Abstract
Background
Human Immuno-deficiency Virus (HIV) associated non-Hodgkin’s lymphoma (NHL) was a special group of disease, which manifests distinct clinical features and prognosis as compared with NHLs in patients without HIV. We performed this study to describe the clinical features of the disease and investigated the potential prognostic factors.
Methods
HIV-infected patients who were newly diagnosed with NHL were enrolled in this study. The selection of anti-lymphoma treatment regimen was mainly dependent on the pathological subtypes of NHLs. Tumor response was reviewed and classified according to the International Workshop Criteria.
Results
A total of 78 patients were enrolled, among whom, 42 (53.8%) were with Diffuse large B cell Lymphoma (DLBCL), and 29 (37.2%) were with Burkitt lymphoma (BL). BL patients presented with higher risk features as compared with DLBCL in terms of numbers of extranodal diseases (P = 0.004) and poor Eastern cooperative oncology group (ECOG) score (P = 0.038). The estimated 2-year overall survival (OS) and progression free survival (PFS) rate was 74.3 ± 8.1%, 28.9 ± 11.0%, and 54.2 ± 8.1%, 19.2 ± 7.5% for DLBCL and BL, respectively. In multivariate analysis, international prognostic index (IPI) score was an independent prognostic factor for predicting both OS (OR = 2.172, 95% CI 1.579–2.987, P < 0.001) and PFS (OR = 1.838, 95% CI 1.406–2.402, P < 0.001).
Conclusions
HIV associated NHLs represents a group of heterogeneous aggressive diseases with poor prognosis. IPI parameters were still effective in predicting the prognosis of HIV associated NHLs.
Electronic supplementary material
The online version of this article (doi:10.1186/s13027-017-0120-2) contains supplementary material, which is available to authorized users.
Keywords: Human immuno-deficiency virus, Lymphoma, Prognosis, International prognostic index, Prognosis
Background
Non-Hodgkin’s Lymphoma (NHL) is one of the most common types of malignancies with high morbidity and mortality in the patients who were infected by Human Immune-deficiency Virus (HIV) [1]. Generally, HIV associated NHLs present with more aggressive clinical behaviors as compared with general population, exemplified with a huge tumor burden, more extranodal diseases, and a tendency of involving genital system [2, 3]. Fortunately, although the prognosis of HIV associated NHLs remains very poor, with the introduction of combined antiretroviral therapy (cART) and high dose chemotherapy, a considerable portion of the patients could be cured [4, 5].
The International Prognostic Index (IPI) [6], which includes age, Eastern Cooperative Oncology Group (ECOG) performance status, Lactate Dehydrogenase (LDH) level, Ann Arbor stage, and extranodal involvement, is used extensively for evaluating patients with aggressive lymphomas, especially for those with diffuse large B cell lymphoma (DLBCL) and receive doxorubicin-containing chemotherapy such as R-CHOP [7, 8]. Although its role as prognostic factor in stratification of the NHL patients in daily practice and clinical trial is well established in general population, its validity remains controversial in HIV associated NHLs.
Another important issue is the complexity of the background of the HIV associated NHLs, such as more aggressive phenotype, immune-deficiency status. What even worse is that treatment factors (cART) also intervene the clinical outcome and prognosis [9–11]. Chronic antigen stimulation (HIV infection) could lead to a polyclonal B-cell expansion and finally promotes the emergence of monoclonal B cells. Both germinal center B cell-like (GCB) active B cell subtype (ABC) could be observed in HIV associated DLBCL. The frequency of double hit mutation could be observed in around 20% in HIV patients with NHLs [12–14]. Thus, a more comprehensive analysis incorporating above mentioned factors is warranted to give more precise stratification of HIV associated NHLs.
In China, with the increasing of incidence of HIV infection in recent years, acquired immunodeficiency syndrome (AIDS) associated NHL became more and more frequently. This subset of the patients might present distinct clinical behavior and treatment outcome as compared with their western counterpart. Hence, we performed this study to describe the clinical features and evaluate the potential prognostic factors of the Chinese AIDS patients with NHLs.
Methods
Patients
The HIV-infected patients who were newly diagnosed NHL were consecutively entered in this study from Jan.2002 to May.2015 in Shanghai Public Health Clinical Center. The subtypes of the lymphoma were defined according to the WHO 2008 classification system.
Systemic evaluation of the patients including complete blood cell count (CBC), biochemical parameters, LDH, viral panel (HIV, HBV, and HCV etc.), CD4 cell count, bone marrow and radiological examinations, was performed to stage the disease and assess the general and disease status.
Anti-lymphoma treatment
Sixty-four out of 78 patients received anti-lymphoma treatment, and the regimen for the first line chemotherapy was mainly dependent on the pathological subtypes of lymphomas. For BL and PBL, Hyper-CVAD A (Cyclophosphamide [CTX] 300 mg/m2 Q12h × 6, D1-3, Dexamethasone [DEX] 40 mg/d, D1-4, D11-14, Vincristine [VCR] 1.4 mg/m2, D4, D11, Adriamycin [ADR] 50 mg/m2, D4) and B regimen (Methotrexate 1 g/m2, D1, Ara-C 2 g/m2 Q12h, D2-3) were given alternatively to the patients. For DLBCL, CHOP regimen (CTX 750 mg/m2, D1, ADR 50 mg/m2, D1, VCR 1.4 mg/m2, D1, Prednisone 60 mg/m2, D1) was initially designed for the patients, initiated from Dec.2014, to enhance the efficacy of young high risk patients (age < 60 years, IPI > 1). DA-EPOCH (Etopside 50 mg/m2 D1-4, VCR 0.4 mg/m2 D1-4, ADR 10 mg/m2 D1-4, all above continuous for 24 h, CTX 750 mg/m2 D5, DEX 40 mg D1-5) was also given to this subgroup of the patients [15]. The patients with FL and IBL received CHOP regimen the same as those with DLBCL. The detailed treatment regimen was depicted in Additional file 1: Table S1. No irradiation therapy was given concurrently during the treatment.
Rituximab 375 mg/m2 was designed to give to the patients with a suitable CD4 cell count (>50 cells/μl) to prevent the deep suppression of both B and T cell immunity. Since rituximab usage is not covered by medical insurance in most provinces in China, the application of treatment with rituximab also depended on patients’ willing. Fifty patients had a CD4 count greater than 50 (2 with PBL without CD20 expression), and 20 patients (40%) received rituximab.
The first line treatment regimen was discontinued if patients experienced any severe adverse event or lymphoma progressing. The initiation of the second line or salvage treatment regimen for relapsed and refractory patients was dependent on the decision of the physicians according to the condition of the patients.
Anti-HIV treatment
ART was given immediately after the diagnosis of HIV with the exception of 5 patients due to rapid progression of lymphoma [16]. They were receiving either a combination of 2 nucleoside reverse transcriptase inhibitors with a protease inhibitor (n = 1) or with a non-nucleoside reverse transcriptase inhibitor (n = 70), or with an integrase inhibitor (n = 2).
Response assessment
Evaluation of the efficacy was performed one month after completion of all the treatment. Thoracic, abdominal, and pelvic computed tomography scans were performed even if those areas were not initially affected [17, 18]. Interim staging was performed after completion of four cycles of treatment for DLBCL and FL patients. For BL and IBL patients, these evaluations were performed every 2 cycles. Tumor response was based on radiographic review and classified as complete response (CR), partial response (PR), overall response (OR, CR + PR), stable disease (SD), or progressive disease (PD) according to the International Workshop Criteria [19].
Statistical analysis
Fisher’s Exact P test and one way ANOVA test were used to compare the clinical parameters in different subtypes of lymphoma. All patients entered into the study were followed up. Overall survival (OS) was measured from the date of disease diagnosis to death (failure) or alive at last follow-up (censored). Progression free survival (PFS) was defined as time from disease diagnosis to treatment failure such as relapse, progressive disease, any new anti-lymphoma treatment, and death of any reasons, or alive in CR at last follow-up (censored). Kaplan-Meier analysis was used to calculate the distribution of OS and PFS. Binary logistic regression was used for the multivariate analysis of prognostic parameters for response, while Cox model was used to identify prognostic variables for OS and PFS. A limited backward selection procedure was used to exclude redundant or unnecessary variants. To provide quantitative information on the relevance of results, 95% confidence intervals (95% CIs) of odds ratios (ORs) was calculated. All above statistical procedures were performed with the SPSS statistical software package, version 16.0.
Results
Patient characteristics
A total of 78 patients were enrolled, among whom, 42 (53.8%) were with DLBCL, 29 (37.2%) were with Burkitt lymphoma (BL), 5 (6.4%) were with plasmablastic lymphoma (PBL) and 1 (1.3%) was with follicular lymphoma (FL) and angioimmunoblastic lymphoma (IBL), respectively. The median age of the patients was 48 (24–74). A strong tendency of male patients was observed (89.7% vs. 10.3%). The clinical characteristics of the patients were shown in Table 1. The BL patients presented with higher risk features as compared with DLBCL patients in terms of numbers of extranodal disease (P = 0.004) and poor ECOG score (P = 0.038), while age (P = 0.151), gender (P = 0.692) and CD4 cell count (P = 0.526) were distributed equally in the two groups.
Table 1.
Characteristics of the patients
| Characteristics | DLBCL (n = 42) |
BL (n = 29) |
PBL and IBL (n = 6) |
|---|---|---|---|
| Age (years) | |||
| Median | 44 | 54 | 48 |
| Range | 24–73 | 25–73 | 33–74 |
| P = 0.151 | |||
| Gender (%) | |||
| Male | 37 (88.1) | 27 (93.1) | 5 (83.3) |
| Female | 5 (11.9) | 2 (6.9) | 1 (6.7) |
| P = 0.692 | |||
| Ann Arbor stage (%) | |||
| I, II | 23 (54.8) | 10 (34.5) | 3 (50.0) |
| III, IV | 19 (45.2) | 19 (65.5) | 3 (50.0) |
| P = 0.146 | |||
| Extranodal diseases (%) | |||
| 0,1 | 33 (78.6) | 15 (51.7) | 4 (66.7) |
| > 1 | 9 (21.4) | 14 (48.3) | 2 (33.3) |
| P = 0.004 | |||
| LDH | |||
| Median | 334 | 373 | 283 |
| Range | 139–6016 | 178–5009 | 182–739 |
| P = 0.257 | |||
| ECOG score (%) | |||
| 0,1 | 18 (42.9) | 5 (17.2) | 3 (50.0) |
| > 1 | 24 (57.1) | 24 (82.8) | 3 (50.0) |
| P = 0.038 | |||
| IPI (%) | |||
| 0,1 | 16 (38.1) | 5 (17.2) | 3 (50.0) |
| > 1 | 26 (61.9) | 24 (82.8) | 3 (50.0) |
| P = 0.069 | |||
| CD4 cell count | |||
| Median | 106 | 108 | 31 |
| Range | 4–483 | 1–549 | 3–69 |
| P = 0.526 | |||
33% (25/78) of the patients was confirmed to be infected with HIV before lymphoma diagnosis. 22% (17/78) of them received ART with a median treatment exposure of 12 months before the diagnosis of lymphoma, whereas 78% (61/78) of the patients were ART-naïve. 67% (53/78) of the patients were simultaneously diagnosed of lymphoma and HIV. Notably, 14% (11/78) of the patients had a plasma HIV-1 RNA viral load below 40 copies/ml at the time of lymphoma diagnosis. Table 2 summarized the baseline characteristics of these patients.
Table 2.
Baseline characteristics of HIV condition of the patients
| Characteristics | |
|---|---|
| AIDS before lymphoma diagnosis (n, %) | 25 (33) |
| ART at lymphoma diagnosis (n, %) | 17 (22) |
| ART exposure from lymphoma diagnosis (months) | 12 (1–84) |
| Patients with CD4 count <200 cells/uL (n, %) | 59 (75) |
| HIV RNA < 40 copies/ml at lymphoma diagnosis (n, %) | 11 (14) |
Treatment response
Among 35 DLBCL patients, 22 (62.9%) and 26 patients (74.3%) achieved CR and OR, respectively. For 23 patients with BL, 5 (21.7%) achieved CR while 8 patients (34.8%) achieved OR. One patient with FL achieved PR. None of the patients with PBL and IBL obtained any response.
Further prognostic analysis for CR induction was performed in 58 patients with DLBCL or BL after anti-lymphoma treatment. As shown in Additional file 1: Table S2, in univariate analysis, DLBCL has significant higher CR rate than BL (P = 0.003). Ann Arbor stage (P < 0.001), number of extranodal disease (P = 0.001), ECOG performance status (P = 0.001), LDH (P = 0.008) and IPI (P < 0.001) were proved to be significantly associated the CR induction results.
IPI score and pathological types were entered into multivariate logistic regression analysis. It was proved that pathological types (DLBCL vs. BL, P = 0.038, OR = 0.240, 95% CI 0.063–0.925) and IPI score (P < 0.001, OR = 0.371, 95% CI 0.229–0.601) were independent prognostic factors of CR (Table 4).
Table 4.
Multivariate analysis of potential prognostic factors for the patients
| Variables | CR | OS | PFS | |||
|---|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | |
| IPI score | <0.001 | 0.371 (0.229–0.601) | <0.001 | 2.172 (1.579–2.987) | <0.001 | 1.838 (1.406–2.402) |
| Pathology subtype (DLBCL vs. BL) |
0.038 | 0.240 (0.063–0.925) | NS | – | NS | – |
Survival analysis
Among 64 patients who received anti-lymphoma treatment, the median follow up time was 13.5 months due to the poor prognosis of the patients. The estimated 2 year OS and PFS rate were 74.3 ± 8.1%, 28.9 ± 11.0%, 20.0 ± 17.9%, and 54.2 ± 8.1%, 19.2 ± 7.5%, and 0% for DLBCL, BL and PBL + IBL, respectively (Fig. 1). One FL patient who achieved PR relapsed after 1 year.
Fig. 1.
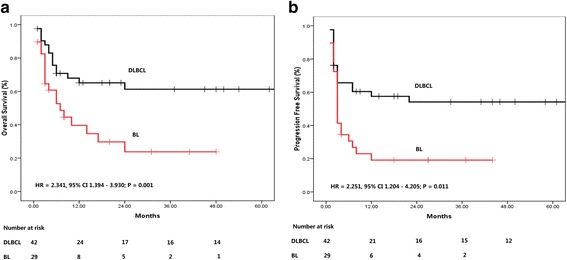
Kaplan-Meier curves for overall survival (OS) and progression free survival (PFS) of treated patients. a OS, (b) PFS
Since a survival plateau could be observed in DLBCL and BL patients (Fig. 1), further prognostic analysis was performed in a group combining the two subsets of the patients. As shown in Figs. 2 and 3, Ann Arbor stage (I, II vs. III, IV), number of extranodal diseases (0, 1 vs. > 1), ECOG performance status (0,1 vs. > 1), and IPI score (0,1 vs. > 1) were all significantly associated the survival (OS and PFS) of the patients. LDH as continuous variable, could predict OS (HR = 1.000, 95% CI 1.000–1.001, P = 0.001) and PFS (HR = 1.000, 95% CI 1.000–1.001, P = 0.002), while CD4 cell count at diagnosis was not associated with the treatment outcome (OS: HR = 1.000, 95% CI 0.996–1.003, P = 0.824; PFS: HR = 0.998, 95% CI 0.992–1.005, P = 0.733). The estimated 2-year OS and PFS rates were shown in Table 3.
Fig. 2.
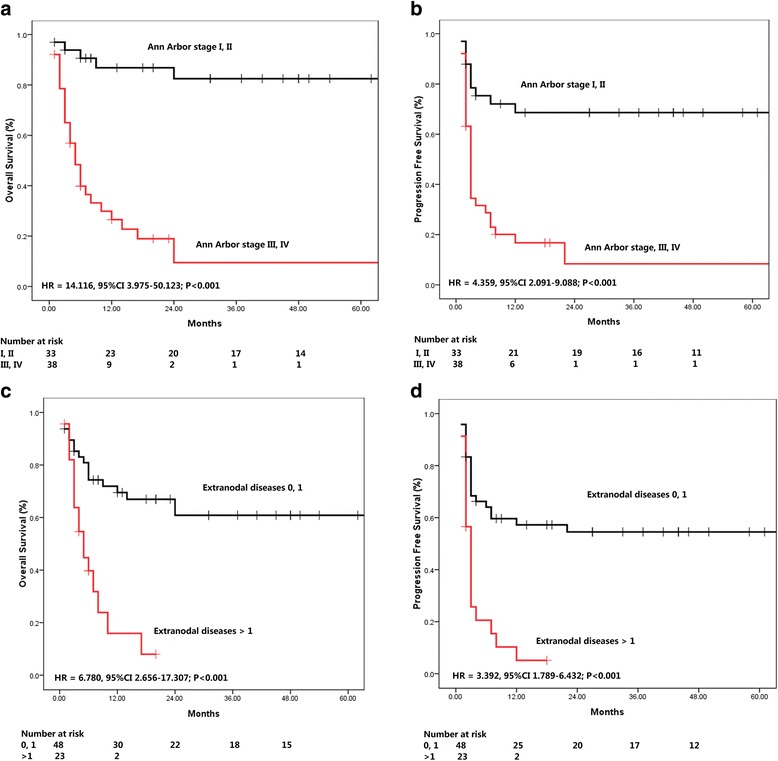
Univariate analysis of potential prognostic clinical factors. a, b Ann Arbor stage for OS and PFS. c, d Extranodal Diseases for OS and PFS
Fig. 3.
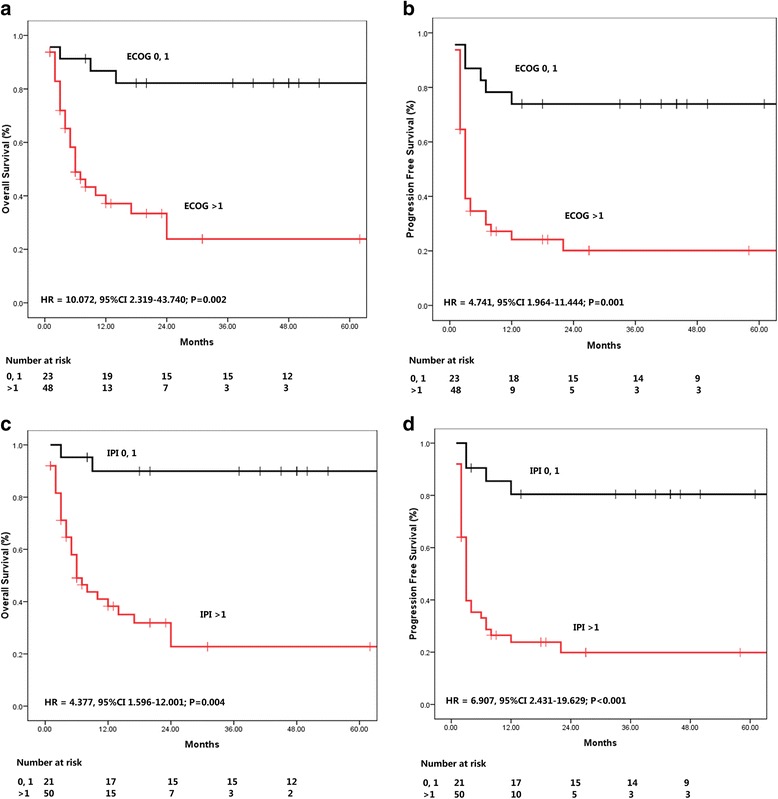
The usefulness of ECOG and IPI in predicting the prognosis of patients. a, b ECOG performance status for OS and PFS. c, d IPI for OS and PFS
Table 3.
The 2 year estimated OS and PFS rate in different subgroups
| OS (%) | PFS (%) | |
|---|---|---|
| Ann Arbor stage | ||
| I, II | 88.6 ± 6.3 | 68.6 ± 8.3 |
| III, IV | 13.0 ± 10.3 | 8.4 ± 6.7 |
| Extranodal diseases | ||
| 0, 1 | 71.7 ± 7.8 | 54.5 ± 7.4 |
| > 1 | 10.3 ± 9.5 | 0 |
| ECOG score | ||
| 0, 1 | 90.2 ± 6.6 | 73.9 ± 9.2 |
| > 1 | 51.4 ± 9.0 | 20.1 ± 6.7 |
| IPI score | ||
| 0, 1 | 95.0 ± 4.9 | 80.4 ± 8.8 |
| > 1 | 49.6 ± 8.8 | 19.9 ± 6.4 |
In multivariate analysis, only IPI score was the independent prognostic factor for predicting both OS (OR = 2.172, 95% CI 1.579–2.987, P < 0.001) and PFS (OR = 1.838, 95% CI 1.406–2.402, P < 0.001) (Table 4).
Discussion
HIV associated NHL represents a type of aggressive malignancies. Although intensive chemotherapy was given, the prognosis of the disease remained very poor. It was controversial whether some important prognostic factors in HIV negative counterpart such as IPI could still work in this special group of the patients [20]. In this study, we included a cohort of 78 patients to analyze the characteristics of AIDS associated NHL and to evaluate the prognostic factors of the disease.
Very similar to the normal HIV negative population, the most common subtype of lymphoma observed in HIV-infected patients was also DLBCL (42/78, 53.8%) [21]. As an important trigger of HIV to activate c-myc, BL was the second common pathologic subtype of the lymphoma (29/78, 37.2%) among this population. Compared to NHL in general population, AIDS patients with NHL presents with a more aggressive features including advanced Ann Arbor stage, significantly elevated LDH, and poor ECOG performance status, and a high IPI score eventually [2]. Of note, BL patients presented with even higher risk features while compared with those with DLBCL [22]. According to our data, BL patients manifested with the feature of more extranodal diseases (P = 0.004) but poorer ECOG scores (P = 0.038).
In this study, it was observed that the Chinese NHL patients presented with the characteristics of very low rate of ART exposure prior to the lymphoma diagnosis. Its negative impact on the anti-tumor treatment outcome could not be excluded. The severe immunosuppression status as well as the more aggressive disease phenotypes of these patients all strongly challenged the health care providers. One of the main reasons for the late ART initiation is that most of AIDS patients in China are from low income class, or so called “grass roots”, who are often less educated, single or separated from their family to work outside to strive. Although Chinese government have made great efforts to provide the basic education of AIDS, making the free ART publicly available, especially to this population, it seemed that a long way still needs to be run to popularize contemporary knowledge of HIV in such a large country with a huge population. The ignorance of the HIV infection of the patients and the prejudice from their family members should be firstly diminished. In this study, most of the patients came to the hospital not for suspicion of HIV infection, but for the complications, including but not limited to fever, unexplained symptoms and signs, or even hematological events like tumor or pancytopenia.
AIDS patients with DLBCL or BL could still achieve a survival plateau. However, it seemed that this plateau is significantly lower than that of the HIV negative counterpart, especially for BL patients [23]. In this study, the 2-year OS and PFS rates for DLBCL and BL were 74.3 ± 8.1%, 28.9 ± 11.0%, and 54.2 ± 8.1%, 19.2 ± 7.5%, respectively. High dose chemotherapy other than Hyper-CVAD A and B using the strategy including more anthracyclin (it was observed that most of BL patients progressed while received B regimen), might be designed. As for DLBCL, CHOP is not enough for the HIV-infected patients. An escalated regimen such as DA-EPOCH or CHOP-E should be considered for further clinical trial.
Various prognostic parameters have been used for predicting the treatment outcome of NHL [7, 11, 24]. Among them, the IPI is probably the most commonly used one [25]. Based on IPI, various systems such as FLIPI were designed for some special subtypes of lymphoma [24]. In this study, we have examined the efficacy of IPI parameters in HIV associated NHL (mostly BL and DLBCL). It was encouraging to prove that IPI parameters still worked in such kind of the patients, almost all the IPI parameters were useful in univariate analysis, and IPI was the independent prognostic factor for predicting both OS and PFS. Interestingly, the initial CD4 count before treatment was not associated with the treatment outcome of lymphoma in terms of CR rate, OS or PFS. It was traditionally believed that a low CD4 cell count was associated with death, which was proved in two large studies in pre-cART and early cART eras [26]. We believed that with the wide application of cART treatment, viral load and other related factors were abrogated.
Conclusion
HIV associated NHLs represents a group of heterogeneous aggressive diseases with poor prognosis. Current anti-lymphoma treatment should be improved in this special group of patients in future clinical practice. IPI still works in the HIV-infected NHL patients in predicting the prognosis during the cART era. However, this study was a retrospective one and the treatment outcome of the patients, especially for BL, need to be further monitored. And the potential usefulness of existing clinical prognostic factors in AIDS patients should be further addressed in future studies.
Acknowledgements
We are grateful Dr. Yan-Ling Feng for the pathologic reviewing and Dr. Hui Zhang for radiological examinations.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 81370653 and 81571977), the 863 project (No.2014AA021403), the Three-Year Action Plan for Public Health (No. 15GWZK0103), and a grant from the Shanghai Municipal Science and Technology Commission (No. 16411960400).
Availability of data and materials
The data and materials are available in the main manuscript.
Authors’ contributions
HZL, YS and RFZ conceived and designed the study. YS, RFZ, LL, YZS, WS, TKQ, YT, ZYW and LQG perform the study, collected and entered the data. YS and RFZ analyzed the data. YS and RFZ wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the independent ethnic board. All patients provided informed consent for both treatment for lymphoma and HIV according to the Declaration of Helsinki.
Abbreviations
- ABC
Activated B-cell subtype
- ADR
Adriamycin
- BL
Burkitt lymphoma
- cART
Combined antiretroviral therapy
- CBC
Complete blood cell count
- CR
Complete response
- CTX
Cyclophosphamide
- DEX
Dexamethasone
- DLBCL
Diffuse large B cell lymphoma
- ECOG
Eastern cooperative oncology group
- FL
Follicular lymphoma
- HIV
Human immune-deficiency virus
- IBL
angioimmunoblastic lymphoma
- IPI
International prognostic index
- LDH
LactateDehydrogenase
- NHL
Non hodgkin’s lymphoma
- OR
Overall response
- ORs
Odds ratios
- OS
Overall survival
- PBL
Plasmablastic lymphoma
- PD
Progressive disease
- PR
Partial response
- SD
Stable disease
- VCR
Vincristine
Additional file
Regimens of chemotherapy. Table S2. Univariate analysis of the factors affecting CR in DLBCL and BL patients. (DOCX 14 kb)
Contributor Information
Yang Shen, Email: shen_yang@126.com.
Renfang Zhang, Email: zrf_1113@163.com.
Li Liu, Email: liulishaphc@163.com.
Yinzhong Shen, Email: 027465@163.com.
Wei Song, Email: songwei@shaphc.org.
Tangkai Qi, Email: qitangkai@shaphc.org.
Yang Tang, Email: tangyang@shaphc.org.
Zhenyan Wang, Email: a1s2r@163.com.
Liqian Guan, Email: guanliqian13@sina.com.
Hongzhou Lu, Email: luhongzhou@fudan.edu.cn.
References
- 1.Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105(16):1221–9. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R, Mitrou P, Arasteh K, Schuermann D, Hentrich M, Duehrsen U, et al. Acquired immunodeficiency syndrome-related lymphoma: simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival--results of the German Multicenter Trial. Cancer. 2006;106(7):1560–8. doi: 10.1002/cncr.21759. [DOI] [PubMed] [Google Scholar]
- 3.Navarro JT, Vall-Llovera F, Mate JL, Morgades M, Feliu E, Ribera JM. Decrease in the frequency of meningeal involvement in AIDS-related systemic lymphoma in patients receiving HAART. Haematologica. 2008;93(1):149–50. doi: 10.3324/haematol.11767. [DOI] [PubMed] [Google Scholar]
- 4.Wolf T, Brodt HR, Fichtlscherer S, Mantzsch K, Hoelzer D, Helm EB, et al. Changing incidence and prognostic factors of survival in AIDS-related non-Hodgkin’s lymphoma in the era of highly active antiretroviral therapy (HAART) Leuk Lymphoma. 2005;46(2):207–15. doi: 10.1080/10428190400015733. [DOI] [PubMed] [Google Scholar]
- 5.Carroll V, Garzino-Demo A. HIV-associated lymphoma in the era of combination antiretroviral therapy: shifting the immunological landscape. Pathog Dis. 2015;73(7):ftv044. [DOI] [PMC free article] [PubMed]
- 6.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–94. [DOI] [PubMed]
- 7.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(14):2373–80. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Yao Y, Li JM, Chen QS, You JH, Zhao HJ, et al. Prognostic factors analysis for R-CHOP regimen therapy in diffuse large B cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2008;29(4):252–7. [PubMed] [Google Scholar]
- 9.Ramaswami R, Chia G, Dalla Pria A, Pinato D, Parker K, Nelson M, et al. Eevolution of HIV associated lymphoma over 3 decades. J Acquir Immune Defic Syndr. 2016;72(2):177–83. doi: 10.1097/QAI.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 10.Mounier N, Spina M, Gabarre J, Raphael M, Rizzardini G, Golfier JB, et al. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107(10):3832–40. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- 11.Barta SK, Xue X, Wang D, Tamari R, Lee JY, Mounier N, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251–62. doi: 10.1182/blood-2013-04-498964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohda S. Overview of lymphoid neoplasms in the fourth edition of the WHO classification. Rinsho byori Jpn J Clin Pathol. 2012;60(6):560–4. [PubMed] [Google Scholar]
- 13.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 14.Li XY, Li JM. Research progress on second-hit in malignant lymphomagenesis-- review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(6):1627–31. [PubMed] [Google Scholar]
- 15.Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017–24. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antinori A, Cingolani A, Alba L, Ammassari A, Serraino D, Ciancio BC, et al. Better response to chemotherapy and prolonged survival in AIDS-related lymphomas responding to highly active antiretroviral therapy. AIDS (London, England) 2001;15(12):1483–91. doi: 10.1097/00002030-200108170-00005. [DOI] [PubMed] [Google Scholar]
- 17.Zhou LL, Wang C, Zhao JH, Yan SK, Gao YR, Cai Q, et al. The role of FDG-PET in staging of lymphoma and evaluation of therapeutic efficiency. Zhonghua Xue Ye Xue Za Zhi. 2009;30(4):233–6. [PubMed] [Google Scholar]
- 18.Zhao J, Qiao W, Wang C, Wang T, Xing Y. Therapeutic evaluation and prognostic value of interim hybrid PET/CT with (18)F-FDG after three to four cycles of chemotherapy in non-Hodgkin’s lymphoma. Hematology (Amsterdam, Netherlands) 2007;12(5):423–30. doi: 10.1080/10245330701393840. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luz E, Marques M, Luz I, Stelitano C, Netto E, Araujo I, et al. Survival and prognostic factors for AIDS and Non-AIDS patients with Non-Hodgkin’s lymphoma in Bahia, brazil: a retrospective cohort study. ISRN Hematol. 2013;2013:904201. doi: 10.1155/2013/904201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PDQ Adult Treatment Editorial Board. AIDS-Related Lymphoma Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002–2015 Apr 2. [PubMed]
- 22.Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS-related Burkitt’s lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol. 2005;23(19):4430–8. doi: 10.1200/JCO.2005.11.973. [DOI] [PubMed] [Google Scholar]
- 23.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS (London, England) 2008;22(4):489–96. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barta SK, Xue X, Wang D, Lee JY, Kaplan LD, Ribera JM, et al. A new prognostic score for AIDS-related lymphomas in the rituximab-era. Haematologica. 2014;99(11):1731–7. doi: 10.3324/haematol.2014.111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Yu Y, Jun-Min L, Jian-Qing M, Qiu-Sheng C, Yu C, et al. Reassessment of the prognostic factors of international prognostic index (IPI) in the patients with diffuse large B-cell lymphoma in an era of R-CHOP in Chinese population. Ann Hematol. 2009;88(9):863–9. doi: 10.1007/s00277-009-0702-1. [DOI] [PubMed] [Google Scholar]
- 26.Bohlius J, Schmidlin K, Costagliola D, Fatkenheuer G, May M, Caro Murillo AM, et al. Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS (London, England) 2009;23(15):2029–37. doi: 10.1097/QAD.0b013e32832e531c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available in the main manuscript.


