Abstract
Background
Microbial consortia composed of autotrophic and heterotrophic species abound in nature, yet examples of synthetic communities with mixed metabolism are limited in the laboratory. We previously engineered a model cyanobacterium, Synechococcus elongatus PCC 7942, to secrete the bulk of the carbon it fixes as sucrose, a carbohydrate that can be utilized by many other microbes. Here, we tested the capability of sucrose-secreting cyanobacteria to act as a flexible platform for the construction of synthetic, light-driven consortia by pairing them with three disparate heterotrophs: Bacillus subtilis, Escherichia coli, or Saccharomyces cerevisiae. The comparison of these different co-culture dyads reveals general design principles for the construction of robust autotroph/heterotroph consortia.
Results
We observed heterotrophic growth dependent upon cyanobacterial photosynthate in each co-culture pair. Furthermore, these synthetic consortia could be stabilized over the long-term (weeks to months) and both species could persist when challenged with specific perturbations. Stability and productivity of autotroph/heterotroph co-cultures was dependent on heterotroph sucrose utilization, as well as other species-independent interactions that we observed across all dyads. One destabilizing interaction we observed was that non-sucrose byproducts of oxygenic photosynthesis negatively impacted heterotroph growth. Conversely, inoculation of each heterotrophic species enhanced cyanobacterial growth in comparison to axenic cultures. Finally, these consortia can be flexibly programmed for photoproduction of target compounds and proteins; by changing the heterotroph in co-culture to specialized strains of B. subtilis or E. coli we demonstrate production of alpha-amylase and polyhydroxybutyrate, respectively.
Conclusions
Enabled by the unprecedented flexibility of this consortia design, we uncover species-independent design principles that influence cyanobacteria/heterotroph consortia robustness. The modular nature of these communities and their unusual robustness exhibits promise as a platform for highly-versatile photoproduction strategies that capitalize on multi-species interactions and could be utilized as a tool for the study of nascent symbioses. Further consortia improvements via engineered interventions beyond those we show here (i.e., increased efficiency growing on sucrose) could improve these communities as production platforms.
Electronic supplementary material
The online version of this article (doi:10.1186/s13036-017-0048-5) contains supplementary material, which is available to authorized users.
Keywords: Synthetic biology, Photoproduction, Synthetic consortia, Microbial communities
Background
Cyanobacteria are under increased investigation as alternative crop species for the production of fuels and other commodity chemicals. While much research focuses on engineering cyanobacterial metabolism towards the synthesis of end products (e.g., biofuels), cyanobacteria are also under consideration for the production of carbohydrate feedstocks to support fermentative bioindustrial processes [1]. In this approach, cyanobacterial biomass is processed to provide organic carbon [2–6], or cyanobacterial cells are manipulated to secrete simple fermentable sugars [7–12]. Multiple groups have recently reported that different cyanobacterial species are capable of secreting soluble sugars in considerable quantities, and continuous production can be sustained over long time periods. The specific productivities of these strains are high in comparison to plant-based feedstocks [12], and cyanobacterial cultivation can use landmass and water that is not suitable for standard agriculture. The promise of this approach has led to investment by biotechnology firms to construct compatible reactors and conduct scaled pilot trials [13, 14]. Yet, as with most cyanobacterial and algal processes, there are barriers to scaled cultivation that may make such strategies economically non-competitive [15–17]. These include the costs to recover dissolved sugars or transport culture media to fermenters as well as potential loss to contaminants. These costs might be mitigated by simultaneous conversion of the cyanobacterial feedstock into higher-value compounds by co-existing microbes in a “one-pot” reaction [18].
We test this premise by characterizing a series of synthetic co-cultures in which engineered cyanobacteria fix and secrete carbon to support growth of a broad range of evolutionarily-unrelated model heterotrophs. To provide organic carbon to engineered consortia, we use a Synechococcus elongatus PCC 7942 strain previously engineered to export up to 85% of the carbon it fixes in the form of sucrose [12], a simple sugar also produced by plant-based feedstocks (e.g., sugarcane) that is readily consumed by many microbes. S. elongatus naturally accumulates sucrose as a compatible solute [1, 19], and can export this carbohydrate through heterologous expression of the proton/sucrose symporter, cscB (hereafter cscB +)[12, 20]. Under mild osmotic shock and slightly alkaline conditions, cscB + S. elongatus continuously generate sucrose at up to 36 mg sucrose L−1 hr−1, a rate predicted to substantially exceed that of sugarcane, if successfully cultivated to scale [12]. This strain therefore demonstrates great promise as the basis for flexible photoproduction of distinct target compounds, especially if the excreted sugars are efficiently harnessed to promote growth of a variety of useful microbes while minimizing additional processing steps.
Our approach to construct cyanobacteria/heterotroph consortia utilizes an inherently modular design: i) the “autotrophic module” (cscB + S. elongatus) fixes carbon and secretes sucrose; while ii) a variety of species (i.e. the “heterotrophic module”) are examined for growth and productivity in pairwise co-cultures (Fig. 1a). Specifically, we examine a number of workhorse model microbes (Escherichia coli, Bacillus subtilis, or Saccharomyces cerevisiae) for their capacity to persist and grow using only cyanobacterially-derived organic products. We chose these species because they are well-researched, phylogenetically well-distributed (i.e. Gram-negative, Gram-positive, or eukaryotic), possess excellent genetic toolkits, a variety of engineered and mutant lines are readily available, and are frequently researched for bioindustrial applications. Finally, these species are not isolated from environments where cyanobacteria dominate, therefore it is expected that no pre-existing evolutionary relationship to cyanobacteria exists.
Fig. 1.
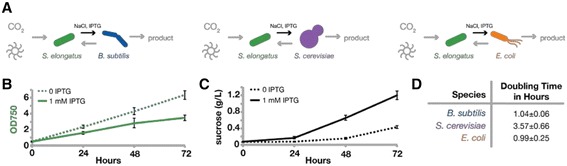
Axenic characterizations of candidate strains. a This schematic shows the engineered microbial community design. CscB + S. elongatus (green) capture light and CO2 via photosynthesis. Fixed carbon is secreted as sucrose (black arrows) when induced with IPTG in the presence of osmotic pressure (NaCl). This secreted carbon then supports the growth of B. subtilis (blue), S. cerevisiae (purple), or E. coli (orange) with the final goal of production of target compounds from those heterotrophs. Axenic cscB + S. elongatus was grown in CoBBG-11 with (solid line) and without IPTG (dashed line) to induce sucrose secretion. Cell density (b) and sucrose levels in culture supernatants (c) were measured. Error bars are standard deviation of 8 biological replicates. For characterization of cyanobacteria in CoYBG-11 see Additional file 1: Figure S1. d Heterotroph growth in isolation was characterized via growth rate in co-culture buffer supplemented with 2% sucrose. Error is standard deviation of ≥ 3 replicates
Taken together, this modular framework permits us to survey the capacity of the cscB + S. elongatus strain for construction of diverse artificial consortia with distinct composition and functions, as well as permits between-species comparison of consortia features. For example, there may be constraints to coexistence of cyanobacteria and heterotrophs that are shared across different microbial species and would be expected to impair co-culture stability. By identifying such bottlenecks, it is then possible to optimize the system for improved performance and bioproduction through strain engineering or culturing techniques.
In this work, we demonstrate that cscB + S. elongatus can promote growth of all tested heterotrophic species in pairwise co-cultures without the addition of external carbohydrates. We identify non-programmed interactions between cyanobacteria and heterotrophic microbes, including phenomena that are shared across all species. In some cases, these emergent interactions limit heterotrophic growth and/or stability. By mitigating bottlenecks to heterotrophic growth, we demonstrate that each of our co-cultures can persist over the long-term (days to months): an important feature that is often missing in synthetic cross-feeding consortia [21–24]. We also observe that the presence of each tested heterotroph species promotes the growth of S. elongatus, despite a lack of pre-existing evolutionary relationships. The modular design of composition allows these consortia to be flexibly functionalized for photoproduction of a target metabolite (polyhydroxybutyrate, PHB) or a target enzyme (alpha-amylase) with appropriate E. coli and B. subtilis strains, respectively. Finally, we examine our results for general design principles for improvement of engineered cyanobacteria/heterotroph consortia, and discuss potential of this platform for the “bottom-up” assessment of naturally-occurring microbial communities.
Results
Cyanobacteria in consortia with heterotrophs
We designed pairwise consortia where cscB + S. elongatus secrete sucrose in response to osmotic pressure and isopropyl β-D-1-thiogalactopyranoside (IPTG)-induced cscB expression [12]. Carbon secreted by cyanobacteria promotes growth of co-cultured heterotrophs (Fig. 1a). Media with optimized compositions of nitrogen, salt, and buffer were developed: termed CoBBG-11 for use in cyanobacteria/bacteria consortia and CoYBG-11 for cyanobacteria/yeast co-culture (see Materials and Methods). We verified that S. elongatus grows and produces sucrose in both CoBBG-11 and CoYBG-11 (Fig. 1b&c, Additional file 1: Figure S1). As previously reported [12], induction of cscB greatly enhances the rate of sucrose export, and this redirection of carbon resources leads to slower growth of cscB + S. elongatus (Fig. 1b&c, Additional file 1: Figure S1). We also verified that all heterotrophs are capable of growth in axenic monocultures in these defined media when provided with exogenous sucrose (2%) as the sole carbon source (Fig. 1d).
CscB + S. elongatus directly support heterotroph growth in co-cultures that contain no external carbon sources (Fig. 2, Additional file 1: Figure S2). In all consortia, cscB + S. elongatus is inoculated with a heterotrophic microbe in the appropriate co-culture media (with or without the addition of 1 mM IPTG to induce cscB expression; see Materials and Methods) and grown over 48 hours in constant light. We tracked the growth of cscB + S. elongatus in co-culture through the use of flow cytometry. Viable heterotrophs were tracked by analyzing the number of colony forming units (CFUs) when plated on the appropriate solid media. More than one strain of E. coli and S. cerevisiae were analyzed in co-culture to determine the effects of particular genetic backgrounds on growth kinetics.
Fig. 2.
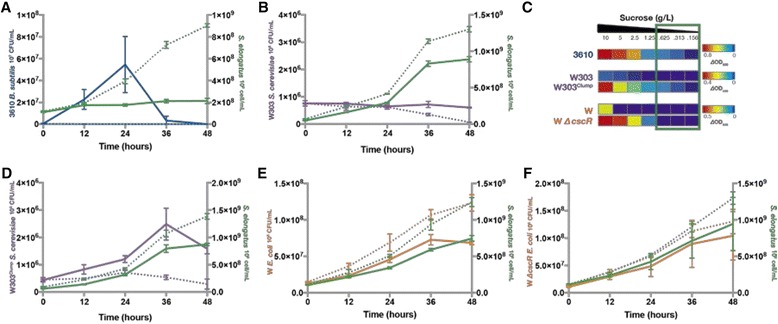
S. elongatus supports microbial communities in batch culture. Batch cultures of cscB + S. elongatus (green) in co-culture with B. subtilis (blue), S. cerevisiae (purple), or E. coli (orange) were grown in constant light. CscB + S. elongatus cells/mL were determined by flow cytometry every 12 hours for co-cultures containing B. subtilis (a; green), S. cerevisiae (b, d; green), and E. coli (e, f; green). Co-cultures with uninduced (dashed lines) or induced cscB expression (solid lines) were tested. Heterotroph viability was monitored by colony forming unit (CFU) for all B. subtilis (a; blue), S. cerevisiae (b strain W303, d strain W303Clump; purple), and E. coli (e; strain W, f; strain W ΔcscR; orange) co-cultures. Data for a, b, d, e, and f, are representative, same-day experiments where error bars are the standard error in 3 biological replicates. Additional replicates in Additional file 1: Figure S2. c Axenic heterotroph growth was tested in defined media with varying concentrations; the range of sucrose that cscB + S. elongatus can secrete in 48 hours is denoted by a green box. Average OD600 is shown as a metric of growth for ≥ 6 biological replicates. OD600 was correlated to viable colony forming units (CFU) in Additional file 1: Figure S3. No contaminants/heterotrophic colonies grew from axenic cyanobacteria controls
B. subtilis growth in co-culture is dependent on IPTG-induced sucrose secretion from cscB + S. elongatus (Fig. 2a, Additional file 1: Figure S2A). Without induction of cscB to enable sucrose secretion, B. subtilis fails to grow, indicating that sucrose availability is limiting at basal levels of sucrose export. However, when IPTG is added to increase sucrose export, B. subtilis growth is nonmonotonic: after an initial increase viability decreases during the second 24 hours of co-culture (Fig. 2a, Additional file 1: Figure S2A).
S. cerevisiae growth in co-culture is dependent on genetic engineering to improve sucrose utilization. Wild type (WT) S. cerevisiae W303 did not grow in co-culture with or without IPTG induction (Fig. 2b, Additional file 1: Figure S2B). We examined the capacity of WT S. cerevisiae W303 to grow axenically at low sucrose concentrations and saw poor/no growth below 2.5 g/L sucrose (Fig. 2c), a higher concentration of sucrose than is produced by cscB + S. elongatus at 48 hours (Fig. 1c). We then turned to an engineered strain, hereafter referred to as W303Clump, derived from previous directed evolution experiments of S. cerevisiae W303 in low sucrose media [25]. W303Clump (originally called Recreated02 in Koschwanez et al. 2013) contains mutations in genes CSE2, IRA1, MTH1, and UBR1 that enhance fitness in dilute sucrose, and also contains a nonsense mutation in ACE2 that compromises the full septation of budding daughter cells from the mother, resulting in small clonal cell aggregates (~6.6 cells per clump on average). These aggregates grow in low sucrose due to increased local cell concentration and increased hexose availability after extracellular cleavage of sucrose by an invertase [26]. Unlike the parental strain, axenic cultures of W303Clump exhibited some growth at all tested sucrose concentrations ≥0.156 g/L (Fig. 2c), as well as when co-cultured with IPTG-induced cscB + S. elongatus (Fig. 2d, Additional file 1: Figure S2C). Similar to B. subtilis, in co-culture W303Clump S. cerevisiae demonstrate declining viability after an initial period of growth (Fig 2d, Additional file 1: Figure S2C).
Finally, E. coli W grows in co-culture independently of induced sucrose secretion from cscB + S. elongatus (Fig. 2e, Additional file 1: Figure S2D). In axenic culture, WT E. coli W exhibits growth only when supplemented with >5 g/L sucrose (Fig. 2c), well above the levels cscB + S. elongatus secrete during 48 hours of growth (Fig. 1c). We therefore tested the growth of an E. coli W strain engineered for growth on sucrose. In this strain the sucrose catabolism repressor, cscR, was deleted (hereafter referred to as ΔcscR E. coli), resulting in more rapid growth at lower sucrose concentrations [27–29]. Indeed, monocultures of ΔcscR E. coli exhibit the capacity to grow on lower concentrations of sucrose ( ≥1.25 g/L; Fig. 2c), yet they still have a relatively low capacity to utilize dilute sucrose comparison to S. cerevisiae and B. subtilis (Fig. 2c), and demonstrate no growth at sucrose concentrations in the range that cscB + S. elongatus can secrete in 48 hours (Fig. 1c, green box Fig. 2c). In co-culture, ΔcscR E. coli exhibited the same monotonic growth pattern as the unmodified strain (Fig. 2e&f, Additional file 1: Figure S2D&E). This suggests that in the first days of co-culture, while exported sucrose concentrations are low (≤1 g/L), E. coli strains cannot utilize sucrose effectively and dominantly depend on other metabolites from S. elongatus; perhaps extracellular polymeric substances [30, 31].
Light driven cyanobacterial metabolism inhibits heterotroph viability
Cyanobacterial light driven metabolism is the source of heterotroph growth inhibition when sucrose is not limiting (Fig. 3a). The lack of monotonic growth in B. subtilis and S. cerevisiae co-cultures indicate that interactions beyond sucrose feeding are occurring between heterotrophs and cyanobacteria (Fig. 2a and d). To focus on products other than fixed carbon that influence heterotrophic viability and eliminate the confounding factor that cyanobacteria only generate sucrose in the light [12], co-cultures were supplemented with exogenous sucrose (2%) and cultivated in the light or dark. After 12 hours of co-cultivation, heterotroph viability of each of the three species was determined, revealing decreased growth or death correlated with increasing concentrations of cyanobacteria solely in illuminated cultures (Fig. 3b-d, Additional file 1: Figure S4). This effect is most apparent in strains of B. subtilis (Fig. 3b, Additional file 1: Figure S4A) where the viability of heterotrophic species decreases by orders of magnitude when co-cultured in the light with high concentrations of S. elongatus. The inhibition of B. subtilis growth while in co-culture with S. elongatus is mitigated when cells are incubated with DCMU, an inhibitor of oxygen evolution from Photosystem II, or thiosulfate, a potent antioxidant (Additional file 1: Figure S10A&B). Likewise, B. subtilis also persists in the presence of dense S. elongatus when oxygen is sparged from the headspace of co-cultures (Additional file 1: Figure S10C).
Fig. 3.
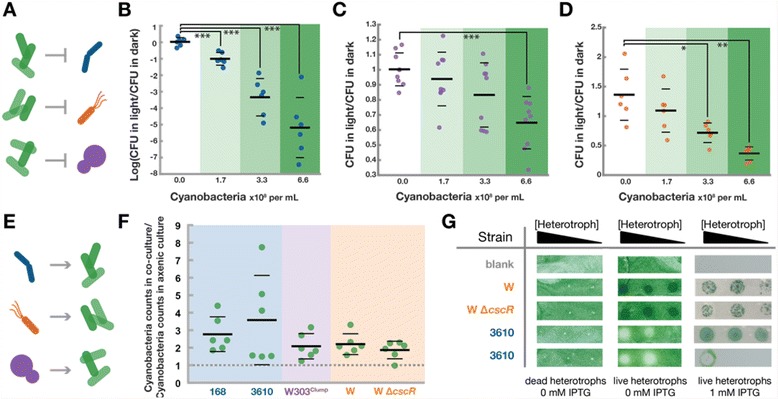
Microbial interactions. Engineered consortia demonstrate un-engineered interactions that can be classified into two categories: negative effects that cyanobacteria have on heterotrophs (a) and positive effects heterotrophs have on cyanobacteria (e). B. subtilis 3610 (b), W303Clump S. cerevisiae (c), and E. coli W ΔcscR (d) were co-cultured with various concentrations of S. elongatus and heterotroph CFU/mL were determined after 12 hours of cultivation in either light or dark. Ratios of CFU in light compared to CFU in dark are reported (b-d). Additional strains were tested in Additional file 1: Figure S4. P-values of two-tailed t-tests with Welch’s correction are denoted with asterisks: * 0.01 to 0.05, ** 0.001 to 0.01, *** 0.0001 to 0.001, **** <0.0001. Positive effects of heterotrophs on cyanobacteria (e) were observed in liquid (f), evidenced by the number of cyanobacteria cells measured in co-cultures relative to axenic controls after 48 hours in constant light. These co-cultures were inoculated with two orders of magnitude fewer cscB + S. elongatus (~1.7x106cells/mL) than the co-cultures depicted in Fig. 2 (~1.7x108cells/mL), and 1 mM IPTG was added to all cultures to induce sucrose export. Thick horizontal lines represent the average measurement for each condition while thin horizontal lines represent one standard deviation from the mean. Positive effects of heterotrophs on cyanobacteria in previous liquid batch experiments is summarized in Additional file 1: Figure S5. The influence of heterotrophs on cyanobacterial growth on solid media (g) was determined by plating a dilute lawn of cscB + S. elongatus on CoBBG-11 agar plates. The cyanobacterial lawn was overlaid with the specified strain in ten-fold serial dilutions of heterotroph and in constant light with or without IPTG
Heterotrophic species stimulate cyanobacterial growth
Conversely, co-culture with heterotrophs can stimulate growth of S. elongatus (Fig. 3e, Additional file 1: Figure S5). This was observed during batch cultures when cyanobacteria counts were higher in co-cultures then in control monocultures of cyanobacteria at various time points (Additional file 1: Figure S5). Because batch cultures of relatively dense S. elongatus can negatively impact heterotrophic viability (Fig. 2a and d and 3a-d), and also lead to significant self-shading, we inoculated low concentrations of cscB + S. elongatus induced with IPTG in co-culture with heterotrophs. After 48 hours of co-culture, cyanobacteria numbers in co-culture were normalized to axenic cyanobacteria controls. We observe significant increases in cyanobacterial growth in the presence of heterotrophic microbes, with total cyanobacterial cell counts increasing by between 80 and 250% on average (Fig. 3f).
The growth-promoting effect of heterotrophs on cyanobacteria is also observed when co-cultivated on solid media. We spotted dilutions of B. subtilis or E. coli on a lawn of dilute cyanobacteria with or without IPTG (Fig. 3g). Areas of the cyanobacterial lawn overlaid with E. coli exhibited more rapid growth than the surrounding lawn of S. elongatus alone. The growth-promoting effect of B. subtilis on cscB + S. elongatus was dependent upon induction of sucrose export. Without IPTG, spots of B. subtilis inhibited cyanobacterial growth (Fig. 3g). However, in the presence of IPTG, B. subtilis was observed to stimulate cyanobacterial growth; cyanobacterial colonies emerged variability either throughout, or at the periphery of the spot B. subtilis was plated (Fig. 3g; “3610” top and bottom panels, respectively). S. cerevisiae was not assayed in this manner because of poor growth of cscB + S. elongatus on CoYBG-11 solid agar plates. Collectively, these experiments indicate that all three evolutionarily unrelated heterotrophs can significantly increase cyanobacterial growth under a range of growth conditions.
Robustness in designed photosynthetic consortia
As the inhibitory effects of cyanobacteria on the viability of heterotrophs are only observed at relatively high density and in the light (Fig. 3a-d), we evaluated co-cultivation strategies that would prevent overgrowth of cyanobacteria to determine if heterotrophic viability could be maintained in the long-term. We first turned to Phenometrics environmental Photobioreactors (ePBRs), which have turbidostat capabilities in addition to control of light, temperature, and culture stirring [32].
When cultivated at a constant density, E. coli/cscB + S. elongatus co-cultures persist over time and heterotrophic viability is maintained. Co-cultures of induced cscB + S. elongatus and W ΔcscR E. coli were grown continuously in ePBRs under constant light (Fig. 4a). By monitoring cscB + S. elongatus cell counts and E. coli CFUs, we determined that co-cultures maintain stable ratios for more than two weeks (Fig. 4a, Additional file 1: Figure S6).
Fig. 4.

Co-cultures persist through time and perturbation. Representative continuous co-cultures of E. coli W ΔcscR/cscB + S. elongatus (a) and W303Clump S. cerevisiae/cscB + S. elongatus (b) were cultured in photobioreactors with 1 mM IPTG. E. coli-containing consortia were grown in constant light while S. cerevisiae communities were exposed to 16:8 hour light/dark photo periods (grey spaces represent darkness). Optical density of the entire culture (black points) as well as counts for the individual cell types were tracked (green S. elongatus, orange E. coli W ΔcscR, purple W303Clump S. cerevisiae). Additional photobioreactor cultures for E. coli W ΔcscR and W303Clump S. cerevisiae are presented in Additional file 1: Figure S6 and S7, respectively. Extended W303Clump S. cerevisiae/cscB + S. elongatus co-cultures are presented in Additional file 1: Figure S8. Recovery of B subtilis 3610/cscB + S. elongatus (c) or E. coli W ΔcscR /cscB + S. elongatus (d) batch cultures following dilution at 24 hours was monitored by viable colony counts and flow cytometry for heterotrophs and cyanobacteria, respectively. Perturbations on to solid media are presented in Additional file 1: Figure S9
Similarly, when cultured in ePBRs, S. cerevisiae W303Clump maintained viability in co-culture with cscB + S. elongatus over time and also persisted through variable light conditions. We induced cscB + S. elongatus to secrete sucrose and inoculated S. cerevisiae W303Clump into ePBRs. To examine the co-culture capacity to persist through light perturbations, these cultures were programmed with an alternating diurnal illumination regime (16 hours light:8 hours dark, Fig. 4b): natural growth environments would inevitably experience changes in light quantity/quality. Sustained growth in these continuous cultures indicates that yeast persist through periods of darkness when cyanobacteria are unable to supply sucrose or other photosynthates (Fig. 4d, Additional file 1: Figure S7). In similar experiments that were extended over longer time periods, S. cerevisiae W303Clump maintains viability in continuous culture with sucrose-secreting S. elongatus for greater than two months (Additional file 1: Figure S8).
Prokaryotic co-cultures with cyanobacteria persist through population bottlenecks and changes in environmental structure. B. subtilis/cscB + S. elongatus and W ΔcscR E. coli/cscB + S. elongatus co-cultures were subjected to large dilutions (1 to 10 or 1 to 100) to determine viability following the introduction of a population bottleneck. Cyanobacterial growth was monitored via flow cytometry following dilution, while heterotroph growth was measured via CFU. In perturbed cultures, heterotrophs can return to pre-dilution levels within three days (Fig. 4c and d). We also examined the persistence of heterotrophs in co-culture following plating onto solid media after growth in liquid co-culture. Co-cultures containing cscB + S. elongatus and B. subtilis 3610 or W ΔcscR E. coli were moved from liquid to solid environments and back again (Additional file 1: Figure S9). This transfer is expected to disrupt the ratio of different species within co-culture and alters any interactions dependent on the co-culture constituents being in well-mixed environments. After incubating co-cultures in the light on agar plates that have no added carbon, green colonies from the agar plate were picked into liquid CoBBG-11. Picked co-cultures were recovered in constant light for 2–5 days; wells with cyanobacterial growth were determined qualitatively by presence of green coloration, and heterotroph persistence was evaluated by plating onto rich media. In the majority of cultures both cyanobacteria and the corresponding heterotroph persisted through these perturbations, although B. subtilis was lost from the co-culture somewhat more frequently than E. coli (Additional file 1: Figure S9).
Bioproduction from functionalized co-cultures
As multiple species can be co-cultured with cscB + S. elongatus, it is possible to exchange heterotrophs to functionalize consortia for a desired activity. In this design, the heterotrophic species of the consortia acts as a “conversion module” to metabolize the products of photosynthesis into target bioproducts in a “one-pot reaction” (Fig. 5a). We tested two heterotrophic strains capable of producing distinct products: enzymes (Fig. 5b&c) and chemical precursors (Fig. 5d).
Fig. 5.
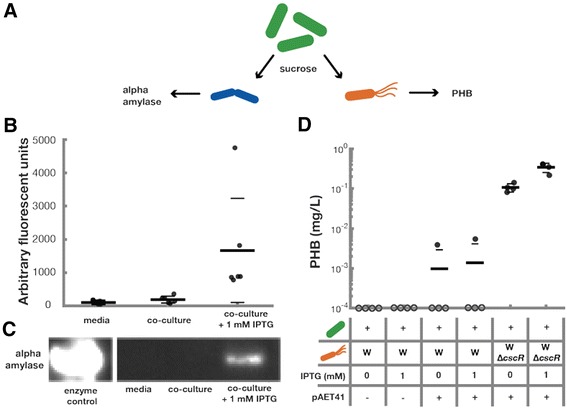
Photoproduction of enzymes and metabolites from co-culture. Flexible functionalization of co-cultures was accomplished via the addition of heterotrophs capable of producing target compounds (a). Alpha-amylase is naturally produced and secreted by B. subtilis strain 168. Supernatants from 24 hour cultures of B. subtilis 168 alone or in co-culture with cscB + S. elongatus were tested for enzymatic activity (b). Western blots also reveal the presence of alpha-amylase in co-cultures containing IPTG (c). E. coli is capable of making PHB when carrying the pAET41 plasmid. Batch co-cultures of E. coli (with or without pAET41 to enable PHB production) and cscB + S. elongatus were cultivated for one week with or without IPTG to induce sugar. PHB content of the total culture was analyzed (d). Filled circles represent measured values; hollow circles placed on the x-axis represent cultures in which no PHB was formed or was produced at levels below the detection limit. Thick horizontal lines represent the average measurement for each condition while thin horizontal lines represent one standard deviation from the mean
Alpha-amylase is produced in co-cultures of B. subtilis strain 168 and cscB + S. elongatus. B. subtilis is a chassis for enzyme production [33] and strain 168 naturally produces active alpha-amylase [34]. In consortia with S. elongatus, B. subtilis 168 produces alpha-amylase after 24 hours in constant light (Fig. 5b and c). The resulting alpha-amylase is functional as determined by enzymatic assay, and accumulates at significantly higher levels in co-cultures with cscB + S. elongatus induced to secrete sucrose (Fig. 5b and c) in comparison to other co-cultures.
Engineered E. coli/S. elongatus communities produce PHB. We co-cultured E. coli strains harboring a previously described PHB production plasmid, pAET41 [35] with cscB + S. elongatus for one week in constant light and measured PHB in the total biomass by liquid chromatagraphy (Fig. 5d). While production from the WT E. coli W strain is similar with and without IPTG, the ΔcscR E. coli W mutants that utilize sucrose more effectively produce significantly more PHB (Fig. 2c, Fig. 5d) [29]. Furthermore, upon the addition of IPTG, the ΔcscR E. coli W strain can produce three times as much PHB in co-culture than in un-induced consortia. Taken together, these results demonstrate that consortia can be flexibly programmed for photoproduction of different bioproducts by employing different heterotrophic organisms.
Discussion
We show that cscB + S. elongatus support diverse heterotrophic microbes in co-culture, demonstrating a flexible autotroph/heterotroph consortia platform. In comparison to other synthetic communities that have been constructed by cross-feeding [21–24, 36], sucrose is a metabolite that is naturally bioavailable to many microbes, and therefore the diversity of species with potential to be supported by cscB + S. elongatus is broad. In constructing these consortia, we observed unforeseen interactions with common features shared across different heterotrophic species. Namely, we observe that light-driven processes of cyanobacteria have negative impacts on all tested heterotrophic species while, conversely, growth of all heterotrophic species simulates cyanobacterial growth. By taking measures to mitigate deleterious interactions, we are able to stabilize consortia over time and demonstrate consortia persistence in the face of fluctuations in light availability, population density, and composition. Finally, we show that these consortia can be functionalized to produce target compounds, where the end product is dictated by the heterotrophic partner.
Because the conceptual design of this platform is modular, we identify desirable features in a heterotrophic partner that could be important to maximize the stability and productivity of cyanobacteria/heterotroph co-cultures more generally. A straightforward engineering target to improve stability and product output from cyanobacterial/heterotroph consortia is to enhance the efficiency of sucrose utilization in the heterotrophic partner. While the sucrose production of cscB + S. elongatus is significant in comparison to traditional plant based feedstocks [12], it is produced continuously and the concentration remains low relative to standard laboratory media (frequently ~2% carbohydrate by volume). We see that use of a derivative yeast strain with mutations conferring enhanced utilization of sucrose (W303Clump S. cerevisiae) greatly improves growth relative to the WT in co-culture with cscB + S. elongatus (Fig. 2b and d), ultimately permitting stable co-culture over months in ePBRs (Fig. 4b, Additional file 1: Figure S7&S8). Similarly, the deletion of the sucrose catabolism operon repressor cscR from the W strain of E. coli improved growth on low sucrose as expected (Fig. 2c) [29], and also greatly increased PHB production during week-long co-cultures experiments (Fig. 5d). However, there is still room for further improvement of sucrose uptake in ΔcscR E. coli, as evidenced by its insensitivity to cscB + S. elongatus sucrose export during 48 hour batch cultures where sucrose concentrations remain <1 g/L (Fig. 2a). It is likely that the relative success of production and long-term stability of ΔcscR E. coli in co-culture with cscB + S. elongatus (Fig. 4a, Additional file 1: Figure S6) is due to an intrinsic capacity of this strain of E. coli to utilize other products cyanobacteria secrete that were not engineered as part of the consortia design.
Similarly, other emergent interactions that we consistently observe between cscB + S. elongatus and all three heterotrophic species influence stability and productivity of a given co-culture pair. All three heterotrophs demonstrated decreased growth and viability when exposed to high densities of cyanobacteria in the light (Fig. 3a-d), suggesting that a cyanobacteria-derived product from active photosynthetic metabolism is the underlying cause. We hypothesize that unavoidable byproducts of photosynthetic light reactions (e.g., O2 and/or reactive oxygen species) may be a large component of this toxicity effect as hyperbaric concentrations of oxygen have previously been shown to detrimentally influence growth of all three of the model organisms we utilize for co-culture in this study [37–39]. Consistent with this hypothesis, we observed that the addition of the herbicide DCMU (which prevents oxygen evolution through inhibition of photosystem II – but does not prevent ROS formation or cyclic electron flow [40]) can rescue growth of B. subtilis, the heterotroph most sensitive to cyanobacteria, when co-cultured with dense cyanobacteria in the light (Additional file 1: Figure S10A). Similarly, supplementing co-cultures with thiosulfate, a potent antioxidant, partially protects B. subtilis in co-culture (Additional file 1: Figure S10B). Finally, sparging oxygen from the media and headspace of co-cultures also increased the viability of B. subtilis when grown with dense S. elongatus cultures in the light (Additional file 1: Figure S10C).
The repressive effect of oxygen, or other toxic cyanobacterial byproducts, likely contributes to the complex growth patterns of heterotrophic microbes in batch co-cultures (i.e., growth followed by decline Fig. 2a and d, Fig. 4). If cscB + S. elongatus density exceeds a threshold, the net effect may be to suppress heterotrophic growth despite increased total production of sucrose. Indeed, in a simplistic mathematical framework we designed to model our co-culture platform (see Additional file 1: Figure S13-S15, Additional file 1: Tables S1-S2), inclusion of a single negative feedback variable representing the negative influence of cyanbacterial density on heterotrophic growth led the model to predict a pattern of heterotrophic “growth, then decline” (Additional file 1: Figure S13). In this context, it is interesting to note that many naturally evolved consortia or symbioses that contain oxygenic phototrophs also have partners with many mechanisms to mitigate reactive oxygen species that may be produced by the phototroph, or within the symbiont due to elevated local oxygen levels [30, 41–44]. As in natural systems, it is likely that imbuing heterotrophic partners with improved oxidative stress mitigation pathways would be one engineering approach to further improve the stability of synthetic consortia. We note that we cannot exclude other pathways that may negatively impact heterotrophic viability in co-culture with dense cscB + S. elongatus: for example some cyanobacterial strains produce protective cyanotoxins [45]. Additionally, cyanobacteria compete for media components and may deplete important nutrients, although we consider this to be a relatively minor factor under our conditions since heterotrophs readily grow in media conditioned by cscB + S. elongatus [11, 12](Additional file 1: Figure S11).
The second emergent interaction that we observe in a species-independent manner is the stimulation of cyanobacterial growth in the presence of each heterotrophic species. These observations are analogous to stimulatory effects of heterotrophs on cyanobacteria and algae in numerous natural examples, such as interactions between Prochlorococcus and “helper heterotrophs” [41], or experiments where microalgae accumulate more biomass in the presence of other microbes than in isolation [46]. However, the generality of the positive effect is somewhat surprising, as the complement of secreted bioproducts from E. coli, B. subtilis, and S. cerevisiae are likely different. Furthermore, these species are not naturally prevalent in environments dominated by cyanobacteria; therefore there is no expectation of evolutionarily-preexisting pathways. These results suggest that many different heterotrophs could boost yields of S. elongatus, a prospect with significant implications for scaled cyanobacterial cultivation. Since all aerobic heterotrophs emit CO2 due to respiration, it is possible this contributes to the enhancement of S. elongatus growth, although this is questionable given that these experiments were conducted in environments already considerably enriched for CO2 (2%). Instead, we hypothesize that these benefits arise from emergent cross-feeding of metabolite(s) and/or absorption of byproducts, a division of labor commonly observed in natural consortia; though this speculative interpretation requires additional investigation. We do note, however, that media conditioned by heterotrophs does not, by itself, guarantee increased growth of cyanobocateria (Additional file 1: Figure S12), suggesting that if a cross-feeding interaction occurs it may involve an intermediate(s) that is volatile or produced at inappropriate levels by axenic heterotrophic cultures.
Our work represents an extension of multiple recent efforts in the design of synthetic microbial consortia: defined here as communities of two or more unrelated microbes that have been engineered to interact with one another through metabolic intermediates or molecular signals [47, 48]. For example, synthetic consortium engineering has been used as a bottom-up approach to gain insight into complex dynamics including population behavior, game theory, pattern formation, and cross-feeding [49–54]. The majority of described synthetic consortia involve the exchange of engineered signals (i.e., quorum sensing) or co-cultivation of complementary auxotrophs, frequently of the same microbial species [54–58]. These types of engineered interactions are relatively inflexible; because of the specialized metabolic signals such consortia designs are constrained to a limited number of species, confounding the identification of species-independent phenomena. Additionally, synthetic consortia are frequently fragile, functioning only for short time frames or requiring artificially structured environments [21, 59, 60]. Continued advancement of synthetic consortia towards academic and industrial application will likely require platforms that address some of these concerns.
The cscB + S. elongatus consortia system exhibits flexibility in that it supports the growth of three distinct “workhorse” model microbial organisms and these co-cultures can be stabilized over time and through perturbation. This flexibility of design allows for modular reconstruction of the consortia platform for a variety of light-driven applications: different heterotrophic organisms can be inoculated with cscB + S. elongatus to reprogram the population for distinct functions. In this work, we show proof-of-principle that co-culture with cyanobacteria can drive the production of alpha-amylase from B. subtilis and PHB from E. coli, both commercially relevant products. Furthermore, while this manuscript was in preparation, it was reported that cscB + S. elongatus can also support the growth and nitrogen fixation capacities of Azotobacter vinelandii [61]. To improve upon the productivity of these early designs and more completely capitalize upon the high sucrose productivities of cscB + S. elongatus, it may be necessary to select or engineer heterotroph species with more favorable co-culture properties (e.g., superior sucrose uptake, resistance to hyperoxyia, or ability to use other cyanobacterial byproducts). Indeed, in related unpublished work, we have demonstrated that cosortia PHB specific productivities can be boosted by nearly three orders of magnitude by selection of a species with naturally favorable characteristics, Halimonas bolievensis (Weiss et al., in preparation). Improved characteristics can also be engineered into a target heterotrophic strain, as we demonstrate here, and the platform provides a methodology to identify genetic determinants that would further improve consortia performance (e.g., laboratory evolution in long-term co-culture populations). Finally, the flexibility of this system allows for the determination of species-independent factors that promote cyanobacterial co-existence with other microbes, which may be useful for determining generalized interactions that underlie the formation and stabilization of natural cyanobacterial symbioses [62–66].
Conclusions
Using a strain of cyanobacteria engineered to secrete a significant proportion of the carbon it fixes in the form of the simple carbohydrate sucrose (cscB+ S. elongatus), we have constructed a variety of synthetic microbial consortia consisting of cyanobacteria/heterotroph pairs. We show that the model heterotrophs E. coli, B. subtilis, and S. cerevisiae can each grow in co-culture with sucrose secreting cyanobacteria where the sole source of carbon in the media is supplied by cyanobacterial photosynthetic metabolism. These synthetic co-cultures can be stabilized over weeks to months, and can persist in the face of selected perturbations (dilution, periods of darkness, and phase changes in growth media). Furthermore, by incorporating heterotrophs engineered for the production of an enzyme (alpha-amylase; B. subtilis) or a target metabolite (PHB; E. coli), co-cultures can be re-programed for photobiological production of different targets. We propose that the unusual flexibility of this consortia platform may allow it to be a useful tool for investigating emergent microbial interactions in both engineered, and natural consortia – particularly in light of the non-programmed interactions we observe between S. elongatus and numerous, evolutionarily-distant, heterotrophic microbes.
Methods
Strains, media, and axenic characterization
S. elongatus PCC 7942 (obtained from ATCC #33912) was engineered to secrete sucrose through the expression of the sucrose/proton symporter cscB [12]. E. coli W was obtained from ATCC (#9637) and the corresponding W ΔcscR strain was generously provided by Dr. Claudia Vicker’s laboratory [29]. B. subtilis 168 was obtained from ATCC (#23857) and B. subtilis 3610 ΔsinI was generously provided by the lab of Dr. Richard Losick [67]. The ΔsinI mutant strain of 3610 was used to minimize chained growth making CFU counts of the strain reproducible [67]. S. cerevisiae strains, WT W303 and W303Clump (previously referred to as Ancestor and Recreated02 strains, respectively) were generously provided by the lab of Dr. Andrew Murray [25]. All strains are listed in Table 1.
Table 1.
Strain and plasmid collection
| Strain | Origin |
|---|---|
| Synechococcus elongatus PCC7942 | ATCC 33912 |
| Synechococcus elongatus trc-lac/cscB | [12] |
| Bacillus subtilis 168 | ATCC 23857 |
| Bacillus subtilis 3610 ΔsinI | [67] |
| Escherichia coli K-12 BW25113 | [70] |
| Escherichia coli W | ATCC 9637 |
| Escherichia coli W ΔcscR | [29] |
| Saccharomyces cerevisiae W303 | Ancestor strain; [25] |
| Saccharomyces cerevisiae W303Clump | Recreated02 strain; [25] |
| Plasmid | |
| pAET41 | [35] |
S. elongatus was propagated in BG-11 (Sigma-Aldrich) plus 1 g/L HEPES, pH 8 in constant light at 35 °C. Axenic cyanobacteria were checked for contamination via plating on rich media. B. subtilis and E. coli were propagated in LB-Miller (EMD Millipore) while S. cerevisiae was maintained in YEPD media (MP Biomedicals). E. coli, B. subtilis, and S. cerevisiae were struck from frozen stocks on rich media plates (LB for bacteria and YEPD for yeast). Co-culture media were optimized for either prokaryotes (CoBBG-11) or S. cerevisiae (CoYBG-11). CoBBG-11 consists of BG-11 supplemented with 106 mM NaCl, 4 mM NH4Cl and 25 mM HEPPSO, pH 8.3-KOH. Indole (100 μM) was added to B. subtilis 168 co-cultures as indicated and in alpha-amylase experiments. CoYBG-11 consists of BG-11 supplemented with 0.36 g/L Yeast Nitrogen Base without amino acids (Sigma Aldrich), 106 mM NaCl, 25 mM HEPPSO, pH 8.3-KOH and 1 mM KPO3. Solid co-culture plates were composed of CoBBG-11 media with 1% autoclaved noble agar (BD Biosciences). Where appropriate, media conditioned by S. elongatus was acquired by taking CoBBG-11 or CoYBG-11 and inoculating it with OD750 0.5 of cscB + S. elongatus in baffled flasks (Additional file 1: Figure S11). This was grown for 48 hours in constant light before filtration. Media conditioned by prokaryotic heterotrophs was made by inoculating CoBBG-11 supplemented with 0.2% sucrose with B. subtilis 3610 or W ΔcscR E. coli at an OD600 of 0.01 and allowing growth for 48 hours in a baffled flask before filtration (Additional file 1: Figure S12).
For characterization of S. elongatus growth and sucrose production, S. elongatus was cultured axenically in baffled flasks of CoBBG-11 or CoYBG-11 and allowed to acclimate for ≥ 12 hours. Then cultures were adjusted to 25 mL with a final density of 0.5 OD750. IPTG (1 mM) was added, as appropriate. This was the start of the experiment and is referred to as time 0. Cultures were monitored at 24 hour intervals by withdrawal of 1 mL culture. OD750was measured via photospectrometer (ThermoScientific NonoDrop 2000c) and culture supernatant was analyzed for sucrose content via a colorimetric Glucose-Sucrose Assay (Megazyme).
To prepare heterotrophic strains, single colonies were picked into their respective rich media and grown until turbid at varying temperatures before co-culture (37 °C for E. coli and B. subtilis; 30 °C for S. cerevisiae). Cells were diluted into the appropriate co-culture media +2% sucrose to acclimate to co-culture media, and maintained within log phase growth (OD600 < 0.70) before use in co-cultures. All acclimating cultures and co-cultures were grown at 35 °C, 150 rpm, 2% CO2, in light (PAR = ~80 μmol with 15 W Gro-Lux Sylvania fluorescent bulbs) within a Multitron Infors HT incubator. Heterotrophic growth was measured by inoculating rinsed cells at 0.01 OD600 (bacteria) or 0.05 OD600 (yeast) into fresh co-culture media at the indicated sucrose concentration. Data for growth rate was collected from 25 mL flask cultures while 96-well plates with 1 mL culture volumes were used to assay growth in a gradient of sucrose concentrations (.156 mg/mL to 10 mg/mL, Fig. 2c) as well as growth in conditioned media; OD600 of plates were read on a BioTek Synergy Neo plate reader.
Batch co-cultivation & quantification
Flask co-cultures were completed in 25 mL volumes in baffled flasks. Cyanobacteria and heterotrophs were acclimated to CoBBG-11 or CoYBG-11 media prior to inoculation into co-cultures. All co-cultures were grown at 35 °C, 150 rpm, 2% CO2, in light (15 W; Gro-Lux; Sylvania) within a Multitron Infors HT incubator. 1 mM IPTG was added when indicated. Growth in co-cultures was monitored every 12 hours: S. elongatus was measured by the count of gated red-fluorescent events on a quantitative flow cytometer (BD Accuri); heterotrophs were assayed by plating dilution series on rich media to count colony forming units (CFU). Estimates of W303Clump cell number were derived by counting CFUs, but numbers were adjusted for the ~6.6 cells/clump as previously reported [25], and as confirmed under our culture conditions. For dilution experiments, co-cultures containing E. coli or B. subtilis were grown for 24 hours before 10 or 100 fold dilutions.
Heterotroph exposure to variable cyanobacteria densities
B. subtilis and E. coli were recovered from rich media as above, washed in CoBBG-11 and inoculated at an OD600 of .01 in CoBBG-11 media + 2% sucrose with cyanobacteria at different densities (OD750 0, 0.5, 1, and 2). S. cerevisiae was treated identically except they were inoculated at ~3x105cells/mL (OD750 = 0.03) and CoYBG-11 was used. These samples were split into two 36-well plates and incubated and exposed to either constant light or dark conditions while maintaining the other growth parameters. Additional cultures of B. subtilis strain 3610 were set up as described above before addition of DCMU (in ethanol) and (thiosulfate) in water to final concentrations of 40 μM and 190 mM, respectively. Vehicle was added to control cultures. Further cultures were split and sealed with septa. One was kept with atmospheric gas. The other was sparged for 5 minutes with gas devoid of oxygen (12:10:82 H2:CO2:N2). Heterotroph counts were determined by plating on rich media for colony counts as above after initial setup (time 0) and after 12 hours of culture. Ratios of the viable cell counts from the light vs. dark cultures or log10 of these ratios after 12 hours are reported.
Structured growth perturbation
To test the ability of co-cultures to withstand environmental perturbation, flask co-cultures were inoculated and grown as previously described for 24 hours before plating of 100 μL on solid co-culture Petri dishes. After five days, uneven lawns of heterotrophs and cyanobacteria arose. Cells were picked from these plates into 96-well plates and allowed to grow for 2–5 additional days. Any well that demonstrated cyanobacterial growth (as judged visually by green appearance) at the end of 48 hours was spotted on rich media to determine the presence or absence of heterotrophic symbionts. Solid culture and 96-well plate growth was completed at 35 °C, 0 rpm, 2% CO2, in constant light (15 W; Gro-Lux; Sylvania) within a Multitron Infors HT incubator.
Heterotroph spotting on cyanobacterial lawns
Lawns of cscB + cyanobacteria were achieved via spreading of 250 μL of cscB + cyanobacteria (OD750 0.5) on solid co-culture plates with or without 1 mM IPTG. After the cyanobacteria had absorbed on to the plate (>3 hours in the dark), 3 μL drops of heterotrophs were spotted on to the lawns. Heterotrophs had been previously grown up in rich media and washed three times to remove any media components before spotting. Media blanks and boiled cells were spotted as negative controls. Plates were then grown at 35 °C, 2% CO2, in constant light (15 W; Gro-Lux; Sylvania) within a Multitron Infors HT incubator.
Long-term continuous co-cultivation
Long-term co-cultures were incubated in Phenometrics Environmental Photo-Bioreactors [32] with 150 mL liquid volumes of a mix of cscB + S. elongatus with either S. cerevisiae W303Clump or E. coli W ΔcscR in the appropriate co-culture BG-11 media + 1 mM IPTG. Reactors were seeded with ~1x108cells/mL of S. elongatus (OD750 = 0.5) and a final concentration of heterotroph equivalent to ~1x106cells/mL S. cerevisiae W303Clump (final OD600 ~ 0.1) or ~5x107cells/mL E. coli W ΔcscR (OD600 ~ 0.05). Light was provided by onboard white, high-power LEDs (400 μmol m2 s2) continuously for E. coli W ΔcscR cultures, and with a 16:8 light:dark photoperiod for S. cerevisiae W303Clump co-cultures. The total density of co-cultures was monitored by onboard infrared diodes, following a brief (3–12 hour) acclimation period where the time-averaged optical density was allowed to settle to a fixed point following culture initiation. This measurement was used to control attached peristaltic pumps that eject fresh media to maintain the set target OD as previously described [32]. Co-culture temperature was maintained at 30 °C by a heated jacket; cells were agitated continuously by a stirbar. Daily, ~2 mL of co-culture volume was withdrawn and cyanobacterial and heterotrophic cell counts determined by flow cytometry and plating, respectively (as described above).
Alpha-amylase production and quantification
For the production of alpha-amylase, co-cultures of cscB + S. elongatus and B. subtilis strain 168 were completed in 8 mL volumes of CoBBG-11 supplemented with 100 μM indole in 6 well dishes. When specified, cyanobacteria were present (OD750 = 0.5) with or without 1 mM IPTG. Control cultures did not contain cyanobacteria. Alpha-amylase production was measured after 24 hours of culture at 35 °C, 0 rpm, 2% CO2, in constant light (15 W; Gro-Lux; Sylvania) within a Multitron Infors HT incubator. Alpha-amylase activity in supernatants was measured immediately after pelleting of cultures with the EnzChek Ultra Amylase Assay Kit, Molecular Probes Life Technologies using the manufacturer’s protocol. Western blots confirmed presence of alpha-amylase in supernatants after addition of NuPAGE LDS sample buffer (Invitrogen) followed by 10 minutes at 100 °C. Protein (10 μL) was run on NuPage 4-12% Bis-Tris gels (Life Technologies) for in MES SDS running buffer for 50 minutes at 185 V. The iBlot 2 Dry Blot System (ThermoScientific) was used to transfer protein to nitrocellulose membranes (iBlot 2 NC Regular Transfer Stacks). Anti-alpha amylase antibodies (polyclonal rabbit; LS-C147316; LifeSpan BioSciences; 1:3,000 dilution) were used as the primary antibody followed by peroxidase-conjugated donkey anti-rabbit antibodies (AffiniPure 711-035-152 lot 92319; Jackson ImmunoResearch; 1:5,000 dilution) as the secondary antibody. The western blot was visualized via Western Lightning® Plus-ECL, Enhanced Chemiluminescence Substrate (PerkinElmer, ProteinSimple FluorChem M). Purified alpha-amylase (Sigma Aldrich) was used as a control in all assays.
PHB production & quantification
E. coli strains were transformed with pAET41 (Table 1) before use in co-cultures for production [35]. Co-cultures were set up as previously described in 25 mL flasks. After one week of growth, the entire culture was spun down, frozen, and stored at −80 °C until PHB content was quantified. PHB content was quantified by standard methods [68, 69]. Briefly: cell pellets were digested with concentrated H2SO4 at 90 °C for 60 min. The digestion solution was diluted with H2O by 500 times and passed through 0.2 μm filter. The solutions were subsequently analyzed by a high performance liquid chromatography (HPLC, Agilent HPLC 1200) equipped with Aminex HPX-87H column and UV absorption detector [69]. The volume of each sample injection was 100 μL. The mobile phase was 2.5 mM H2SO4 aqueous solution, with a flow rate of 0.5 mL/min for 60 min. 5 mM sodium acetate (Sigma Aldrich) was added as an internal standard. The concentrations of PHB were determined by comparing the peak area with that in standard curves from 0.1 to 30 mM.
Mathematical framework, statistics, and figures
All equations were modeled in Mathematica (Wolfram Research, Inc., Mathematica, Version 11.0). Statistics were completed in GraphPad Prism version 7, GraphPad Software, La Jolla California USA, www.graphpad.com.
Acknowledgements
Special thanks to Chong Liu and the Nocera laboratory for quantifying PHB accumulation and Charleston Noble from the Nowak laboratory for helping us explore mathematical frameworks of our communities. Additionally, we are grateful to the Betenbaugh, Guarnieri, and Zengler groups for their conversations and collaboration. We must also thank the members of the Ducat (especially Jingcheng Huang, Brad Abramson, Eric Young, Josh MacCready, Derek Fedeson, Taylor Weiss), and Silver (especially John Oliver, Brendan Colon, Marika Ziesack) labs as well as Jenna Chandler, Igor Vieira, and Henry Wettersten for their critical reviews of the manuscript.
Funding
This work was supported by the National Science Foundation, Award Numbers 1437657 and DGE1144152, Department of Energy DE-SC0012658 ‘Systems Biology of Autotrophic-Heterotrophic Symbionts for Bioenergy’, SynBERC, and the Wyss Institute for Biologically Inspired Engineering.
Availability of data and materials
All data generated or analyzed during this study are included in the figures of this published article [and its supplementary information files].
Authors’ contributions
DD and SH conceived and designed experiments. SH, DD, and LY performed experiments. DD, SH, and PS wrote the manuscript. All authors read and edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- CFU
Colony forming unit
- ePBR
Environmental Photobioreactors
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- PHB
Polyhydroxybutyrate
- WT
Wild type
Additional file
Contains supplementary figures S1-S15 as well as the mathematical framework used to investigate microbial community interactions. (DOCX 75744 kb)
References
- 1.Hays SG, Ducat DC. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth Res. 2015;123:285–95. doi: 10.1007/s11120-014-9980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Möllers KB, Cannella D, Jørgensen H, Frigaard N-U. Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol Biofuels. 2014;7:64. doi: 10.1186/1754-6834-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikawa S, Joseph A, Yamada R, Izumi Y, Yamagishi T, Matsuda F, Kawai H, Chang J-S, Hasunuma T, Kondo A, Ducat DC, Way JC, Silver PA, Lopez PJ, Desclés J, Allen AE, Bowler C, Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ, Melis A, Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC, Harun R, Jason WSY, Cherrington T, et al. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ Sci. 2013;6:1844. doi: 10.1039/c3ee40305j. [DOI] [Google Scholar]
- 4.Markou G, Angelidaki I, Georgakakis D. Carbohydrate-enriched cyanobacterial biomass as feedstock for bio-methane production through anaerobic digestion. Fuel. 2013;111:872–879. doi: 10.1016/j.fuel.2013.04.013. [DOI] [Google Scholar]
- 5.John RP, Anisha GS, Nampoothiri KM, Pandey A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour Technol. 2011;102:186–93. [DOI] [PubMed]
- 6.Rosgaarda L, Jara de Porcellinisa A, Jacobsenc JH, Frigaardc NU, Sakuragia Y. Bioengineering of carbon fixation, biofuels, and biochemicals in cyanobacteria and plants. J Biotechnol. 2012;162(1):134–47. [DOI] [PubMed]
- 7.Song K, Tan X, Liang Y, Lu X. The potential of Synechococcus elongatus UTEX 2973 for sugar feedstock production. Appl Microbiol Biotechnol. 2016;100:7865–7875. doi: 10.1007/s00253-016-7510-z. [DOI] [PubMed] [Google Scholar]
- 8.Du W, Liang F, Duan Y, Tan X, Lu X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab Eng. 2013;19:17–25. doi: 10.1016/j.ymben.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Duan Y, Luo Q, Liang F, Lu X. Sucrose secreted by the engineered cyanobacterium and its fermentability. J Ocean Univ China. 2016;15:890–896. doi: 10.1007/s11802-016-3007-8. [DOI] [Google Scholar]
- 10.Xu Y, Guerra LT, Li Z, Ludwig M, Dismukes GC, Bryant DA. Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC 7002: Cell factories for soluble sugars. Metab Eng. 2013;16:56–67. doi: 10.1016/j.ymben.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Niederholtmeyer H, Wolfstädter BT, Savage DF, Silver PA, Way JC. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl Environ Microbiol. 2010;76:3462–6. doi: 10.1128/AEM.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducat DC, Avelar-Rivas JA, Way JC, Silver PA. Rerouting carbon flux to enhance photosynthetic productivity. Appl Environ Microbiol. 2012;78:2660–8. doi: 10.1128/AEM.07901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aikens J, Turner RJ. Transgenic photosynthetic microorganisms and photobioreactor. Patent US 8367379 B2. Grant, USA. 2013.
- 14.Aikens J, Turner RJ. Method of producing a fermentable sugar. Patent US 8597914 B2. Grant, USA. 2013.
- 15.Chisti Y. Constraints to commercialization of algal fuels. J Biotechnol. 2013;167:201–14. doi: 10.1016/j.jbiotec.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Bux F, Chisti Y (Eds): Algae Biotechnology. Cham: Springer International Publishing; 2016. [Green Energy and Technology]
- 17.Singh J, Gu S. Commercialization potential of microalgae for biofuels production. Renew Sustain Energy Rev. 2010;14:2596–2610. doi: 10.1016/j.rser.2010.06.014. [DOI] [Google Scholar]
- 18.Ortiz-Marquez JCF, Do Nascimento M, Zehr JP, Curatti L. Genetic engineering of multispecies microbial cell factories as an alternative for bioenergy production. Trends Biotechnol. 2013;31:521–529. doi: 10.1016/j.tibtech.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Klähn S, Hagemann M. Compatible solute biosynthesis in cyanobacteria. Environ Microbiol. 2011;13:551–562. doi: 10.1111/j.1462-2920.2010.02366.x. [DOI] [PubMed] [Google Scholar]
- 20.Bockmann J, Heuel H, Lengeler JW. Characterization of a chromosomally encoded, non-PTS metabolic pathway for sucrose utilization in Escherichia coli EC3132. MGG Mol Gen Genet. 1992;235:22–32. doi: 10.1007/BF00286177. [DOI] [PubMed] [Google Scholar]
- 21.Song H-S, Renslow RS, Fredrickson JK, Lindemann SR. Integrating Ecological and Engineering Concepts of Resilience in Microbial Communities. Front Microbiol. 2015;6:1298. doi: 10.3389/fmicb.2015.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wintermute EH, Silver PA. Dynamics in the mixed microbial concourse. Genes Dev. 2010;24:2603–14. doi: 10.1101/gad.1985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mee MT, Collins JJ, Church GM, Wang HH. Syntrophic exchange in synthetic microbial communities. Proc Natl Acad Sci U S A. 2014;111:E2149–56. doi: 10.1073/pnas.1405641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koschwanez JH, Foster KR, Murray AW. Improved use of a public good selects for the evolution of undifferentiated multicellularity. Elife. 2013;2013:1–27. doi: 10.7554/eLife.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koschwanez JH, Foster KR, Murray AW, Keller L. Sucrose Utilization in Budding Yeast as a Model for the Origin of Undifferentiated Multicellularity. PLoS Biol. 2011;9(8):e1001122. [DOI] [PMC free article] [PubMed]
- 27.Archer CT, Kim JF, Jeong H, Park J, Vickers CE, Lee S, Nielsen LK. The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics. 2011;12:9. doi: 10.1186/1471-2164-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabri S, Nielsen LK, Vickers CE. Molecular control of sucrose utilization in Escherichia coli W, an efficient sucrose-utilizing strain. Appl Environ Microbiol. 2013;79:478–487. doi: 10.1128/AEM.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arifin Y, Sabri S, Sugiarto H, Krömer JO, Vickers CE, Nielsen LK. Deletion of cscR in Escherichia coli W improves growth and poly-3-hydroxybutyrate (PHB) production from sucrose in fed batch culture. J Biotechnol. 2011;156:275–8. doi: 10.1016/j.jbiotec.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Villa F, Pitts B, Lauchnor E, Cappitelli F, Stewart PS. Development of a laboratory model of a phototroph-heterotroph mixed-species biofilm at the stone/air interface. Front Microbiol. 2015;6:1–14. doi: 10.3389/fmicb.2015.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi F, De Philippis R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life. 2015;5:1218–1238. doi: 10.3390/life5021218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucker BF, Hall CC, Zegarac R, Kramer DM. The environmental photobioreactor (ePBR): An algal culturing platform for simulating dynamic natural environments. Algal Res. 2014;6(PB):242–249. doi: 10.1016/j.algal.2013.12.007. [DOI] [Google Scholar]
- 33.van Dijl JM, Hecker M. Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact. 2013;12:3. doi: 10.1186/1475-2859-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green DM, Colarusso LJ. The physical and genetic characterization of a transformable enzyme: Bacillus subtilis α-amylase. Biochim Biophys Acta - Spec Sect Enzymol Subj. 1964;89:277–290. doi: 10.1016/0926-6569(64)90216-0. [DOI] [PubMed] [Google Scholar]
- 35.Peoples OP, Sinskey AJ. Poly-??-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC) J Biol Chem. 1989;264:15298–15303. [PubMed] [Google Scholar]
- 36.Song H, Ding M-Z, Jia X-Q, Ma Q, Yuan Y-J. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev Chem Soc Rev. 2014;6954:6954–6981. doi: 10.1039/C4CS00114A. [DOI] [PubMed] [Google Scholar]
- 37.BOEHME DE, VINCENT K, BROWN OR. Oxygen and toxicity inhibition of amino acid biosynthesis. Nature. 1976;262:418–420. doi: 10.1038/262418a0. [DOI] [PubMed] [Google Scholar]
- 38.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–54. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregory EM, Fridovich I. Oxygen Toxicity and the Superoxide Dismutase. J Bacteriol. 1973;114:1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das PK, Bagchi SN. Bentazone and bromoxynil induce H+ and H2O2 accumulation, and inhibit photosynthetic O2 evolution in Synechococcous elongatus PCC7942. Pestic Biochem Physiol. 2010;97:256–261. doi: 10.1016/j.pestbp.2010.03.005. [DOI] [Google Scholar]
- 41.Morris JJ, Kirkegaard R, Szul MJ, Johnson ZI, Zinser ER. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by “helper” heterotrophic bacteria. Appl Environ Microbiol. 2008;74:4530–4534. doi: 10.1128/AEM.02479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beliaev AS, Romine MF, Serres M, Bernstein HC, Linggi BE, Markillie LM, Isern NG, Chrisler WB, Kucek LA, Hill EA, Pinchuk GE, Bryant DA, Wiley S, Fredrickson JK, Konopka A. Inference of interactions in cyanobacterial– heterotrophic co-cultures via transcriptome sequencing. ISME J. 2014;869:2243–2255. doi: 10.1038/ismej.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venn AA, Loram JE, Douglas AE. Photosynthetic symbioses in animals. J Exp Bot. 2008;59:1069–1080. doi: 10.1093/jxb/erm328. [DOI] [PubMed] [Google Scholar]
- 44.Kihara S, Hartzler DA, Savikhin S. Oxygen Concentration Inside a Functioning Photosynthetic Cell. Biophys J. 2014;106:1882–1889. doi: 10.1016/j.bpj.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LEFLAIVE J, TEN-HAGE L. Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshw Biol. 2007;52:199–214. doi: 10.1111/j.1365-2427.2006.01689.x. [DOI] [Google Scholar]
- 46.Do Nascimento M, Dublan M de los A, Ortiz-Marquez JCF, Curatti L. High lipid productivity of an Ankistrodesmus-Rhizobium artificial consortium. Bioresour Technol. 2013;146:400–7. doi: 10.1016/j.biortech.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein HC, Carlson RP. Microbial Consortia Engineering for Cellular Factories: in vitro to in silico systems. Comput Struct Biotechnol J. 2012;3 doi: 10.5936/csbj.201210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hays SG, Patrick WG, Ziesack M, Oxman N, Silver PA. Better together: Engineering and application of microbial symbioses. Curr Opin Biotechnol. 2015;36:40–49. doi: 10.1016/j.copbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Balagaddé FK, Song H, Ozaki J, Collins CH, Barnet M, Arnold FH, Quake SR, You L. A synthetic Escherichia coli predator–prey ecosystem. Mol Syst Biol. 2008;4:187. doi: 10.1038/msb.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yurtsev EA, Conwill A, Gore J. Oscillatory dynamics in a bacterial cross-protection mutualism. Proc Natl Acad Sci U S A. 2016;113:6236–41. doi: 10.1073/pnas.1523317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gore J, Youk H, van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–6. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Kim JK, Hirning AJ, Josić K, Bennett MR. Emergent genetic oscillations in a synthetic microbial consortium. Science. 2015;80-:349. doi: 10.1126/science.aaa3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu S, Gerchman Y, Collins CH, Arnold FH, Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005. [DOI] [PubMed]
- 54.Shou W, Ram S, Vilar JMG: Synthetic cooperation in engineered yeast populations. Proc Natl Acad Sci USA. 2007;104(6):1877–82. [DOI] [PMC free article] [PubMed]
- 55.Tamsir A, Tabor JJ, Voigt CA. Robust multicellular computing using genetically encoded NOR gates and chemical “wires.”. Nature. 2011;469:212–215. doi: 10.1038/nature09565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott SR, Hasty J. Quorum Sensing Communication Modules for Microbial Consortia. ACS Synth Biol. 2016;5(9):969–77. [DOI] [PMC free article] [PubMed]
- 57.Wintermute EH, Silver PA. Emergent cooperation in microbial metabolism. Mol Syst Biol. 2010;6:407. doi: 10.1038/msb.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mee MT, Collins JJ, Church GM, Wang HH. Syntrophic exchange in synthetic microbial communities. Proc Natl Acad Sci. 2014;20. [DOI] [PMC free article] [PubMed]
- 59.Moffitt JR1, Lee JB, Cluzel P. The single-cell chemostat: an agarose-based, microfluidic device for high-throughput, single-cell studies of bacteria and bacterial communities. Lab Chip. 2012;12(8):1487–94. [DOI] [PMC free article] [PubMed]
- 60.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci U S A. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MJ, Francis MB: A Designed A. vinelandii-S. elongatus Coculture for Chemical Photoproduction from Air, Water, Phosphate, and Trace Metals. ACS Synth Biol. 2016;5(9):955–61. [DOI] [PubMed]
- 62.Rai AN, Bergman B, Rasmussen U. Cyanobacteria in Symbiosis. Dordrecht: Springer Netherlands; 2002. [Google Scholar]
- 63.Martínez-Pérez C, Mohr W, Löscher CR, Dekaezemacker J, Littmann S, Yilmaz P, Lehnen N, Fuchs BM, Lavik G, Schmitz RA, LaRoche J, Kuypers MMM. The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat Microbiol. 2016;1:16163. doi: 10.1038/nmicrobiol.2016.163. [DOI] [PubMed] [Google Scholar]
- 64.Aschenbrenner IA, Cernava T, Berg G, Grube M. Understanding microbial multi-species symbioses. Front Microbiol. 2016;7:180. doi: 10.3389/fmicb.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hom EFY, Murray AW. Plant-fungal ecology. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science. 2014;345:94–8. doi: 10.1126/science.1253320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramanan R, Kim B-H, Cho D-H, Oh H-M, Kim H-S: Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol Adv. 2016;34:14–29. [DOI] [PubMed]
- 67.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–49. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 68.Torella JP, Gagliardi CJ, Chen JS, Bediako DK, Colón B, Way JC, Silver PA, Nocera DG. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc Natl Acad Sci. 2015;112:2337–2342. doi: 10.1073/pnas.1424872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, Gallagher JJ, Sakimoto KK, Nichols EM, Chang CJ, Chang MCY, Yang P: Nanowire–Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals. Nano Lett. 2015;15(5):3634–9. [DOI] [PMC free article] [PubMed]
- 70.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the figures of this published article [and its supplementary information files].


