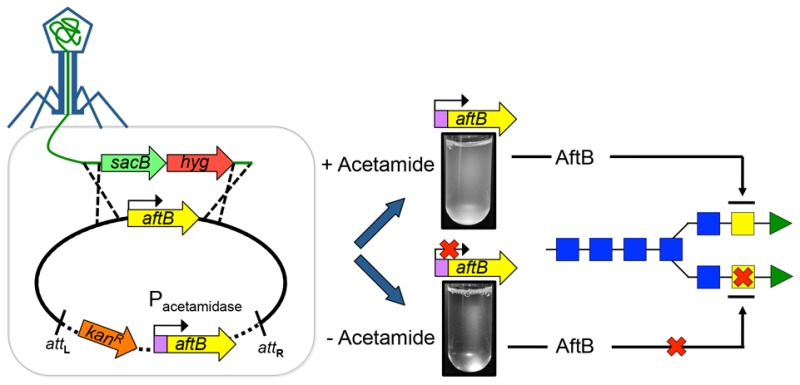
Our earlier studies demonstrated that the nonessential AftB from C. glutamicum (Cg-AftB) adds the terminal β(1 → 2)-linked Araf residues to the arabinan domain of AG before its decoration with mycolic acids.15 In this study, we investigated the potential role of AftB in the biosynthesis of LAM in M. smegmatis. For this purpose, we constructed a knockout phage phMSMEG6400 designed to replace the chromosomal M. smegmatis aftB (Ms-aftB) with a hygromycin resistance cassette (Figure 2a). Several attempts to obtain a Ms-aftB null mutant were unsuccessful, suggesting that Ms-aftB, unlike its homologue in C. glutamicum, is an essential gene.15 We then employed CESTET and generated a Ms-aftB conditional mutant by transducing a merodiploid M. smegmatis::pMV306-aftB strain containing a second, inducible copy of aftB to yield M. smegmatisΔaftB::pMV306-aftB.16 The correct replacement of the native chromosomal copy of aftB with a hygromycin cassette in the transductants was confirmed by Southern blot analysis (Figure 2b). In order to confirm aftB essentiality and to study the fate of the mycobacterial cells depleted of AftB, we monitored the growth of M. smegmatisΔaftB::pMV306-aftB in liquid medium in the presence and absence of acetamide over 28 h (Figure 2c). Optical density (OD) measurements revealed normal growth kinetics for the mutant strain grown in media containing acetamide. However, cells cultured in the absence of inducer showed a decrease in OD after 16 h of growth and cell lysis after 28 h of incubation. This decrease in OD correlated with a decrease in the number of CFUs (Figure 2c). Although, the aftB homologue in C. glutamicum has been reported to be nonessential,15 the failure to obtain a aftB null mutant in the absence of second inducible copy of aftB, coupled with the inability of M. smegmatis to grow in the media without acetamide, an inducer that activates aftB expression, shows that AftB is crucial for the viability of M. smegmatis. In addition, previous studies employing high density mutagenesis studies suggest that Rv3805c in M. tuberculosis is an essential gene.17 It is reasonable to conclude that aftB is essential for M. tuberculosis viability and therefore represents an attractive drug target.
Figure 2.
Generation of a conditional aftB mutant in M. smegmatis. (a) A map of the aftB region in the parental M. smegmatis strain, its corresponding region in the conditional M. smegmatisΔaftB::pMV306-aftB mutant and merodiploid strain; res, resolvase site; hyg, hygromycin resistance gene from Streptomyces hygroscopicus; sacB, sucrose counter-selectable gene from Bacillus subtilis. Digoxigenin-labeled probes were derived from ∼1 kb upstream and downstream flanking sequences that were used to construct the knockout plasmid and are indicated by thick lines with square ends. SacI-digested bands expected in a Southern blot are indicated in I, II, III, and *. (b) The Southern blot of SacI-digested genomic DNA from the merodiploid and conditional aftB mutant with expected bands I, II, and III. The asterisk (*) indicates a band appearing as a result of a second aftB copy integration into mycobacterial chromosome. Representative lanes are from the same Southern blot and are grouped next to each other for clarity. (c) Log CFU obtained from cultures of M. smegmatisΔaftB pMV306-aftB grown with and without acetamide (Ac). Log10 CFU values were calculated from different dilutions at different time points from a single experiment. The images on the right show cultures after 28 h of growth in the presence and absence of Ac. Results are mean ± SD from n = 3 colonies per strain and are from one representative of two independent experiments.
Initial Characterization of Lipoglycans Extracted from the M. smegmatis aftB Conditional Mutant
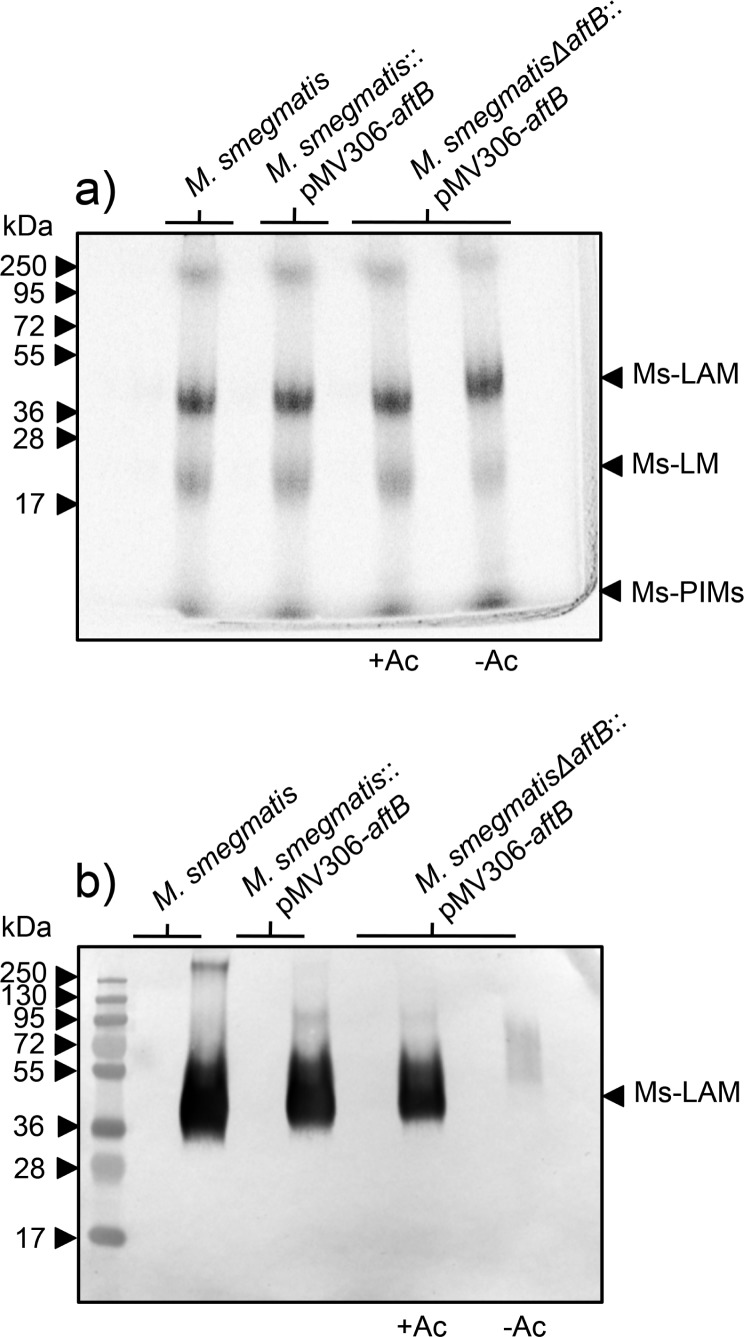
Strains of M. smegmatis, M. smegmatis::pMV306-aftB, and conditional M. smegmatisΔaftB::pMV306-aftB mutant grown in the presence and absence of acetamide were examined for their ability to synthesize LAM. Exponentially growing cultures including M. smegmatisΔaftB::pMV306-aftB depleted of AftB were labeled with [1, 2-14C]-glucose for 10 h followed by lipoglycan extraction. [14C]-LAM and [14C]-LM were profiled using SDS-PAGE analysis and the lipoglycans revealed and quantified by phosphorimaging (Figure 3a). Extracts from all strains showed the presence of [14C]-LAM and [14C]-LM (Figure 3a). Densitometry analysis revealed no significant difference in lipoglycan profiles extracted from different strains. Subsequently, [14C]-lipoglycans were assessed for the reactivity with an anti-AraLAM (α-AraLAM) monoclonal antibody (mAb, F30-5), which recognizes the terminal arabinan branches of LAM.18,19 All lipoglycan preparations reacted with the antibody F30-5, with an exception of LAM isolated from the M. smegmatisΔaftB::pMV306-aftB depleted of AftB (Figure 3b). These results strongly indicate that AftB is involved in the synthesis of the nonreducing end of arabinan domain of LAM in M. smegmatis.
Figure 3.
Lipoglycan profiles of M. smegmatis, M. smegmatis pMV306-aftB, and M. smegmatisΔaftB pMV306-aftB strains grown with and without acetamide. (a) Equivalent aliquots of [14C]-lipoglycans (20,000 cpm) were analyzed using SDS-PAGE and visualized by phosphorimaging. The three major bands represented by Ms-LAM, Ms-LM and Ms-PIMs are indicated. The protein molecular weight standards are provided on the left for comparison. (b) Equivalent aliquots (50 000 cpm) of [14C]-lipoglycans from each strain were immunoblotted with α-AraLAM antibody F30-5 that recognizes the branches of the arabinan domain of LAM. The major band represented by Ms-LAM is indicated. The protein molecular weight standards are provided on the left for comparison.
Structural Characterization of LAM Isolated from the M. smegmatis aftB Conditional Mutant
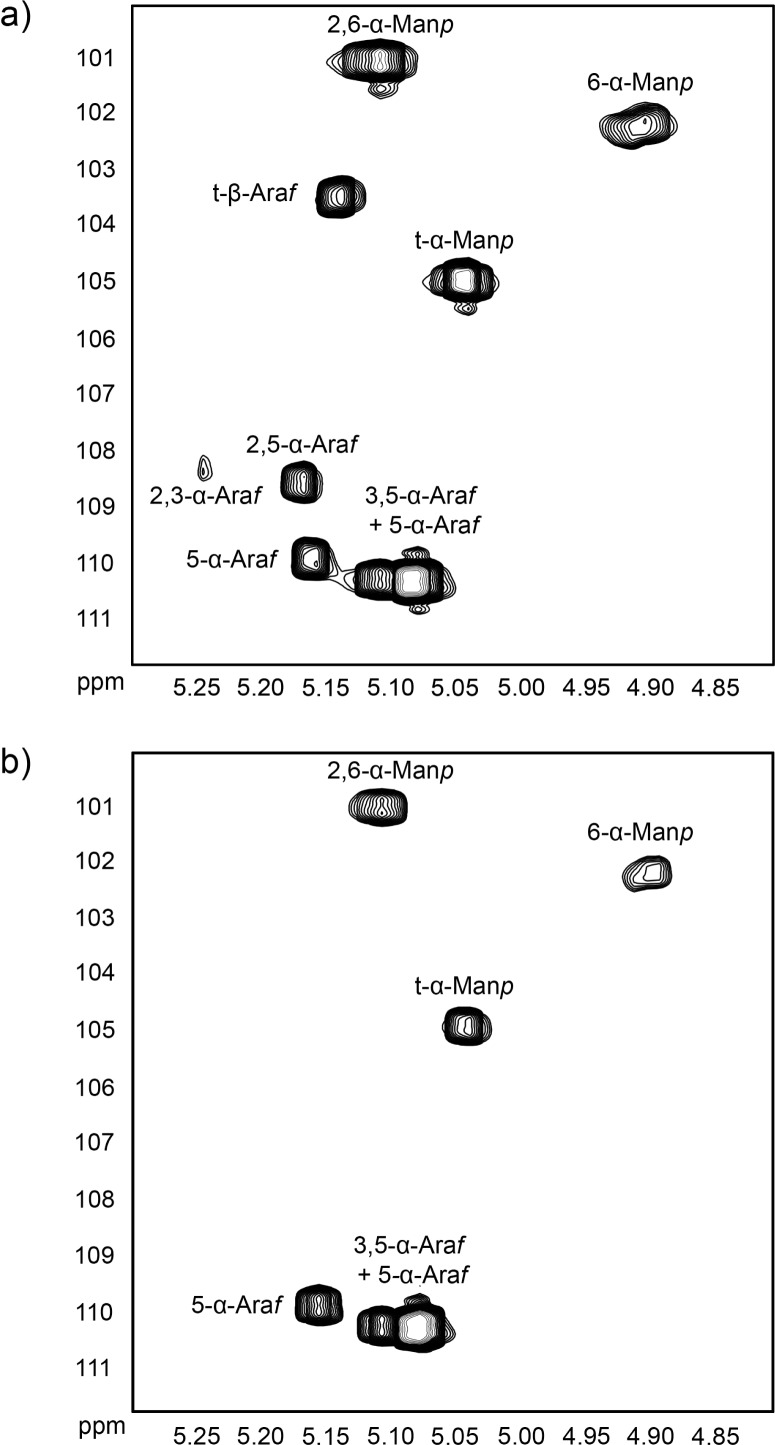
One of the disadvantages in generating conditional mutants by employing CESTET is the restricted time scale where cells have to be cultured in the absence of acetamide long enough to deplete the intracellular protein of interest but short enough not to yield persister cells, which results in a modest yield of cell mass. In order to further analyze the different LAM preparations by NMR spectroscopy and improve the carbon signal intensity due to poor cell yield, we labeled M. smegmatisΔaftB pMV306-aftB cultured in the presence and absence of acetamide with [13C]-glucose for 10 h, followed by lipoglycan extraction. Purified [13C]-lipoglycans were recovered from the crude extracts by hydrophobic and gel exclusion chromatography before subjecting samples to 1H–13C HSQC analysis. On the basis of our earlier studies with mycobacterial LAM13 and previously published work,20,21 we assigned the proton and carbon resonances of the different spin systems using 1H–13C HSQC experiments. The 13C resonances at δ101.1 ppm and δ102.3 ppm correlating to protons at δ5.11 ppm and δ4.91 ppm were assigned as 2,6-α-Manp and 6-α-Manp linkages, respectively (Figure 4a,b). The resonance at δ105.0 ppm, which correlated to an anomeric proton at δ5.04 ppm, was designated as t-α-Manp. As expected, the resonances associated with the mannan core (2,6-α-Manp, 6-α-Manp, and t-α-Manp) remained unaffected by the disruption of AftB activity and as a result were visible in both 1H–13C HSQC spectra (Figure 4a,b). The resonances associated with the arabinan domain, however, were notably more complex in the spectra of LAM isolated from the M. smegmatisΔaftB::pMV306-aftB grown in the presence of acetamide (Figure 4a) than compared to the resonances of LAM extracted from M. smegmatisΔaftB::pMV306-aftB grown in the absence of the inducer (Figure 4b). Several spin systems with 13C resonances at δ110.0 ppm and δ110.4 ppm were assigned to anomeric protons at δ5.16 ppm and δ5.08 ppm, respectively, and designated as 5-α-Araf in different chemical environments. The 3,5-α-Araf residues corresponded to δ110.4 ppm and were assigned to protons at δ5.09 ppm. The 2,3-α-Araf and 2,5-α-Araf linkages corresponded to δ108.4 ppm and δ108.6 ppm with protons at δ5.24 ppm and δ5.17 ppm, respectively (Figure 4a). Finally, the 13C resonance at δ103.5 ppm was designated to δ 5.14 ppm as the t-β-Araf linkage (Figure 4a). Most importantly, only 5-α-Araf and 3,5-α-Araf residues were conserved in the LAM of M. smegmatisΔaftB::pMV306-aftB depleted of AftB, but 2,3-α-Araf, 2,5-α-Araf, and t-β-Araf linkages were absent due to the loss of β(1 → 2) linkages (Figure 4a,b). The 1H–13C HSQC experiments confirmed that AftB acts as a β(1 → 2) ArafT in the biosynthesis of LAM in M. smegmatis. The full HSQC spectra are available in the Supporting Information (Supporting Figure 1). Further chemical characterization, such as gas chromatography analysis, could provide accurate glycosyl composition of the truncated LAM; however due to the nature of the conditional mutant and a modest yield of cell mass generated, such analysis has proved to be extremely difficult to perform.
Figure 4.
Expanded region (δ 1H: 4.80–5.30, δ 13C: 100–112) of the two-dimensional 1H–13C HSQC spectra in D2O at 313 K of M. smegmatis LAM extracted from the aftB conditional mutant in the presence (a) and absence (b) of AftB.
Analysis of Toll-like Receptor 2 Activation and Cytokine Production by Truncated LAM
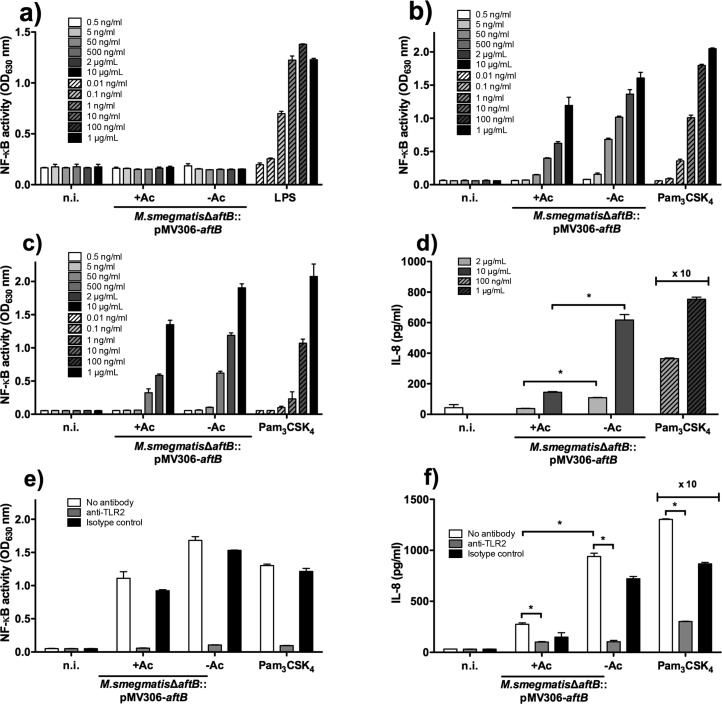
Both LM and LAM display immunoregulatory and anti-inflammatory properties that affect the host immune response.22,23 A family of pattern recognition receptors, named TLR2 in association with TLR1 and TLR6, detect a wide range of ligands including mycobacterial LAM. Studies have demonstrated that the lipidic part of the molecule is required for its activity, whereas the glycosidic moiety was shown to contribute by directly controlling the extent of this activity.24 Thus, we explored the capacity of truncated LAM extracted from the conditional M. smegmatisΔaftB::pMV306-aftB mutant depleted of AftB to induce TLR2-dependent innate immune responses. Previous studies demonstrated that a positive relation exists between the length of the mannan chain and the ability of the lipoglycan to activate TLR2.24 Lipoglycans with accessible long mannan domains, such as LM, were shown to be potent inducers of TLR2, whereas LAM molecules were poor activators of the TLR2 signaling due to their large arabinan domain somehow obstructing the availability of the mannan chain.13,25,26 Crude lipoglycan preparations extracted from M. smegmatisΔaftB::pMV306-aftB, cultured in the presence and absence of acetamide, were subsequently subjected to hydrophobic and size exclusion chromatography to yield pure LAM fractions (Figure 5a,b).27,28 Earlier studies have reported that some of the LAM preparation from M. smegmatis may have been contaminated with lipopeptides and as a result had an immune stimulatory activity.13,24 Therefore, we have assessed both purified truncated and full-length LAM fractions for their activity on HEK293 cells that stably express human TLR4, which is the receptor for bacterial lipopolysaccharide (LPS) and lipid A.29 Cells were incubated with LPS, which served as a positive control, at concentrations ranging from 0.01 ng mL–1 to 1 μg mL–1 and both LAM fractions at concentrations ranging from 0.5 ng mL–1 to 10 μg mL–1. In the presence of LPS, a dose-dependent effect was observed, whereas HEK293-TLR4 cells stimulated with the purified full-length and truncated LAM resulted in similar results to those of the noninduced cells (Figure 6a). Therefore, we have concluded that the isolated LAM fractions were devoid of lipopeptide contamination. Subsequently, a derivative of HEK293 cells that stably expresses human TLR2 along with a NF-κB-inducible reporter system were incubated with full-length and truncated LAM extracted from M. smegmatisΔaftB::pMV306-aftB grown in the presence and absence of acetamide, respectively, at concentrations ranging from 0.5 ng mL–1 to 10 μg mL–1. The synthetic Pam3CSK4 lipopeptide was used as a positive control of TLR2 activation at concentrations ranging from 0.01 ng mL–1 to 1 μg mL–1. As shown in Figure 6b, both purified LAM molecules induced NF-κB activation in a dose-dependent fashion with the truncated LAM exhibiting a stronger TLR2 activation than compared to the full length LAM. A similar dose-dependent effect was observed when human monocyte/macrophage THP-1 cells, which also stably express a NF-κB-inducible reporter system, were incubated with full length LAM, truncated LAM, and Pam3CSK4 ligands (Figure 6c). As activation of TLR2 results in production of various cytokines and ultimately regulates the adaptive immunity, TLR2-dependent IL-8 production was measured in the supernatants of stimulated THP-1 cells (Figure 6d). Cells were incubated with full length and truncated LAM at concentrations of 2 μg mL–1 and 10 μg mL–1 and a positive reference ligand Pam3CSK4 at concentrations of 100 ng mL–1 and 1 μg mL–1. The TLR2 dependent activation of NF-κB and subsequently the production of IL-8 by truncated LAM was significantly stronger than compared to the one induced by full-length LAM (Figure 6d). The potency of truncated LAM to induce TLR2 response in both HEK293 and THP-1 cells suggests that a more exposed mannan chain may allow TLR2 to more readily recognize the lipoglycan. This is also consistent with the previous reports.13 In addition, we assessed if the induction of NF-κB and production of IL-8 is a direct result of TLR2 activation by using an anti-TLR2 antibody in THP-1 cells (Figures 6e, 6f). Cells were preincubated with the anti-TLR2 or isotype control antibodies (10 μg mL–1) for 30 min at 37 °C followed by incubation with full length LAM (5 μg mL–1), truncated LAM (5 μg mL–1), or Pam3CSK4 lipopeptide (1 μg mL–1). Both full length and truncated LAM induced activation of NF-κB (Figure 6e) and as a result production of IL-8 (Figure 6f) that was almost completely abolished by an anti-TLR2 antibody, thus demonstrating signaling through the TLR2.
Figure 5.
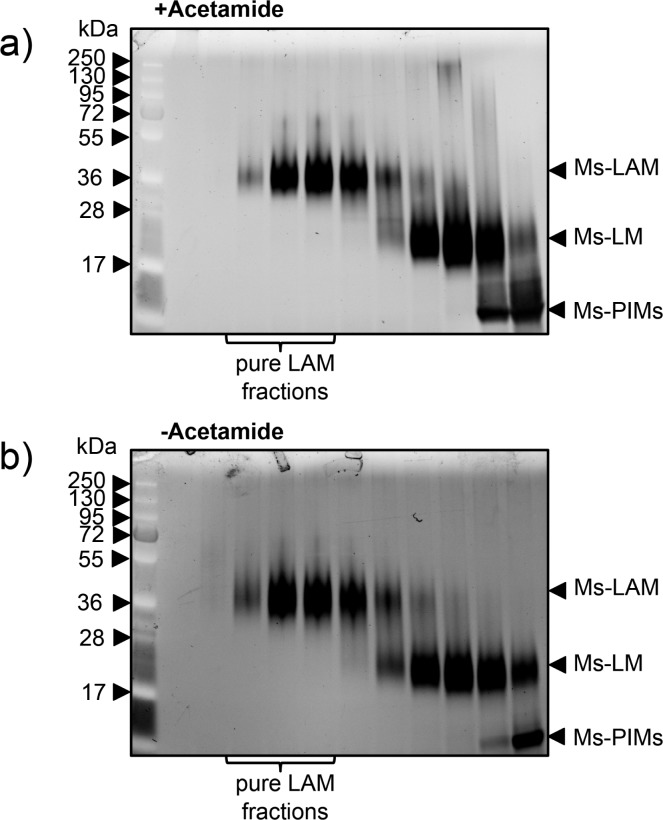
LAM purification profiles of M. smegmatisΔaftB pMV306-aftB grown with (a) and without (b) acetamide by gel exclusion chromatography. Eluted samples were analyzed using SDS-PAGE and visualized by Pro-Q emerald glycoprotein stain. The three major bands represented by Ms-LAM, Ms-LM, and Ms-PIMs are indicated. The protein molecular weight standards are provided on the left for comparison. The indicated fractions that contained pure LAM lipoglycan were pooled, extensively dialyzed in water, and dried.
Figure 6.
Immune response profiles elicited by LAM extracted from conditional aftB mutant cultured in the presence and absence of acetamide. (a) TLR4-dependent NF-κB activity by human HEK293-TLR4 cell line in response to the lipoglycans. (b) TLR2-dependent NF-κB activity by human HEK293-TLR2 cell line in response to the lipoglycans. TLR2-dependent NF-κB (c) activation and IL-8 (d) production by human monocyte/macrophage THP1 cell line in response to lipoglycans. TLR2-dependent NF-κB (e) activation and IL-8 (f) production by human THP-1 cells in response to lipoglycans were tested using blocking antibody anti-TLR-2. Results are mean ± SD from n = 2 (a–c, e) or n = 3 (d, f) and are from one representative of two (d, f) or three (a–c, e) independent experiments. The ×10 means that the concentration of cytokine detected is amplified 10-fold. *< 0.05; n.i., not induced.
A substantial number of biological activities have been associated with phosphatidyl-myo-inositol-based glycolipids, notably LM and LAM.22,23,30 LM, a precursor of LAM, is composed of the conserved mannosyl-phosphate inositol anchor and mannan core. It was demonstrated to carry a dual modulatory function where it acts as a strong agonist for TLR2-dependent stimulation as well as exhibits TLR2 independent inhibition of cell activation and proinflammatory cytokine synthesis in murine primary macrophages.25 Further studies revealed that the degree of LM acylation plays an important role in pro- and anti- inflammatory properties31,32 whereas its mannan chain directly determines the lipoglycan activity.24 Specifically, the activity of lipoglycans seemed to increase with the number of Manp units composing the mannan core. Interestingly, mycobacterial LAMs have been shown to be poor agonists of TLR2 despite containing the LM glycosidic moiety. It is unclear how, but the large arabinan domain in LAM perturbs the biological activity of the mannan core. This was further confirmed by the chemical degradation of the arabinan domain of LAM and the regain of proinflammatory properties.26 The data presented here are in agreement with these findings as the truncated LAM isolated from the conditional aftB mutant demonstrated an increase in TLR2-dependent proinflammatory activity compared to the full-length LAM. It was suggested that the arabinan domain may exert its inhibitory effect by steric hindrance, which prevents interaction between TLR2 and the underlying mannan domain.26 The arrangement of the arabinofuranose residues in LAM is largely dependent on the flexibility of the glycosidic linkages and puckering of the rings. It is known that ring puckering along with the more rigid β(1 → 2) linkages promote water mediated hydrogen bonding between different arabinofuranose residues. A structural study using fragments of the nonreducing termini of LAM and a murine antibody CS-35Fab has suggested that such hydrogen bonding between the arabinofuranose rings might contribute to the stabilization and orientation of the arabinan component of LAM.33 Therefore, we hypothesize that removal of the terminal β(1 → 2) linkages can lead to a decrease in the propensity of hydrogen bonding and subsequently to a less organized and structurally compact arabinan domain as well as offer additional flexibility. This in turn can result in a more exposed “bioactive” mannan core. Further investigation is required to fully understand arabinan’s role of LAM in relation to TLR2 activation.26
Methods
Plasmid Construction
The 1938 bp coding region aftB (MSMEG_6400) was amplified by PCR from M. smegmatis mc2155 genomic DNA using the primer pair (restriction sites underlined): 5′-GAT CGA TCG GAT CCG TGC GCA TCA GCC TGT GGC-3′ and 5′-GAT CGA TCA TCG ATC TAC GGT CCC GTT GCC GGC-3′. A single copy integrating plasmid pMV306-aftB was generated by ligating a 2.6 kb inducible acetamide promoter digested with XbaI-BamHI and a 1.9 kb aftB digested with BamHI-ClaI into pMV306 digested with XbaI-ClaI resulting in a pMV306 construct containing aftB gene cloned downstream of the acetamide promoter. The merodiploid strain was generated by electroporation of M. smegmatis with pMV306-aftB and selecting for kanamycin resistant colonies.
Generation of ΔaftB Conditional Mutant in M. smegmatis
Approximately 1 kb upstream and downstream, flanking sequences of MSMEG_6400 (aftB) were PCR amplified from M. smegmatis mc2155 genomic DNA using the primer pairs: MS6400LL (5′-TTT TTT TTC CAT AAA TTG GGA GTT ACA CCA GCA GCT ACC-3′) and MS6400LR (5′-TTT TTT TTC CAT TTC TTG GAC CAG CAC ACC ATC ATC C-3′) as well as MS6400RL (5′-TTT TTT TTC CAT AGA TTG GAT GGG CAT GCT GGG CAT GAA CG-3′) and MSMEG6400RR (5′-TTT TTT TTC CAT CTT TTG GCA CCG AGA TGC CCG AGT TGT AG-3′). Following restriction digestion of the primer incorporated Van91I sites, the PCR fragments were cloned into Van91I-digested p0004S to yield the plasmid pΔMSMEG6400. The resultant plasmid was then packaged into the temperature sensitive phage phAE159 as described previously34 to yield the knockout phage phΔMSMEG6400. Specialized transduction was performed as described earlier,34 except that the host strain was the merodiploid strain and half of the transduction mix was spread on TSB agar plates containing kanamycin, hygromycin, and 0.2% (w/v) acetamide while the other half was spread on plates lacking acetamide. Deletion of the aftB gene was confirmed using Southern blot analysis.
Conditional Depletion of AftB
Strains to be tested were grown in either TSB or minimal medium supplemented with 0.05% (v/v) Tween-80 and 0.2% (w/v) acetamide to an OD 600 nm of 0.5. Cells were washed twice with media to remove traces of acetamide and resuspended in the original volume of appropriate media. Culture was used as a 20% inoculum in minimal media and grown for 12 h to deplete intracellular AftB. The depleted subculture served as inoculum (5%) for cultures with or without 0.2% (w/v) acetamide. Aliquots were taken and labeled with either [1, 2-14C]-glucose 1 μCi mL–1 or [13C6]-glucose for 10 h. Cells were harvested, washed with phosphate buffered saline, and dried.
Cell Stimulation Assays
For stimulation assays, HEK293 and THP-1 cells were plated at 50 000 cells per well. Cells were stimulated with LAM at 0.5 ng mL–1 to 10 μg mL–1 in a final volume of 200 μL. Nonstimulated cells and cells stimulated with Pam3CSK4 (InvivoGen) or LPS (InvivoGen) served as negative and positive controls, respectively. Alkaline phosphatase activity was measured after 16–18 h by mixing 20 μL of the culture supernatant and 180 μL of Quanti-BlueTM, reading absorbance at OD 630 nm. To investigate TLR2 dependence, THP-1 cells were preincubated for 30 min at 37 °C with 5 or 10 μg mL–1 of anti-TLR2 monoclonal antibody (clone T2.5, InvivoGen, mab-mtlr2, lot # MT2-37-01) or isotype control (IgG1, eBioscience, 16-4714–85, lot # 4295108).
Cytokine ELISA
Culture supernatants from THP-1 cells were harvested and assayed for IL-8 cytokine production using commercially available ELISA kit and according to the manufacturer’s instructions (Ready Set-Go ELISA kits, eBioscience).
Acknowledgments
We acknowledge the national NMR facility (HWB-NMR) at the University of Birmingham for access to high-field NMR spectrometers and the Microbiology Society for a Research Visit Grant. We also thank B. J. Appelmelk for the gift of the monoclonal antibody F30-5 and M. Parny (IPBS, Toulouse) for help with the cell culture. G.S.B. acknowledges support in the form of a Personal Research Chair from James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow. We acknowledge funding from the MRC (grant MR/K012118/1) and the Wellcome Trust (grant 081569/Z/06/Z). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.6b00898.
Materials and Methods section (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization. (2015) Global Tuberculosis Report 2015.
- Chatterjee D.; Hunter S. W.; McNeil M.; Brennan P. J. (1992) Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 267, 6228–6233. [PubMed] [Google Scholar]
- Nigou J.; Gilleron M.; Cahuzac B.; Bounéry J. D.; Herold M.; Thurnher M.; Puzo G. (1997) The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette Guérin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J. Biol. Chem. 272, 23094–23103. 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- Chatterjee D.; Bozic C. M.; McNeil M.; Brennan P. J. (1991) Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J. Biol. Chem. 266, 9652–9660. [PubMed] [Google Scholar]
- Chatterjee D.; Khoo K. H.; McNeil M. R.; Dell A.; Morris H. R.; Brennan P. J. (1993) Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology 3, 497–506. 10.1093/glycob/3.5.497. [DOI] [PubMed] [Google Scholar]
- Angala S. K.; McNeil M. R.; Zou L.; Liav A.; Zhang J.; Lowary T. L.; Jackson M. (2016) Identification of a novel mycobacterial arabinosyltransferase activity which adds an arabinosyl residue to α-d-mannosyl residues. ACS Chem. Biol. 11, 1518–1524. 10.1021/acschembio.6b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo K. H.; Dell A.; Morris H. R.; Brennan P. J.; Chatterjee D. (1995) Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J. Biol. Chem. 270, 12380–12389. 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- Guérardel Y.; Maes E.; Elass E.; Leroy Y.; Timmerman P.; Besra G. S.; Locht C.; Strecker G.; Kremer L. (2002) Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with α 1,3-mannopyranose side chains. J. Biol. Chem. 277, 30635–30648. 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- Mishra A. K.; Alves J. E.; Krumbach K.; Nigou J.; Castro A. G.; Geurtsen J.; Eggeling L.; Saraiva M.; Besra G. S. (2012) Differential arabinan capping of lipoarabinomannan modulates innate immune responses and impacts T helper cell differentiation. J. Biol. Chem. 287, 44173–44183. 10.1074/jbc.M112.402396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A.; Philipp W. J.; Sreevatsan S.; Bernasconi C.; Stockbauer K. E.; Wieles B.; Musser J. M.; Jacobs W. R. (1997) The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3, 567–570. 10.1038/nm0597-567. [DOI] [PubMed] [Google Scholar]
- Belanger A. E.; Besra G. S.; Ford M. E.; Mikusová K.; Belisle J. T.; Brennan P. J.; Inamine J. M. (1996) The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. U. S. A. 93, 11919–11924. 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch H. L.; Alderwick L. J.; Bhatt A.; Rittmann D.; Krumbach K.; Singh A.; Bai Y.; Lowary T. L.; Eggeling L.; Besra G. S. (2008) Biosynthesis of mycobacterial arabinogalactan: identification of a novel α(1→3) arabinofuranosyltransferase. Mol. Microbiol. 69, 1191–1206. 10.1111/j.1365-2958.2008.06354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch H. L.; Alderwick L. J.; Appelmelk B. J.; Maaskant J.; Bhatt A.; Singh A.; Nigou J.; Eggeling L.; Geurtsen J.; Besra G. S. (2010) A truncated lipoglycan from mycobacteria with altered immunological properties. Proc. Natl. Acad. Sci. U. S. A. 107, 2634–2639. 10.1073/pnas.0915082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovierová H.; Larrouy-Maumus G.; Zhang J.; Kaur D.; Barilone N.; Korduláková J.; Gilleron M.; Guadagnini S.; Belanová M.; Prevost M.-C.; Gicquel B.; Puzo G.; Chatterjee D.; Brennan P. J.; Nigou J.; Jackson M. (2009) AftD, a novel essential arabinofuranosyltransferase from mycobacteria. Glycobiology 19, 1235–1247. 10.1093/glycob/cwp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel M.; Alderwick L. J.; Birch H. L.; Sahm H.; Eggeling L.; Besra G. S. (2007) Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 282, 14729–14740. 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- Bhatt A.; Jacobs W. R. (2009) Gene essentiality testing in Mycobacterium smegmatis using specialized transduction. Methods Mol. Biol. 465, 325–336. 10.1007/978-1-59745-207-6_22. [DOI] [PubMed] [Google Scholar]
- Sassetti C. M.; Boyd D. H.; Rubin E. J. (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84. 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Appelmelk B. J.; den Dunnen J.; Driessen N. N.; Ummels R.; Pak M.; Nigou J.; Larrouy-Maumus G.; Gurcha S. S.; Movahedzadeh F.; Geurtsen J.; Brown E. J.; Eysink Smeets M. M.; Besra G. S.; Willemsen P. T. J.; Lowary T. L.; van Kooyk Y.; Maaskant J. J.; Stoker N. G.; van der Ley P.; Puzo G.; Vandenbroucke-Grauls C. M. J. E.; Wieland C. W.; van der Poll T.; Geijtenbeek T. B. H.; van der Sar A. M.; Bitter W. (2008) The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell. Microbiol. 10, 930–944. 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Driessen N. N.; Ummels R.; Maaskant J. J.; Gurcha S. S.; Besra G. S.; Ainge G. D.; Larsen D. S.; Painter G. F.; Vandenbroucke-Grauls C. M. J. E.; Geurtsen J.; Appelmelk B. J. (2009) Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect. Immun. 77, 4538–4547. 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron M.; Bala L.; Brando T.; Vercellone A.; Puzo G. (2000) Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. J. Biol. Chem. 275, 677–684. 10.1074/jbc.275.1.677. [DOI] [PubMed] [Google Scholar]
- Driessen N. N.; Stoop E. J. M.; Ummels R.; Gurcha S. S.; Mishra A. K.; Larrouy-Maumus G.; Nigou J.; Gilleron M.; Puzo G.; Maaskant J. J.; Sparrius M.; Besra G. S.; Bitter W.; Vandenbroucke-Grauls C. M. J. E.; Appelmelk B. J. (2010) Mycobacterium marinum MMAR_2380, a predicted transmembrane acyltransferase, is essential for the presence of the mannose cap on lipoarabinomannan. Microbiology (London, U. K.) 156, 3492–3502. 10.1099/mic.0.037507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. K.; Driessen N. N.; Appelmelk B. J.; Besra G. S. (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol. Rev. 35, 1126–1157. 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankute M.; Cox J. A. G.; Harrison J.; Besra G. S. (2015) Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol. 69, 405–423. 10.1146/annurev-micro-091014-104121. [DOI] [PubMed] [Google Scholar]
- Nigou J.; Vasselon T.; Ray A.; Constant P.; Gilleron M.; Besra G. S.; Sutcliffe I.; Tiraby G.; Puzo G. (2008) Mannan chain length controls lipoglycans signaling via and binding to TLR2. J. Immunol. 180, 6696–6702. 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- Quesniaux V. J.; Nicolle D. M.; Torres D.; Kremer L.; Guérardel Y.; Nigou J.; Puzo G.; Erard F.; Ryffel B. (2004) Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172, 4425–4434. 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Vignal C.; Guérardel Y.; Kremer L.; Masson M.; Legrand D.; Mazurier J.; Elass E. (2003) Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-α and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J. Immunol. 171, 2014–2023. 10.4049/jimmunol.171.4.2014. [DOI] [PubMed] [Google Scholar]
- Lanéelle M.-A.; Nigou J.; Daffé M. (2015) Lipid and lipoarabinomannan isolation and characterization. Methods Mol. Biol. 1285, 77–103. 10.1007/978-1-4939-2450-9_5. [DOI] [PubMed] [Google Scholar]
- Mishra A. K.; Alderwick L. J.; Rittmann D.; Wang C.; Bhatt A.; Jacobs W. R. Jr.; Takayama K.; Eggeling L.; Besra G. S. (2008) Identification of a novel α(1→6) mannopyranosyltransferase MptB from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomannan- and lipoarabinomannan-deficient mutant. Mol. Microbiol. 68, 1595–1613. 10.1111/j.1365-2958.2008.06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. C.; Young D. W.; Golenbock D. T.; Christ W. J.; Gusovsky F. (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692. 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Angala S. K.; Belardinelli J. M.; Huc-Claustre E.; Wheat W. H.; Jackson M. (2014) The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 49, 361–399. 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doz E.; Rose S.; Nigou J.; Gilleron M.; Puzo G.; Erard F.; Ryffel B.; Quesniaux V. F. J. (2007) Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 282, 26014–26025. 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- Gilleron M.; Nigou J.; Nicolle D.; Quesniaux V.; Puzo G. (2006) The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem. Biol. 13, 39–47. 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Murase T.; Zheng R. B.; Joe M.; Bai Y.; Marcus S. L.; Lowary T. L.; Ng K. K. S. (2009) Structural insights into antibody recognition of mycobacterial polysaccharides. J. Mol. Biol. 392, 381–392. 10.1016/j.jmb.2009.06.074. [DOI] [PubMed] [Google Scholar]
- Bardarov S.; Bardarov S. Jr; Pavelka M. S. Jr; Sambandamurthy V.; Larsen M.; Tufariello J.; Chan J.; Hatfull G.; Jacobs W. R. Jr. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology (London, U. K.) 148, 3007–3017. 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.