Abstract
Rationale
Preclinical studies indicate that gonadal hormones are important determinants of drug self-administration. To date, little is known about the influence of sex and estrous cycle on drug self-administration in ecologically relevant social contexts.
Objective
Examine the role of sex and estrous cycle in a rat model during cocaine and heroin self-administration with male-female and female-female social dyads.
Methods
Male and female virgin rats were trained to self-administer cocaine and heroin in operant conditioning chambers that permitted two rats to self-administer concurrently but prevented physical contact. Experiment 1 examined cocaine self-administration on a progressive-ratio schedule in male-female dyads. Experiments 2 and 3 examined heroin self-administration on a fixed-ratio schedule in male-female dyads at constant and varying doses, respectively. Experiment 4 examined heroin self-administration in female-female dyads on a fixed-ratio schedule.
Results
Cocaine-maintained breakpoints increased by ~17% in females during estrus but remained consistent in males. Heroin self-administration decreased by ~70% during proestrus in females whether they were isolated, housed with males, or housed with females. Heroin self-administration was lower in males than females under some conditions and was not consistently associated with the responding of females.
Conclusions
Cocaine and heroin self-administration is influenced by the estrous cycle in females when in the presence of a male partner. As a novel finding, these data illustrate that heroin self-administration is reduced in females during proestrus regardless of the social context tested. Finally, these data suggest that drug self-administration in males is only minimally influenced by the hormonal status of a female partner.
Keywords: cocaine, estrous, heroin, self-administration, sex, social
Introduction
Clinical studies indicate that sex plays a critical role in the etiology and treatment of substance use disorders. For example, women transition to patterns of abuse more rapidly, relapse more readily, and report greater craving for cocaine than men (Becker et al. 2005; Kosten et al. 1993; Westermeyer et al. 1997). Similarly, women meet criterion for dependence to opioids more rapidly and enter treatment for opioid abuse sooner than men (Anglin et al. 1987; Hser et al. 1987). Preclinical studies also indicate that sex plays a critical role in the acquisition, maintenance, escalation, and reinstatement of drug self-administration in laboratory animals, with females exhibiting greater susceptibility to the effects of multiple drugs as compared to males (Caggiula et al. 2002; Campbell and Carroll 2000; Lynch 2006; Lynch and Carroll 1999; Lynch et al. 2002; Marusich et al. 2015; Roth and Carroll 2004; Wissman et al. 2011). Female rats are also more sensitive than male rats to the effects of social defeat-induced stress on measures of cocaine-induced locomotor activity and binge-like cocaine self-administration (Holly et al 2012). Furthermore, in females, a social defeat-induced anhedonic phenotype is predictive of binge-like cocaine self-administration (Shimamoto et al. 2015).
Studies using human participants have reported increased subjective ratings of euphoria and craving during the follicular phase (high estrogen) compared to the luteal phase (moderate estrogen) following administration of amphetamine and smoked cocaine (Evans and Foltin 2006; Evans et al. 2002; Justice and de Wit 1999; White et al. 2002). Similarly, studies conducted in rats reveal that drug sensitivity and drug self-administration are dependent on gonadal sex hormones and vary across the rat estrous cycle (Caine et al. 2004; Carroll et al. 2004; Carroll and Smethells 2015; Grimm and See 1997; Larson et al. 2007; Lynch et al. 2001; Segarra et al. 2014). For instance, Roberts and colleagues (1989) reported that higher cocaine-maintained breakpoints were observed in female rats during the estrus phase as compared to other phases. Lynch (2008) reported similar results and found significant correlations between estradiol concentrations and cocaine-maintained breakpoints on a progressive-ratio (PR) schedule. Similar effects have been found for cocaine-induced locomotion and stereotypy, with the highest levels of both behaviors occurring during the estrus phase relative to all other phases (Quinones-Jenab et al. 1999). Taken together, these studies indicate a critical role of estrus in cocaine-related behaviors.
Fewer studies have examined the role of the estrous cycle during opioid self-administration, and research to date has failed to demonstrate a consistent effect of the estrous cycle and gonadal hormones on opioid intake. For example, Stewart and colleagues (1996) reported no effects of estrogen in ovariectomized (OVX) rats relative to vehicle-injected controls during acquisition and maintenance of heroin self-administration on a PR schedule. In contrast, Roth and colleagues (2002) reported that estrogen treatment increased both acquisition and heroin-maintained responding on a fixed-ratio (FR) schedule compared to vehicle-treated controls, but no effect of estrous cycle was observed on the acquisition of heroin self-administration. The apparent inconsistency may be the result of differential testing procedures (e.g., maintenance relative to acquisition phases and the duration of test sessions).
Changes in drug-maintained responding as a function of estrous cycle may be of particular interest when placed in the context of the growing body of literature indicating that drug taking can be influenced by a social partner (for reviews see Strickland and Smith 2014; 2015). Until recently, studies examining the effects of social contact on measures of drug self-administration were limited by the lack of commercially available equipment allowing more than one animal to engage in drug self-administration in close proximity to a partner. Our laboratory recently developed operant conditioning chambers that allow rats full visual, auditory, olfactory, and limited tactile contact during drug self-administration. Using these chambers, we reported that cocaine self-administration can either be facilitated or inhibited by social contact based on whether a partner is also self-administering cocaine (Smith 2012). These findings are consistent with epidemiological studies reporting the influence of peers (and the behavior of peers) on drug use among adolescents and young adults (Bot et al. 2005; Salvy et al. 2014). These chambers thus provide an ecologically valid model of drug use by which drug effects may be examined within a social context.
Previous research on the effects of social contact on intravenous drug self-administration has relied mostly on males and male-male dyads. This represents a significant limitation of the literature given the increasing prevalence of drug use disorders in females (Brady et al. 2009; Greenfield et al. 2010) and the ubiquitous nature of mixed-sex dyads in humans. Accordingly, we designed a series of experiments to assess these features of human drug use in social operant conditioning chambers. To this end, we first examined cocaine breakpoints on a PR schedule and heroin intake on a FR schedule, both common tests of reinforcing efficacy for these drugs (Arnold and Roberts 1997). Importantly, we used male-female dyads and later female-female dyads to assess the role of social contact and the estrous cycle on drug self-administration. Additional experiments were conducted to elucidate the novel finding regarding the effects of estrous cycle on heroin intake in social contexts.
Methods
Animals
Nulliparous male and female rats arrived to the vivarium at ~70 days old. The rats were single-housed for one week to acclimate to the vivarium. Except during a brief period of food restriction required for lever press training, rats had free access to food and water throughout the experiment. Animals were maintained on a 12:12 light/dark cycle (lights on: 0500). All subjects were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 2011) and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Davidson College.
Apparatus
Rats were trained in commercially available operant conditioning chambers (Med Associates Inc., St. Albans, VT) during lever press training. The custom-built, operant conditioning chambers used throughout self-administration training and testing are modular in design, constructed with stainless steel and aluminum, have a wire screen bisecting the chambers, and are IACUC-approved for the long-term housing of rats (Faircloth Machine Shop, Winston-Salem, NC). The primary advantage of these chambers is they allow rats full visual, auditory, olfactory, and limited tactile contact during drug self-administration sessions. Subjects were moved into the custom-built chambers one week after arrival and remained in the chambers for the remainder of the experiment. Importantly, in the case of the male-female dyads, the wire screen dividing the chamber prevented copulation. These animals had no previous sexual experience or established dominance hierarchy.
Lever Press Training
Lever press training occurred according to previously published methodologies (Smith 2012; Smith et al. 2008; 2014). Importantly, operant training sessions continued until rats acquired 40 food pellets during three consecutive training sessions; in general, this required one week of training. Following completion of lever press training, rats were placed back on unrestricted access to food for the remainder of the study.
Estrous Cycle Monitoring
Concurrent with the beginning of lever-press training, daily collection of vaginal cells (via lavage) began in female subjects. Samples were collected and analyzed using light microscopy (100×) less than one hour before each self-administration session. The cells were categorized into one of four estrous phases: metaestrus, diestrus, proestrus and estrus (Goldman et al. 2007; Hubscher et al. 2005; Marcondes et al. 2002;). Data were used from cycles in which all phases were represented. Overall, 18.4% of cycles were not used because of incomplete cycles; however, all females (100%) showed multiple complete cycles both before and during drug exposure. We found no evidence that either cocaine or heroin suppressed cycles. Data were obtained from 3–7 cycles from each rat per experiment.
Catheter Implantation
Briefly, rats were anesthetized with a combination of ketamine/xylazine and a catheter was implanted in the right jugular vein that exited on the dorsal surface between the scapulae (Lacy et al. 2014a; Smith et al. 2008). Catheters were flushed with ticarcillin for 7 days and only heparinized saline thereafter. Rats were allowed a minimum of three days of recovery before self-administration began.
Self-administration Training
Male-female dyads were given access to 1.0 mg/kg/infusion cocaine (Experiment 1) or 0.01 mg/kg/infusion heroin (Experiments 2–4) on a fixed-ratio (FR1) schedule during 4-hour sessions. Rats were restricted to a maximum of 40 reinforcers during this time. Rats were moved to the testing phase if they received more than 20 reinforcers in three consecutive sessions. All training and testing sessions began promptly at 1700, at the beginning of the dark phase of the light/dark cycle.
Self-administration Testing
Experiment 1: Male-female dyads (N=24; 12 male/female dyads) were tested using a progressive-ratio (PR) schedule, which successively increases the ratio value required to receive cocaine reinforcement, i.e., 1, 2, 4, 9, 12, 15, 20, 25, 32, 40, 50, 62…603 (Richardson and Roberts 1996). Breakpoints were defined as the number of infusions received. Sessions terminated automatically when one hour elapsed with no infusions. Rats were repeatedly tested on this PR schedule for approximately 30 days at a constant dose of 0.5 mg/kg/infusion cocaine. The number of PR sessions animals completed ranged from 21–32 (mean = 29.5).
Experiment 2: Male-female dyads (N=12; six male/female dyads) were tested on an FR1 schedule with a constant dose of 0.0075 mg/kg/infusion heroin for a minimum of 20 daily test sessions. All sessions were two hours in duration and no limit was placed on the number on infusions that could be obtained. Importantly, an FR schedule was used in this experiment (as well as Experiments 3 & 4) because opioid self-administration is not amenable to within-session PR procedures and requires modified (e.g., between-session) procedures in order to obtain breakpoints greater than vehicle infusion alone (Arnold and Roberts 1997; Grasing et al. 2003; Roberts and Bennett 1993). Consequently, results from different experiments are not necessarily directly comparable and should be considered exploratory.
Experiment 3: Male-female dyads (N=10; five male/female dyads) were tested on an FR1 schedule with 0.0003, 0.001, 0.003, 0.01, and 0.03 mg/kg/infusion heroin. Doses were tested in a pseudo-random order such that no more than two ascending or descending doses were tested in a row. Each dose was tested across an entire estrous cycle in each female and the same dose was tested simultaneously in her male social partner. In this way, each of the five doses were tested for at least four consecutive days (to accommodate each estrous phase), leading to at least 20 daily test sessions. Pairs of animals changed to a new dose following the completion of the female’s estrus cycle. Males and females were tested at the same time with access to the same concentrations of heroin for the same duration. Again, all sessions were two hours in duration and no limit was placed on the number of infusions that could be obtained.
Experiment 4: Female-female dyads (N=12; six female/female dyads) and three isolated females were tested with a constant dose of 0.0075 mg/kg/infusion heroin in the same manner as that described in Experiment 2 (see above).
Data Analysis
For all experiments, the metaestrus and diestrus phases were averaged together, as means, in a manner similar to that described in previous studies (referred to as M/D below; Feltenstein and See 2007; Roberts et al. 1989). For the PR studies, data were defined as the number of infusions obtained as opposed to the final ratio value completed because the latter are not normally distributed and show high heterogeneity of variance (Green et al. 2002; Liu et al. 2005). In Experiment 1, a mixed-factor ANOVA (Sex × Cycle) was performed to determine differences between males and females on cocaine-maintained breakpoints. Experiment 2 also employed a mixed-factor ANOVA (Sex × Cycle) to examine males and females during heroin self-administration. A mixed-factor ANOVA (Sex × Cycle × Dose) was used to analyze data gathered in Experiment 3. Area under the curve calculations were also determined in Experiment 3 using the trapezoidal rule (Cooper et al. 2008) and the effect of Cycle was examined via repeated-measures ANOVA. In Experiment 4, a repeated-measures ANOVA (Cycle) was used to examine heroin self-administration in female-female dyads and isolated females. For all analyses, self-administration data were averaged over multiple cycles for each rat resulting in a mean value for each of the three phases of estrous: meta/diestrus (M/D), proestrus (P), and estrus (E). Mean values were taken in this manner to prevent some rats (i.e., those completing a greater number of cycles) from being over-represented in the analyses. Post-hoc t-tests, using the Holm-Bonferroni correction, were used to examine between and within-group differences following significant effects in the omnibus analysis. An alpha level of 0.05 was used to determine statistical significance for all analyses.
Results
Experiment 1: The role of estrous cycle in male-female dyads during cocaine self-administration on a PR schedule
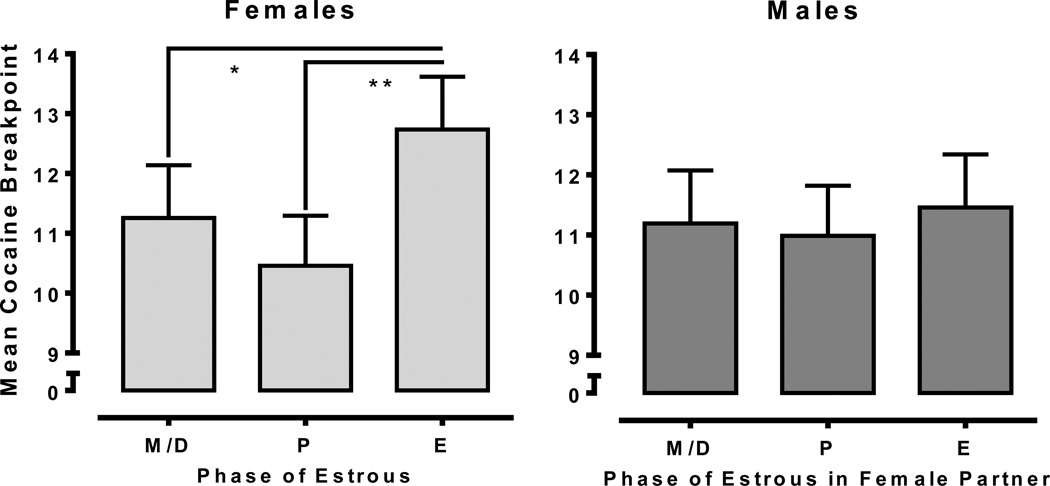
Figure 1 shows breakpoints maintained by cocaine on a PR schedule in male-female dyads. A 2×3 mixed-factor ANOVA revealed a significant main effect of Cycle [F(2, 44) = 6.02, p<.01], indicating that cocaine-maintained breakpoints were greatest during estrus. Analyses based upon a priori predictions in females revealed a significant main effect of Cycle [F(2, 22) = 6.61, p<.01], with the highest breakpoints reached during estrus. Post-hoc analyses indicated that breakpoints during estrus were significantly higher than those during meta/diestrus and proestrus. Conversely, analyses of males revealed no differences in breakpoints across the estrous cycle of their partners. These data indicate that estrus is associated with increased cocaine-maintained breakpoints in females but not in their male partners.
Fig. 1.
Mean cocaine-maintained breakpoints (0.5 mg/kg cocaine) for females (left; n=12) and males (right; n=12) as a function of the female’s estrous cycle. Females reached significantly higher breakpoints during the estrus (E) phase relative to other phases. Male partners did not differ across the estrous cycle of their female partner (*<.05; **<.01).
Experiment 2: The role of estrous cycle in male-female dyads during heroin self-administration on an FR1 schedule (constant dose)
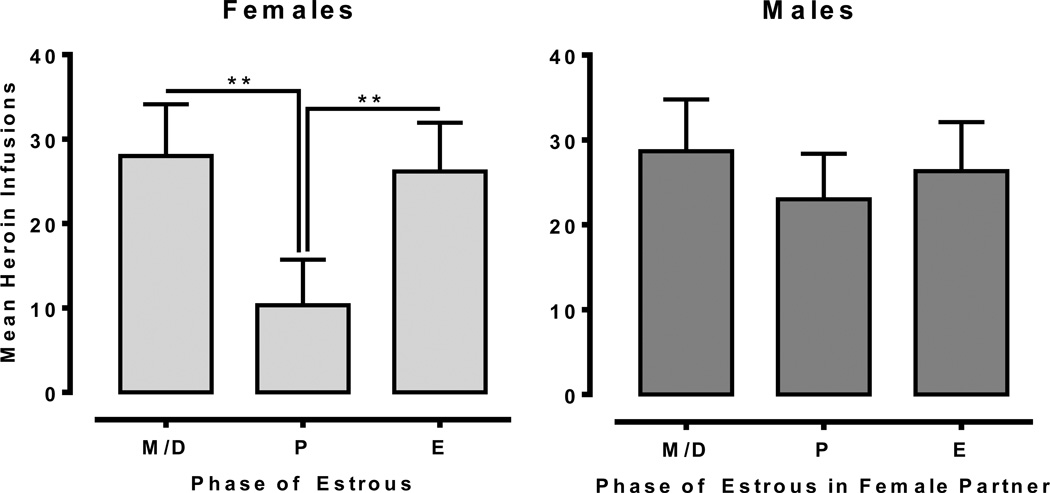
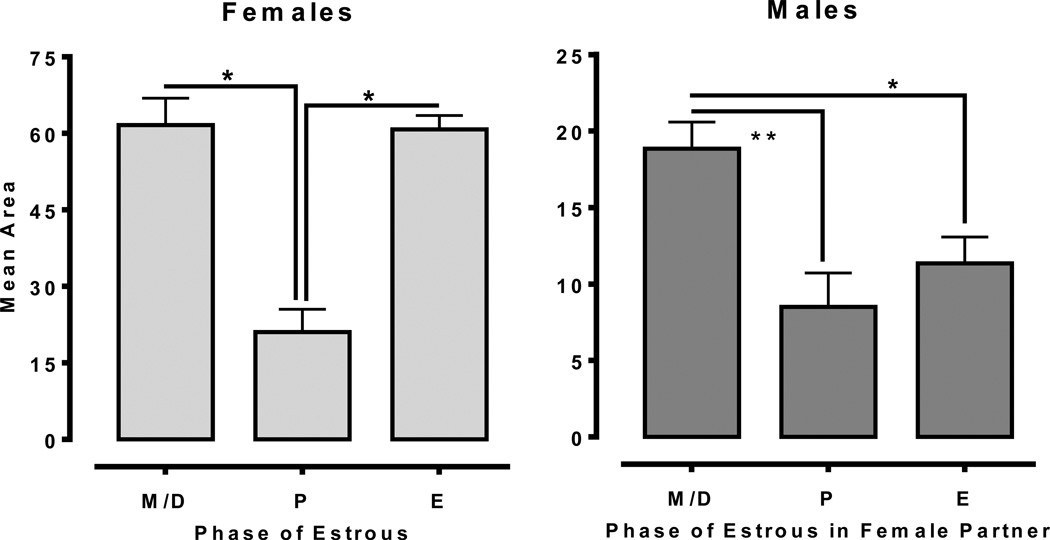
Figures 2 and 3 shows responding maintained by 0.0075 mg/kg/infusion heroin on an FR schedule in male-female dyads. The 2×3 mixed-factor ANOVA revealed a significant main effect of Cycle [F(2, 20) = 22.46, p<.001] and a significant Sex × Cycle interaction [F(2, 20) = 7.60, p<.01]. Further analysis in females revealed a significant main effect of Cycle [F(2, 10) = 21.83, p<.001], with post-hoc tests indicated a significant reduction in heroin infusions during proestrus relative to estrus and meta/diestrus (p<.01). In contrast, heroin self-administration in males was consistent across sessions and did not match the pattern of infusions received by their female partners.
Fig. 2.
Mean heroin infusions for females (left; n=6) and males (right; n=6) at a constant dose of 0.0075 mg/kg/infusion. Females showed significantly lower heroin self-administration during the proestrus (P) phase of the estrous cycle. Males; however, did not show any differences in heroin infusions as a function of their female partner’s estrous cycle (**<.01).
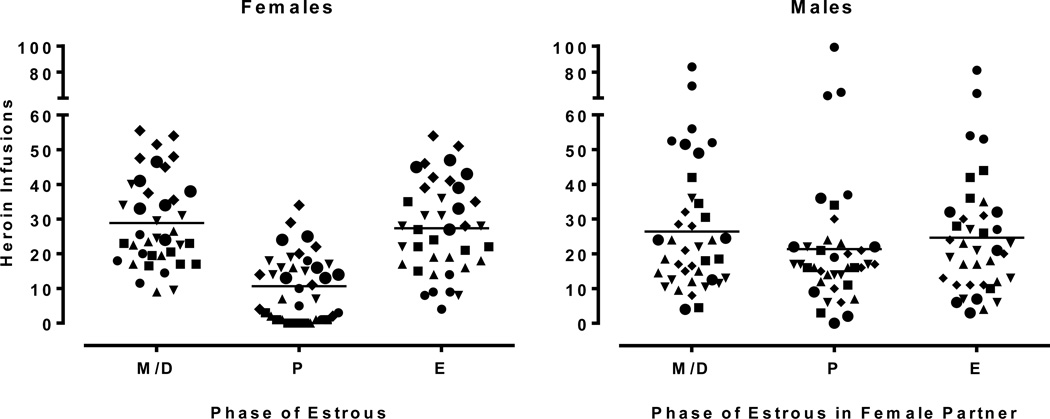
Fig. 3.
Scatterplot of the data from Experiment 2. Unique symbols represent each rat, the symbols of female (n=6) and male (n=6) partners match (across the figures) indicating a social dyad in the experiment. Data represent self-administration from 5–8 complete estrous cycles.
Experiment 3: The role of estrous cycle in male-female dyads during heroin self-administration on an FR1 schedule (dose-response analysis)
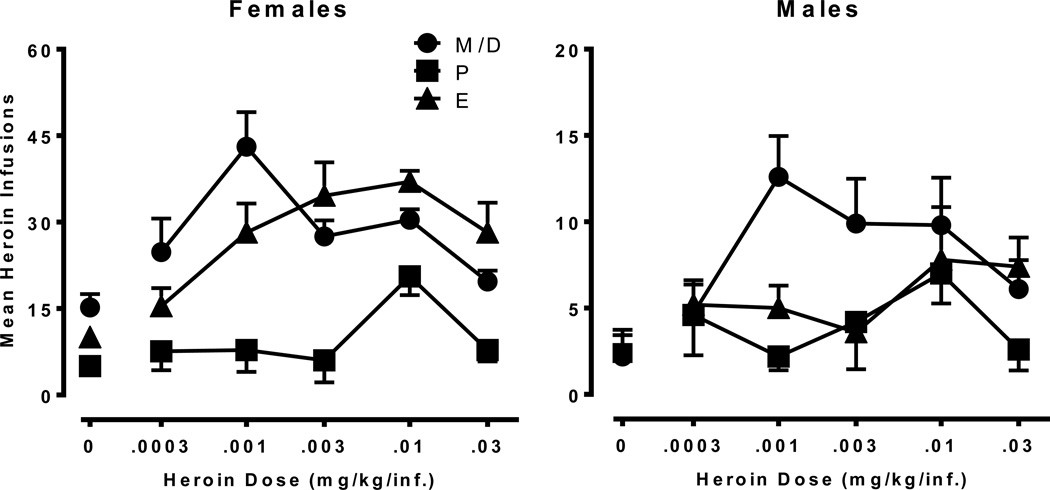
Figure 4 shows the dose-response curve of heroin on an FR schedule in male-female dyads. A 2×3×5 mixed-factor ANOVA revealed significant main effects of Dose [F(4,32) = 4.78, p<.01], Cycle [F(2,16) = 39.08, p<.001], and Sex [F(1,8) = 105.71, p<.001]. The interactions of Cycle × Sex [F(2,16) = 20.48, p<.001] and Dose × Cycle [F(8,64) = 3.59, p<.05] were also significant. Heroin self-administration was generally characterized by an inverted “U-shaped” dose-effect curve in both sexes regardless of the phase of estrous. The mean number of heroin infusions during proestrus was significantly lower than meta/diestrus or estrus. The significant interaction of Cycle × Sex indicated that females self-administered more heroin than males with significant reductions during proestrus. Males, on the other hand, showed reduced responding during the proestrus and estrus phase of their female partner.
Fig. 4.
Mean heroin infusions across a range of heroin doses for females (left; n=5) and males (right; n=5) presented as a function of estrus cycle. The data points represent the average number of infusions based on one data point per animal at a given dose and phase of estrous. Results from females indicate a reduction in heroin self-administration during proestrus (P) compared to meta/diestrus (M/D) and estrus (E). Males show reductions in heroin self-administration during the estrus (E) and proestrus (P) phases of their female partners compared to meta/diestrus (M/D). Please note difference in Y-axis between females and males.
Separate analyses in females revealed a significant main effect of Cycle [F(2,8) = 31.06, p<.001], which is concordant with Experiment 2 and indicates a reduction in heroin self-administration during proestrus. The dose-effect curve was shifted downward, with the greatest reductions observed at low to moderate doses [Dose: F(4,16) = 4.17, p<.05; Dose × Cycle: F(8,32) = 3.05, p<.05]. An analysis of data from males revealed that heroin functioned as a reinforcer, with the .001, .003, and .03 mg/kg/infusion doses differing significantly from saline. Males exhibited reductions in self-administration during both the estrus and proestrus phase of their partner [Cycle: F(2,8) = 15.40, p<.01]. Area under the curve analyses (Figure 5) yielded significant effects of Cycle for females [F(2,8) = 24.06, p<.001] and males [F(2,8) = 11.63, p<.01]. These data indicate that females showed reductions in heroin self-administration during proestrus and males showed reductions in heroin self-administration during the proestrus and estrus phases of their female partners.
Fig. 5.
Area under the curve values derived from the heroin dose-response curve in Experiment 3. Data were calculated using the trapezoidal rule. Females (n=5) show significant reductions during proestrus (P) compared to other phases. Males (n=5) had significantly lower values during both proestrus (P) and estrus (E) compared to meta/diestrus (M/D) of their female partners (*<.05; **<.01). Please note difference in Y-axis between males and females.
Experiment 4: The role of estrous cycle in female-female dyads during heroin self-administration on an FR1 schedule (constant dose)
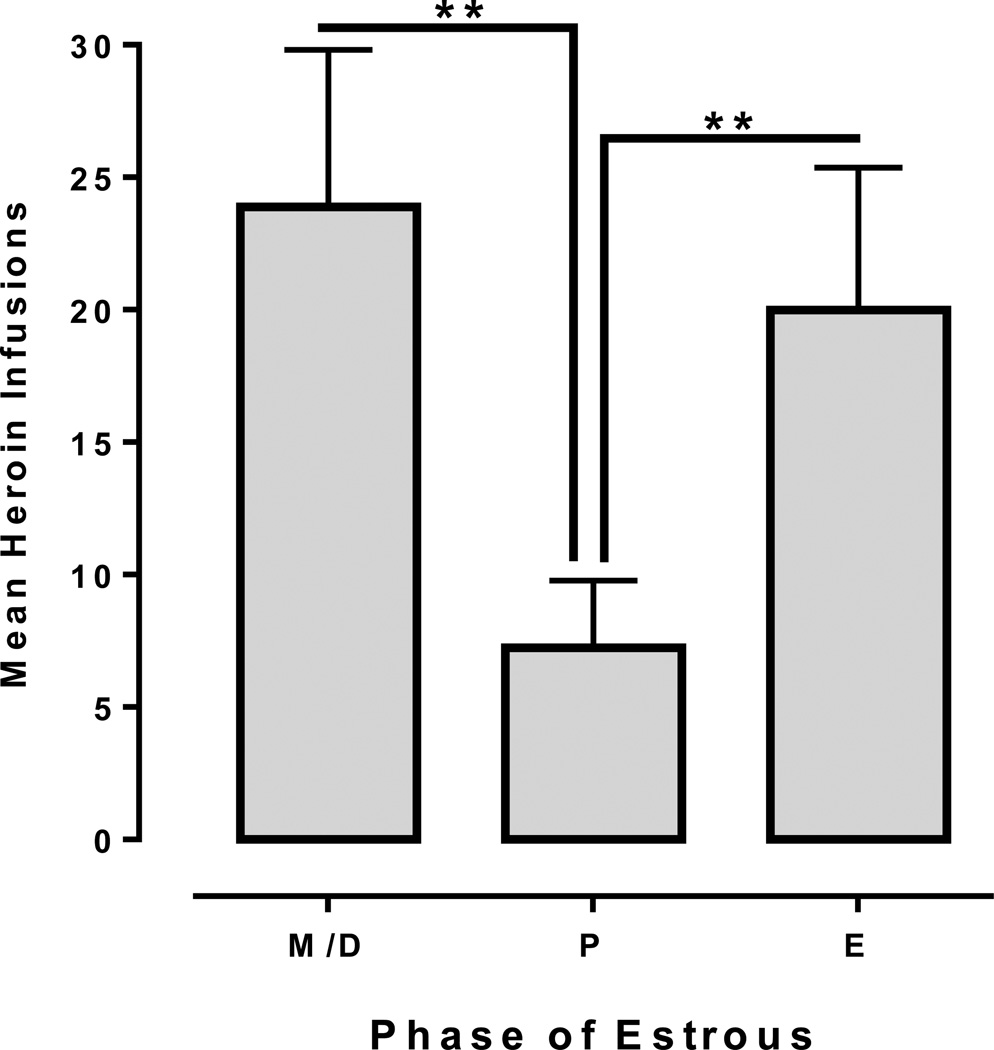
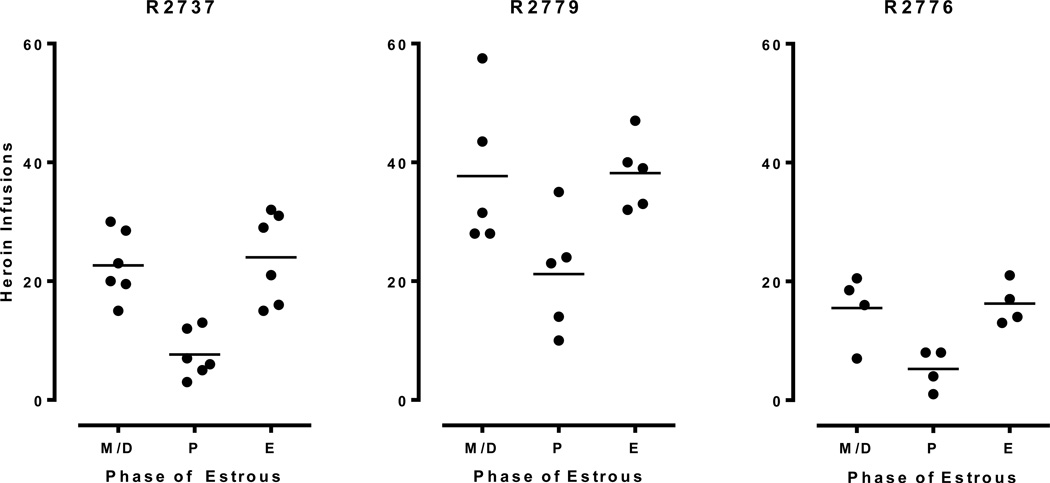
Figure 6 depicts responding maintained by 0.0075 mg/kg/infusion heroin in female-female dyads. A repeated-measures ANOVA revealed a significant main effect of Cycle [F(2,22)=12.18, p<.001], with significantly lower heroin infusions obtained during proestrus than other phases. Cohen’s kappa coefficients (i.e., a measure of inter-subject agreement for categorical data) indicated that the estrous cycles were not synchronized within dyads (mean k = −.04 for individual dyads). Additional analysis of data obtained from three individually housed females was in accord with the findings from the social dyads. A significant effect of Cycle [F(2, 4) = 65.07, p<.01] was found with post-hoc analyses revealing a significant reduction in heroin infusions during proestrus compared to other phases (Figure 7).
Fig. 6.
Mean heroin infusions obtained from female-female dyads (n=12). The mean number of infusions during proestrus (P) was significantly lower than during estrus (E) and meta/diestrus (M/D; *<.05; **<.01).
Fig. 7.
Data obtained from three isolated female rats (R2737, R2779, and R2776) presented as scatterplots. The mean number of infusions was significantly lower during proestrus than during estrus and meta/diestrus.
Discussion
The primary findings of this study were that drug self-administration frequently differed between males and females, and drug self-administration varied across the estrous cycle in females. We found that cocaine self-administration increased during estrus in females when a male partner was present and that heroin self-administration decreased during proestrus regardless of the social context. This latter effect was observed across a 100-fold range of heroin doses and across multiple estrous cycles, indicating a robust and reliable effect of proestrus on the positive reinforcing effects of heroin. Finally, we found that drug self-administration in males was generally not related to the drug-reinforced responding of their female partner, but may be influenced by their partner’s estrous cycle under some conditions. These data were collected in an ecologically relevant context in which rats were able to interact socially with either same-sex or opposite-sex partners. Epidemiological reports indicate that individuals are more likely to use drugs in the presence of others than when they are alone (Center for Behavioral Health Statistics and Quality 2015). The examination of male-female dyads is particularly relevant given the increased prevalence and importance of opposite-sex relationships during development (Dunphy 1963; Poulin and Pedersen 2007) and data suggesting that an opposite-sex relationship serves as a risk factor for drug use during adolescence (Dick et al. 2007; Molly et al. 2014; Mrug et al. 2011).
Sex-based differences in drug self-administration are a critical area of research given the increased susceptibility to drug abuse in female populations and the lesser amount of research focused on drug abuse in females (Becker et al. 2005; Brady and Randall 1999; Clayton and Collins 2014; Roth et al. 2004). In this study, we observed greater rates of heroin self-administration in females relative to males in Experiment 3. This finding is concordant with previous studies demonstrating greater rates of drug self-administration in females (Cicero et al. 2003; Lynch and Carroll 1999; Roth and Carroll 2004), and supports the hypothesis that females may be particularly sensitive to the reinforcing effects of drugs and may be at a higher risk for developing a substance use disorder.
Cocaine self-administration varied across the estrous cycle in females, peaking on the night of estrus. These data are consistent with those of previous studies (Lynch 2008; Roberts et al. 1989), and extend those findings to an ecologically relevant social model. In contrast, cocaine self-administration in males was remarkably consistent across sessions, and did not vary according to the behavior or hormonal status of their female partners. These data suggest that the effects of estrous on cocaine self-administration were specific to females and did not extend to their male partners, at least under conditions in which social contact was permitted but sexual contact was prevented.
Heroin self-administration also varied significantly across the estrous cycle in females. Specifically, we found a robust (~70%) reduction in heroin self-administration in females during proestrus that was reliable across repeated estrous cycles. To our knowledge, this effect has not been reported previously. These decreases were consistent across a 100-fold range of doses and reflected a downward shift in the dose-effect curve, suggesting a reduction in the efficacy of heroin (rather than a change in its potency) to function as a positive reinforcer. Moreover, this effect was apparent in every female tested, whether they were housed in isolation (3 out of 3 female rats), with a male partner (11 out of 11 female rats), or with a female partner (12 out of 12 female rats). Proestrus is of particular biological interest because of the relative increases in estradiol and progesterone during this period (Butcher et al. 1974).
The effects of the estrous cycle on drug self-administration are presumed to be due to variations in circulating ovarian hormones. Female rats show significantly more cocaine-induced locomotor behavior and cocaine self-administration during behavioral estrus, a period shortly after estradiol peaks in which females engage in proceptive/receptive behaviors (e.g., lordosis), than during other phases of the cycle (Jackson et al. 2006). Relative to non-estrus females, females in estrus showed greater responding during self-administration, extinction, and cocaine-primed reinstatement (Feltenstein and See 2007). Interestingly, this effect is selectively attenuated by progesterone (Feltenstein et al. 2009). Indeed the highest progesterone levels were noted at the time of lowest cocaine seeking (proestrus) and the lowest levels of progesterone occurred at the time of highest cocaine seeking (estrus; Feltenstein and See 2007). In ovariectomized rats, estradiol treatment increased cocaine sensitization (Festa and Quinones-Jenab 2004; Hu et al. 2004), cocaine self-administration (Jackson et al. 2006; Yang et al. 2007), and cocaine-seeking behavior during reinstatement (Larson et al. 2005). In contrast, progesterone attenuated the effects of estradiol, acting as a protective factor. For example, acute progesterone pretreatment reversed the effects of estradiol on the acquisition of cocaine self-administration (Jackson et al. 2006) and cocaine-primed reinstatement (Anker et al. 2007) in ovariectomized rats. In addition, the progesterone metabolite, allopregnanolone, blocked the escalation of cocaine self-administration (Anker et al. 2010), cocaine-primed reinstatement (Anker et al. 2009), and stress-induced reinstatement (Anker and Carroll 2010) in female rats. Taken together, these studies provide strong evidence for the role of gonadal hormones in cocaine abuse vulnerability, with estradiol increasing susceptibility and progesterone decreasing susceptibility.
The effects of gonadal hormones on heroin self-administration are less clear, and previous studies have failed to observe a consistent relationship. For instance, Roth and colleagues (2002) reported that estrogen-treated ovariectomized rats acquired heroin self-administration faster and obtained more heroin infusions than vehicle-treated rats. In contrast, Stewart and colleagues (1996) failed to find an effect of estradiol on the acquisition of heroin self-administration in ovariectomized rats and failed to find a difference in heroin-maintained breakpoints between estrogen-treated and progesterone-treated rats. These conflicting findings may be explained by procedural differences between the two studies, including the doses and schedules of reinforcement used. Interestingly, Roth and colleagues (2002) also reported no significant effect of the estrous cycle in control rats treated with vehicle on either the rate of acquisition or the number of heroin infusions obtained. It should be noted that sessions lasted 12 hours in that study (6 hours of autoshaping + 6 hours of self-administration), which likely meant that some test sessions overlapped multiple phases of estrous.
In the present study, we found that drug self-administration in males was influenced by the estrous cycle of their female partner, but only under very limited conditions. We previously reported that drug self-administration can either be facilitated or inhibited by social contact (Smith 2012; Smith et al. 2014) and that patterns of drug-reinforced responding become more similar over time within social dyads (Lacy et al. 2014b). We have also reported that rats without access to cocaine will press an inactive response lever in a way that “mimics” the responding of a social partner that is self-administering cocaine (Smith 2012). Such findings led us to hypothesize that variations in the responding of females across the estrous cycle would similarly affect the behavior of their male partners. Contrary to our hypothesis, males in Experiments 1 and 2 did not exhibit variations in their responding despite significant variations in the responding of their female partners across the estrous cycle. Interestingly, these data may assuage concerns by investigators about housing males and females in the same room for fear of the presence of opposite-sex animals influencing responding. In Experiment 3, heroin self-administration in males varied across the estrous cycle of their partners, but followed a different pattern relative to that of females. Specifically, whereas responding decreased only during proestrus in females, responding decreased during both proestrus and estrus in males. We emphasize that heroin self-administration was uniformly low in males, and never reached the levels seen in females. Collectively, these data do not support the hypothesis that drug self-administration in males varies according to that of their female partner, but does allow for a limited role of a female’s hormonal status under some conditions. As an additional consideration, the reinforcement schedules, procedures, and reinforcers varied between each of the experiments. This does not allow for direct comparison of cocaine versus heroin infusions, for example. Thus, each experiment should be interpreted individually, but with the commonality of the effects of estrous cycle on drug self-administration in social contexts. Similarly, it should be noted that all animals underwent lever press training prior to catheter implantation (see Methods), preventing an unbiased examination of the effects of these manipulations on the acquisition of drug-reinforced responding.
The present findings support previous studies describing significant sex differences in drug self-administration. As a novel finding, heroin self-administration was markedly and consistently reduced during proestrus, a time in which circulating concentrations of both estradiol and progesterone are high. In contrast to our hypothesis, the response patterns of males did not consistently track those of females, and there was only limited evidence that drug self-administration in males was influenced by the hormonal status of their female partners. Collectively, these findings demonstrate the influence of sex and the estrous cycle on measures of drug self-administration in an ecologically valid model of the social environment.
Acknowledgments
This study was funded by NIH Grants DA027485 and DA031725.
The authors thank Sarah Bills for expert technical assistance and the National Institute on Drug Abuse for supplying the study drugs.
Footnotes
The authors report no financial conflicts of interest.
References
- Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse. 1987;13:59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology (Berl) 2010;212:419–429. doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bot SM, Engels RC, Knibbe RA, Meeus WH. Friend's drinking behaviour and adolescent alcohol consumption: the moderating role of friendship characteristics. Addict Behav. 2005;30:929–947. doi: 10.1016/j.addbeh.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Greenfield SF. Women and addiction: a comprehensive handbook. New York: Guilford Publications; 2009. [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8:312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Smethells JR. Sex differences in behavioral dyscontrol: role in drug addiction and novel treatments. Front Psychiatry. 2015;6:175. doi: 10.3389/fpsyt.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends’ influences on adolescent drinking: a genetic epidemiological study. Alcohol Clin Exp Res. 2007;32:2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Dunphy DC. The social structure of urban adolescent peer groups. Sociometry. 1963;26:230–246. [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Grasing K, Li N, He S, Parrish C, Delich J, Glowa J. A new progressive ratio schedule for support of morphine self-administration in opiate dependent rats. Psychopharmacology (Berl) 2003;168:387–396. doi: 10.1007/s00213-003-1442-x. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance Abuse in Women. The Psychiatric clinics of North America. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, DeBold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology (Berl) 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Booth MW. Sex differences in addict careers. 3. Addiction. Am J Drug Alcohol Abuse. 1987;13:231–251. doi: 10.3109/00952998709001512. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals NIH publication. Bethesda, Md: U.S. Dept. of Health and Human Services, Public Health Service; 2011. p. v. [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Brophy MK, Witte MA, Smith MA. Exercise decreases speedball self-administration. Life Sci. 2014a;114:86–92. doi: 10.1016/j.lfs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Smith MA. Cocaine self-administration in social dyads using custom-built operant conditioning chambers. J Neurosci Methods. 2014b;236:11–18. doi: 10.1016/j.jneumeth.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW, Wiley JL. The impact of gonadal hormones on cannabinoid dependence. Exp Clin Psychopharmacol. 2015;23:206–216. doi: 10.1037/pha0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molly LE, Gest SD, Feinberg ME, Osgood DW. Emergence of mixed-sex friendship groups during adolescence: developmental associations with substance use and delinquency. Dev Psychol. 2014;50:2449–2461. doi: 10.1037/a0037856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrug S, Borch C, Cillessen AHN. Other-sex friendships in late adolescence: risky associations for substance use and sexual debut? J Youth Adolescence. 2011;40:875–888. doi: 10.1007/s10964-010-9605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin F, Pederson S. Developmental changes in gender composition of friendship networks in adolescent girls and boys. 2007;43:1484–1496. doi: 10.1037/0012-1649.43.6.1484. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA. Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1993;111:215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–318. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Salvy SJ, Pedersen ER, Miles JN, Tucker JS, D'Amico EJ. Proximal and distal social influence on alcohol consumption and marijuana use among middle school adolescents. Drug Alcohol Depend. 2014;144:93–101. doi: 10.1016/j.drugalcdep.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Torres-Diaz YM, Silva RD, Puig-Ramos A, Menendez-Delmestre R, Rivera-Bermudez JG, Amadeo W, Agosto-Rivera JL. Estrogen receptors mediate estradiol's effect on sensitization and CPP to cocaine in female rats: role of contextual cues. Horm Behav. 2014;65:77–87. doi: 10.1016/j.yhbeh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto A, Holly EN, Boyson CO, DeBold JF, Micezk KA. Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology (Berl) 2015;232:825–834. doi: 10.1007/s00213-014-3725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lacy RT, Strickland JC. The effects of social learning on the acquisition of cocaine self-administration. Drug Alcohol Depend. 2014;141:1–8. doi: 10.1016/j.drugalcdep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Woodside B, Shaham Y. Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology. 1996;24:154–159. [Google Scholar]
- Strickland JC, Smith MA. The effects of social contact on drug use: behavioral mechanisms controlling drug intake. Exp Clin Psychopharmacol. 2014;22:23–34. doi: 10.1037/a0034669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. Animal models of social contact and drug self-administration. Pharmacol Biochem Behav. 2015;136:47–54. doi: 10.1016/j.pbb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, Kopka S, Nugent S. Course and severity of substance abuse among patients with comorbid major depression. Am J Addict. 1997;6:284–292. [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]